Abstract
Primates tend to be long-lived, and, except for humans, most primate females are able to reproduce into old age. Although aging in most mammals is accompanied by dental senescence due to advanced wear, primates have low-crowned teeth that wear down before old age. Because tooth wear alters crown features gradually, testing whether early dental senescence causes reproductive senescence has been difficult. To identify whether and when low-crowned teeth compromise reproductive success, we used a 20-year field study of Propithecus edwardsi, a rainforest lemur from Madagascar with a maximum lifespan of >27 years. We analyzed tooth wear in three dimensions with dental topographic analysis by using Geographical Information Systems (GIS) technology. We report that tooth wear exposes compensatory shearing blades that maintain dental function for 18 years. Beyond this age, female fertility remains high; however infants survive only if lactation seasons have elevated rainfall. Therefore, low-crowned teeth accommodate wear to a point, after which reproductive success closely tracks environmental fluctuations. These results suggest a tooth wear-determined, but rainfall-mediated, onset of reproductive senescence. Additionally, our study indicates that even subtle changes in climate may affect reproductive success of rainforest species.
Keywords: lemur, longevity, wild population, Ranomafana Park, life history
Although mammalian dentitions have features that are adapted to quickly and thoroughly reduce food particle size (1), mastication results in a gradual loss of crown features through wear. Several solutions have evolved that prolong the functional durability of teeth (1, 2), including hypsodonty, or high-crowned teeth, which has appeared repeatedly in connection with increased seasonality and drying of environments (3, 4). Despite living in diverse environments, primates seem to lack extreme adaptations against tooth wear and have brachydont, or low-crowned teeth.
Few primate species are dietary specialists, and most can be classified as generalized herbivores, or as omnivores, eating both plants and animals. Nevertheless, primate species with predominantly frugivorous diets have cheek teeth with rounded cusps and shallow basins, whereas more folivorous species tend to have teeth with steeply angled cusps that frequently join to form shearing crests or blades (1, 5–9). However, these dental differences are manifested most clearly in young individuals because brachydont tooth shapes can change rapidly through wear (1, 10–12).
Early dental senescence due to wear raises questions of how primates are able to support their energy-demanding brains and long lifespans (13, 14) and at what stage dental senescence begins to affect their life history and reproductive success. Although only humans outlive their reproductive senescence substantially, many primates, and mammals in general, show declining reproductive success in old age (15, 16), suggesting that dental senescence could be hypothesized to be a factor in reproductive senescence.
Here, we investigate the loss of tooth functional capacity and its consequences across the primate lifespan by combining data from a detailed long-term field study (17, 18) of the Milne-Edwards' sifaka (Propithecus edwardsi) with dental topographic analysis (19, 20). P. edwardsi is found in the eastern rainforest of Madagascar and is primarily folivorous with seasonal peaks of fruit and seed consumption during the austral summer rains (21). Although the unworn molar morphology of this 6-kg sifaka closely conforms to the primate ideal for folivory, the teeth appear to undergo significant wear over time (Fig. 1A). Yet, the maximum lifespan of P. edwardsi in the wild has been estimated to be at least 27 years, conforming to the captive records showing individuals of several lemur species living >30 years (18).
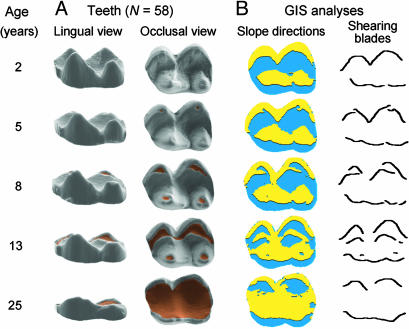
Fig. 1.
Low-crowned (brachydont) cheek teeth of Milne-Edwards' sifaka (P. edwardsi) lose high cusp relief quickly. (A) Lingual and occlusal views of right lower second molar show first signs of exposed dentine (brown color) at 5 years old, and by 25 years of age, the whole crown is worn away. (B) From 3D DEMs, GIS analyses were used to classify buccal and lingual slopes (yellow and blue color) from which shearing blades, including compensatory blades exposed by tooth wear, were delineated. Mean molar length is 7.4 mm.
Fifty-eight mandibular dental impressions from 32 anesthetized sifakas were taken at Ranomafana National Park between 1993 and 2004. To analyze tooth shape changes in three dimensions, we used GIS to measure wear-related tooth shape changes by modeling tooth crowns as landscapes with eroding mountains and valleys (11, 22). We then identified the onset of dental senescence and examined whether fertility or offspring survival are affected by the loss of “dental prime” (onset of dental senescence). Finally, with the 20-year field data, we tested what effect yearly differences in rainfall, proximating environmental quality, have on reproductive success of sifakas at different stages of tooth wear. Our results implicate dental senescence in linking infant survival to rainfall.
Materials and Methods
Field Site. A field study of the arboreal P. edwardsi was initiated in 1986 in the southeastern rainforest of Madagascar, now part of Ranomafana National Park (21°02′–21°25′ S and 47°18′– 47°37′ E). The altitude of the rainforest ranges from 600 m to 1513 m, and rainfall averages 3,090 mm a year, with half of the rain falling between December and April. Currently, a trail network >30 km in length and covering 2.1 km2 is used to study sifakas and other sympatric taxa. Study animals are habituated to human observers and are periodically anesthetically darted, captured, and released (17, 23). Sifakas are marked with collars to facilitate the identification of individuals from a distance.
Age Estimations. Dental impressions (Express, low-viscosity, regular set, 3M Dental Products) were taken of cleaned and dried right mandibular tooth rows from 9, 11, 12, 13, 2, and 11 individuals in 1993, 1994, 1995, 2002, 2003, and 2004, respectively. Casts made from these impressions were then scanned in three dimensions, and digital elevation models (DEMs) of the tooth crowns were used in the GIS analysis. Because the eruption and wear of all molars are nearly synchronous in P. edwardsi, we confined our analysis to the second lower molar (24).
Of the 32 individual sifakas studied, eight had at least three consecutive dental impressions taken. Seventeen were born during the course of the field study, of which the oldest was 18 years in 2004. We used the 2D area of exposed molar dentine of known-aged individuals to estimate ages of all remaining individuals. Rates of dentine exposure are similar in individuals of different age. For the 17 individuals with known ages, a least-squares regression analysis provided the relationship between age and exposed dentine [Age (yrs) = 4.879 × Sqrt (area of exposed dentine) + 1.911; R2 = 0.87].
Using this regression, the oldest living individual, which was fully adult when the study began, was estimated to be 27 years old in 2005. We consider this age to be a reasonable estimate because this individual had to be a minimum of 23 years old, based on the earliest age of first parturition, which is four years in P. edwardsi (17). A second female was also estimated to have been 27 years old when she disappeared and was presumed dead in 1993. Regressions calculated separately for females [5.994 × Sqrt (area of exposed dentine) + 1.892; R2 = 0.97] and males [4.104 × Sqrt (area of exposed dentine) + 2.022; R2 = 0.84] indicate that males appear to have faster tooth wear. Using the sex-specific relationships would decrease the age estimate of the oldest male from 22 to 20 years and increase the estimate of the oldest female from 27 to 32 years. The overall pattern of results is not affected by the regression used for age estimates. For robust age categories, we present our results by using the combined regression and after grouping individuals into four age categories, comprising six-year intervals to age 18 and a single interval thereafter. For data matrix, see Table 1, which is published as supporting information on the PNAS web site.
GIS Analysis of Tooth Wear. High-quality plaster casts (Fujirock, GC Europe, Leuven, Belgium) of mandibular tooth rows were scanned by using a three-dimensional piezo scanner (MDX-15, Roland) with 100-μm resolution. Teeth were oriented manually to maximize crown-base projection (resulting in a roughly horizontal occlusal plane), which remains relatively constant through wear. Three-dimensional point files were exported to GIS software (mfworks 3.0, KeiganSystems, London, ON, Canada) and interpolated by using inverse distance weighting to produce DEMs. The analyses were carried out by using the portion of the second molar crowns that projects above the horizontal plane, defined when the casts were oriented for scanning, through the lowest point in the talonid basin. All of the GIS procedures refer to routines in mfworks 3.0 (25). The surface relief of each tooth is defined as the ratio of 3D area/2D area. Equivalent results were obtained by calculating the average slope across the surface of each tooth by using the grade function.
For detection of shearing blades, we applied the following GIS procedures. DEMs were smoothed (by using the scan function with a circular four-cell moving window) to eliminate artefactual surface roughness introduced by modeling a continuous surface as a collection of cells. The profile function was used to discriminate between those cells that slope buccally and those that slope lingually. To isolate cells corresponding to blades from cells belonging to cusp slopes, we used the recode function to reassign cells with values two and three to have value one, whereas all of the remaining parts of the crown were removed by recoding them to be void. These procedures identify more efficiently shearing blades that are longitudinally oriented, reflecting the lateral, more selenodont pattern of shearing crest formation in P. edwardsi. For a conservative measure of shearing blades, we set the minimum recognizable slope at 5°. Using different cutoff points or manually delineating shearing blades from DEMs did not change the pattern of results.
We calculated blade lengths in two ways. The first definition requires that blade elements consist of at least two contiguous cells, and the more conservative definition (presented in the figures) requires a minimum of four contiguous cells. Adjacent cells were first grouped by using the clump function (with maximum distance set to 1.7 cells), followed by coding the grouped cells based on their length (by using score total function where the zone/region map is the clumped map and the data map is the recoded map). Short blades (shorter than two or four cells long) are removed by recoding them to be void. Finally, the 2D and 3D lengths of all blades were calculated and standardized by using the mesiodistal length of the tooth. For the data matrix, see Table 1. We randomized the age assignments 1,000 times to test differences in tooth wear among age groups.
Additional Tooth Data. Tooth wear marks were quantified by using high-resolution epoxy casts (26). Wear scratch frequencies on enamel were scored from the second molar protoconid cusp in a square that is 0.4 mm on each side at ×35 magnification (26). Additionally, we examined fecal samples collected in 1993 containing easily identifiable seed particles of rahiaka (Chrysophyllum boivinianum; ref. 27). We calculated the proportion of the number of large rahiaka fragments (>2 mm) out of all rahiaka fragments (>1 mm) in the feces of a dentally senescent individual (198 fragments, n = 1) and in the feces of three individuals that were in their dental prime (342–1,181 fragments, n = 6).
Life History Analysis. Data on behavior and infant survival have been collected by using both focal animal and scan sampling techniques. In 34 of the 41 infants born, disappearance date was known within a month. In other cases, a mean survival was tabulated by using observed last presence and first absence. Infanticides were assessed from either direct observations or inferred based on behavioral observations. We randomized the age assignments 1,000 times to test differences in infant survival among age groups. Rainfall data are recorded daily at the field station located within the study area.
To test whether a significant correlation could be obtained between rainfall and infant survival of the dentally senescent females chosen randomly, we randomized age assignments 10,000 times and calculated the correlation coefficient after each randomization (altogether 26 infants, including only those born in years with both dentally prime and senescent females giving birth). From the random probability distribution, we calculated the frequency of the observed or higher correlations.
Results and Discussion
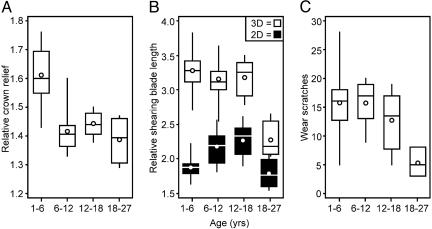
Onset of Dental Senescence. First, we determined how wear affects the overall morphology of brachydont tooth crown in sifakas. Using the digitized tooth crowns, we calculated crown relief (11, 22), which is the ratio of the 3D surface area to the 2D planometric (or “footprint”) area of each tooth crown. Whereas relief was high in young sifakas, as is typical of folivorous primates, relief decreased rapidly with age, reaching minimal values soon after 6 years of age (Fig. 2A). This initial flattening of the tooth crown, to be expected to happen in brachydont teeth, does not seem to be detrimental to sifakas because demographic data (18) indicate that they typically survive well past this age, for example, 44% of the studied individuals had reached 10 years of age.
Fig. 2.
With wear, sifaka teeth quickly lose their crown relief but retain shearing blades and wear characteristics until 18 years of age. (A) The high cusp relief is mostly lost before 7 years of age, but the wear exposes dentine (Fig. 1) and compensatory shearing blades form (visible as an increase in 2D blade length in B), resulting in the maintenance of the overall 3D blade length. (B) Compensatory blades are present until the whole enamel crown is worn down at ≈18 years of age when, without the high crown relief, the 3D blade length plummets (P = 0.001, tested by using 1,000 randomizations). (C) The drop in blade lengths also marks a drop in low-magnification use wear scratches tabulated in a square that is 0.4 mm on each side (P = 0.002, tested by using 1,000 randomizations). The circles denote means. The boxes, with the median indicated, enclose 50% of the observations, and the whiskers denote range. The numbers of individuals in each age group (starting from the youngest) are 22, 10, 6, and 3, and the corresponding number of dental casts (due to multiple captures) are 29, 16, 9, and 4.
We next examined more closely what happened to teeth as the crowns wear flat. Across mammalian species, efficient particlesize reduction has been shown to correlate with the length of shearing blades, irrespective of exact cusp relief (1, 8, 12), and, recently, Ungar and M'Kirera (28) demonstrated that the overall angularity of tooth crown surfaces is conserved despite tooth wear. We therefore used GIS to delineate and measure shearing blades by identifying where buccally facing surfaces give way to lingually facing surfaces (Fig. 1). We measured the 3D lengths of crests and the 2D lengths of their projections onto the xy plane. Blade lengths were standardized by the mesiodistal lengths of teeth; thus, a tooth crown with a blade length of two has twice its length in shearing blades.
The results show that, despite the rapid flattening of tooth crowns (Fig. 2 A), the relative 3D length of shearing blades remains nearly constant for >10 additional years (Fig. 2B). Our GIS analysis shows that this constancy is accomplished by the formation of compensatory shearing blades as the enamel cover wears, revealing islands of dentine surrounded by newly exposed cutting edges (Fig. 1B). As crown relief diminishes, overall shearing blade length is maintained as long as blades that are folded into vertical planes in unworn teeth are supplemented by the compensatory blades in worn teeth (Figs. 1 and 2 A and B). It is noteworthy that, to the extent that the compensatory blades show species-specific differences, as shown for crown surface angularity (28), 3D blade lengths may be useful in identifying extinct species even from worn teeth.
As more of the compensatory blades are formed, the sifaka molars become reminiscent of the more selenodont herbivore morphology of marsupial koala teeth (see the age 13 tooth in Fig. 1). In the latter, wear-exposed compensatory blades substitute for the high crests of unworn teeth until these blades are also lost and additional chewing strokes are required to process the same amount of food (29, 30). In sifakas, this point of dental senescence is reached after 18 years of age (Fig. 2B) when the occlusal surface has been reduced to a shallow dentine bowl surrounded by a low-relief enamel band (Fig. 1 A).
These results on tooth wear (Fig. 2B) suggest that, unlike the loss of high crown relief early in life, the loss of compensatory blades at two-thirds of the maximum lifespan signifies the transition from dental prime to dental senescence. This inference about the onset of dental senescence is supported by our analysis of low-magnification use wear (26). Wear scratches calculated in an enamel covered square reveals that the frequency of wear features decreases dramatically in concert with plummeting blade lengths (Fig. 2C). Furthermore, our field observations of fecal samples indicate a poorer food particle size reduction in a dentally senescent sifaka compared with three sifakas in their dental prime (ratios of >2-mm large seed fragments to >1-mm fragments are 0.48 and 0.04–0.19, respectively).
Tooth Wear, Fertility, and Infant Survival. Despite their loss of the dental prime after 18 years of age, some sifakas seem able to survive almost another 10 years with teeth that are not only worn flat, but that also have 30% shorter shearing blades (Fig. 2). Because these individuals are few in number, they may contribute little to population growth. However, during the 20-year study, an average of 14% of adult sifakas were past their dental prime, indicating that long-lived individuals may in fact play an important role in population growth.
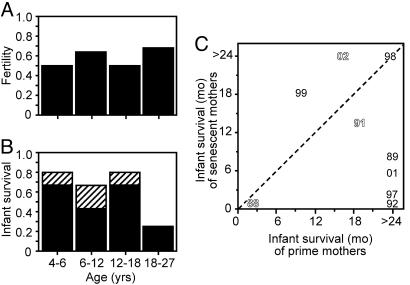
Next, we searched for signs of reproductive senescence in the three oldest dentally senescent females. First, we examined fertility and observed that females of all age groups have approximately one infant every other year (Fig. 3A). Thus, both the early loss of crown relief (Fig. 2 A) and the loss of compensatory blades (Fig. 2B) seem to be irrelevant to fertility. This apparent lack of reproductive senescence sensu stricto in P. edwardsi conforms to earlier studies on lemurs showing no gradual decline in fertility with age (15, 31). Measuring fertility alone, however, may not capture all aspects of reproductive senescence. Indeed, compared with other primates of similar body mass, lemurs have relatively small newborns, suggesting a limited investment in the gestation phase of reproduction (21). Because small newborns may signify that infant survival, rather than fertility, may be sensitive to aging, we examined next whether dental senescence is associated with infant survival.
Fig. 3.
Fertility remained high, whereas infant survival declined in dentally senescent females. (A) Females in all age groups have had one infant in every other year. (B) More than half of these infants survived 24 months when born to dentally prime mothers, whereas a quarter survived when born to dentally senescent mothers (age 18 and above, P = 0.073, tested by using 1,000 randomizations). Calculating infant survival after excluding infanticidal mortality increases infant survival of only prime mothers (hatched, P = 0.009, tested by using 1,000 randomizations). (C) A pairwise comparison of years when both females in and past their dental prime had infants shows that in the majority of years, the dentally senescent mothers lost infants first (years with infanticide are in outline lettering). The dashed line represents equal infant longevity of dentally prime and dentally senescent females. The numbers of females in each age group (starting from the youngest) are 4, 5, 2, and 3, and the corresponding total observation years are 12, 25, 12, and 19.
One identified mortality factor of newborn lemurs is infanticide where immigrating lemurs kill infants of resident females (18, 21, 32). Because the role of dental senescence in infanticide is likely to be very indirect, we analyzed infant survival both with and without infanticides. The results show that infant survival drops in the oldest, dentally senescent, females (Fig. 3B). Furthermore, all incidents of infanticide affect females in their dental prime, suggesting that dental senescence is associated with an age-specific mortality factor for infants (Fig. 3B). Increased predation is unlikely to be this mortality factor because the only known P. edwardsi predator, a nocturnal viverrid (Cryptoprocta ferox) does not appear to preferentially target old individuals, typically killing both the mother and the infant (17, 18).
A distinct possibility for the drop in infant survival in our data is that the three oldest females happened by chance to give birth during years when younger females also lost their infants.
However, infant-survival data tabulated yearly show that, even together with infanticides that affect only females in their dental prime, old females lose their infants earlier than younger females in 67% of cases (Fig. 3C). It is noteworthy that also in P. verreauxi verreauxi, which lives in harsher and more seasonal dry forests in Madagascar, infant survival of old females drops, but, additionally, some of the oldest females show a decline in fertility (31).
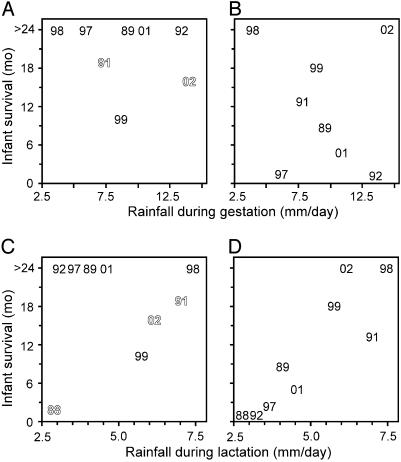
Tooth Wear, Infant Survival, and Rainfall. Because our field observations indicated that, apart from infanticide, infants tend to die after exhibiting signs of general weakening, we examined whether environmental conditions, as measured by monthly rainfall, might account for the higher infant mortality of dentally senescent mothers. P. edwardsi typically breeds in November to January and has a 6-month gestation period. Infants are usually born by the second half of June, during the austral winter (17, 18). Prime lactation takes place during the dryer season when sifakas survive mostly on leaves, and weaning becomes conspicuous in January during peaking austral summer rains and fruit availability (17, 21). Furthermore, production of new leaves, which require less dental processing than dryer and more fibrous mature leaves (1), corresponds with rainfall (33). To identify the period of the year critical for infant survival, we tested separately whether rainfall during the gestation or lactation season correlates with infant survival. The results show that rainfall during gestation does not correlate with infant survival for either young or old females (Fig. 4 A and B).
Fig. 4.
Rainfall during lactation correlates with infant survival in dentally senescent females. In dentally prime (A) and senescent (B) females, infants survive even during years with little rain during the gestation season. However, in dentally prime (C) and senescent (D) females, rain during lactation season is critical for infant survival of dentally senescent females (D). The correlations (rs) between rainfall and infant survival for A, B, C, and D, respectively, are –0.19, –0.16, 0.04, and 0.88, and the corresponding P values are 0.61, 0.68, 0.92, and 0.01. Correlations calculated separately for each age class in their dental prime range from –0.35 to 0.28, all not significant. In B and D, the infant survival data from –88 to –92 and from –97 to –02 are for two different females except in –02, which includes infant survival for a third female as well. Years with infanticide are in outline lettering.
However, although infants of young females survive even during years with little rain during lactation (Fig. 4C), the infants of old females appear to require >5 mm rain per day during lactation to survive past 1 year of age (Fig. 4D; rs = 0.88, P = 0.01). Furthermore, to test how likely this significant correlation between rainfall and infant survival of dentally senescent mothers is in our data, we assigned ages randomly 10,000 times and calculated rs after each randomization. The probabilities are P = 0.0042 (randomizing all of the years together) and P < 0.0001 (randomizing age assignments within each year separately), suggesting that dental senescence is a key factor in linking infant survival to rainfall.
Although future work is needed to identify the exact mechanism of how dental senescence may affect infant survival, the role of lactation in primate life history is indicative of how tooth wear could compromise reproductive success. Compared with gestation, lactation is a more energy-demanding period of reproduction (34, 35), when both environmental conditions and maternal characteristics, such as dominance rank, can have lasting effects on survival and fitness of the offspring (34, 36, 37). Our results indicate that maternal dental function during lactation, in connection with environmental conditions, may be critical for infant survival. Milk of many lemurs is dilute, and as the source of both nutrition and water, lactation is crucial for an infant's growth and the prevention of dehydration (35). Because P. edwardsi appears to obtain all water through its diet (33), years with an especially dry lactation season may be demanding both in terms of nutrition and water. We therefore propose that, even in rainforest settings, aging primates are not immune to environmental fluctuations and that dental senescence can play a role in the senescence of reproductive success. Based on our link between the relatively subtle variation in rainforest rainfall and infant survival, we predict that the effects of dental senescence may be more pronounced in mammals living in more seasonal habitats. For example, even small morphological differences in teeth affecting the onset of dental senescence can be hypothesized to contribute to the different levels of frailty found among baboon populations (38). Furthermore, in koalas, which also live in dryer habitats than P. edwardsi, individuals with worn teeth have been shown to decrease their reproductive effort (30).
In conclusion, we have shown that despite the rapid early loss of crown relief in a rainforest primate with typical low-crowned teeth, compensatory shearing blades form to maintain dental function until two-thirds of the maximum lifespan. After this age, dental function plummets but without directly affecting the fertility of older females. Instead, loss of dental capacity appears ultimately to limit fitness by rendering old females unable to buffer their reproductive success against environmental fluctuations. Thus, sifakas seem to have an incipient tendency to live beyond their reproductive senescence, a condition that is fully manifested in human females (and to lesser degree in macaques and other taxa; refs. 15, 16, and 39). Such a gap between reproductive and dental senescence may, over evolutionary time, result in changes that prolong functional durability of teeth but may, over ecological time, render endangered rainforest species vulnerable to rapid climate change.
Supplementary Material
Acknowledgments
We thank K. Glander, T. Morelli, Raymond Ratsimbazafy, Rémí Rakotovao, Georges René Randrianirina, Laurent “Raleso” Randrianasolo, Telo Albert, J. Wyatt, and the late Georges Rakotonirina, who was instrumental in the initiation of this study, for excellent technical help in the field; the staff of the Centre ValBio Research Station, Association Nationale pour la Gestion des Aires Protégées, Madagascar Institute pour la Conservation des Ecosystèmes Tropicaux, and Institute for the Conservation of Tropical Enviroments for logistical support; and A. Evans, M. Fortelius, I. Salazar-Ciudad, P. Ungar, and G. Wilson for comments and advice on this work. Field research was authorized by Ministère de l'Environnement, des Eaux et Forêts. This study was supported by the Academy of Finland, Earthwatch Institute, a J. William Fulbright Award, the L. S. B. Leakey Foundation, the National Science Foundation (including Doctoral Dissertation Improvement Grants), Primate Conservation, Inc., the St. Louis Zoological Society (Field Research Council Grant), the National Geographic Society, and the Wenner-Gren Foundation for Anthropological Research.
Author contributions: S.J.K., P.C.W., and J.J. designed research; S.J.K., S.J.A.-N., S.T.P., G.M.S., P.C.W., and J.J. performed research; L.R.G., P.C.W., and J.J. contributed new reagents/analytic tools; S.J.K., S.J.A.-N., S.T.P., G.M.S., L.R.G., P.C.W., and J.J. analyzed data; and S.J.K., L.R.G., P.C.W., and J.J. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: DEMs, digital elevation models; GIS, Geographical Information Systems.
See Commentary on page 16533.
References
- 1.Lucas, P. W. (2004) Dental Functional Morphology (Cambridge Univ. Press, Cambridge, U.K.).
- 2.Janis, C. M. & Fortelius, M. (1988) Biol. Rev. 63, 197–230. [DOI] [PubMed] [Google Scholar]
- 3.Williams, S. H. & Kay, R. F. (2001) J. Mammal. Evol. 8, 207–229. [Google Scholar]
- 4.Jernvall, J. & Fortelius, M. (2002) Nature 417, 538–540. [DOI] [PubMed] [Google Scholar]
- 5.Kay, R. F. (1975) Am. J. Phys. Anthropol. 43, 195–216. [DOI] [PubMed] [Google Scholar]
- 6.Walker, P. & Murray, P. (1975) in Primate Functional Morphology and Evolution, ed. Tuttle, R. (Mouton, The Hague, The Netherlands), pp. 135–150.
- 7.Kay, R. F. & Hylander, W. L. (1978) in The Ecology of Arboreal Folivores, ed. Montgomery, G. G. (Smithsonian Institution Press, Washington, DC), pp. 173–192.
- 8.Sheine, W. S. & Kay, R. F. (1982) J. Morphol. 172, 139–149. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita, N. (1998) Am. J. Phys. Anthropol. 106, 169–188. [DOI] [PubMed] [Google Scholar]
- 10.Rensberger, J. M. (1973) J. Paleontol. 47, 515–528. [Google Scholar]
- 11.Dennis, J. C., Ungar, P. S., Teaford, M. F. & Glander, K. E. (2004) Am. J. Phys. Anthropol. 125, 152–161. [DOI] [PubMed] [Google Scholar]
- 12.Evans, A. R. (2005) Biol. J. Linn. Soc. Lond. 85, 81–96. [Google Scholar]
- 13.Sacher, G. A. (1975) in Primate Functional Morphology and Evolution, ed. Tuttle, R. (Mouton, The Hague, The Netherlands), pp. 417–441.
- 14.Deaner, R, O., Barton, R. A. & van Schaik, C. P. (2003) in Primate Life Histories and Socioecology, eds. Kappeler, P. M. & Pereira, M. E. (Univ. of Chicago Press, Chicago) pp. 233–265.
- 15.Caro, T. M., Sellen, D. W., Parish, A., Frank, R., Brown, D. M., Voland, E. & Borgerhoff Mulder, M. (1995) Int. J. Primatol. 16, 205–220. [Google Scholar]
- 16.Cohen, A. A. (2004) Biol. Rev. 79, 733–750. [DOI] [PubMed] [Google Scholar]
- 17.Wright, P. C. (1995) Int. J. Primatol. 16, 835–854. [Google Scholar]
- 18.Pochron, S. T., Tucker, W. T. & Wright, P. C. (2004) Am. J. Phys. Anthropol. 125, 61–72. [DOI] [PubMed] [Google Scholar]
- 19.Zucotti, L. F., Williamson, M. D., Limp, W. F. & Ungar, P. S. (1998) Am. J. Phys. Anthropol. 107, 137–142. [DOI] [PubMed] [Google Scholar]
- 20.Jernvall, J. & Selänne, L. (1999) Palaeontologia Electronica. Vol. 2, Issue 1 (March 15). Available at www-odp.tamu.edu/paleo. Accessed October 10, 2005.
- 21.Wright, P. C. (1999) Yearb. Phys. Anthropol. 42, 31–72. [Google Scholar]
- 22.Ungar, P. S. & Williamson, M. (2000) Palaeontologia Electronica. Vol. 3, Issue 1 (March 15). Available at www-odp.tamu.edu/paleo. Accessed October 10, 2005.
- 23.Glander, K. E., Fedigan, L. M., Fedigan, L. & Chapman, C. (1991) Folia Primatol. 57, 70–82. [DOI] [PubMed] [Google Scholar]
- 24.Godfrey, L. R., Samonds, K. E., Jungers, W. L., Sutherland, M. R. & Irwin M. T. (2004) Am. J. Phys. Anthropol. 123, 250–276. [DOI] [PubMed] [Google Scholar]
- 25.Tomlin, C. D. (1990) Geographic Information Systems and Cartographic Modelling (Prentice Hall, Englewood Cliffs, NJ).
- 26.Semprebon, G. M., Godfrey, L. R., Solounias, N., Sutherland, M. R. & Jungers, W. L. (2004) J. Hum. Evol. 47, 115–144. [DOI] [PubMed] [Google Scholar]
- 27.Schatz, G. E. (2001) Generic Tree Flora of Madagascar (Missouri Botanical Garden, St. Louis, and Royal Botanic Gardens, Kew, U.K.).
- 28.Ungar, P. S. & M'Kirera, F. (2003) Proc. Natl. Acad. Sci. USA 100, 3874–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanyon, J. M. & Sanson, G. D. (1986) J. Zool. Ser. A 209, 169–181. [Google Scholar]
- 30.Logan, M. & Sanson, G. D. (2002) Aust. J. Zool. 50, 621–626. [Google Scholar]
- 31.Richard, A. F., Dewar, R. E., Schwartz, M. & Ratsirarson, J. (2002) J. Zool. Lond. 256, 421–436. [Google Scholar]
- 32.Erhart, E. M. & Overdorff, D. J. (1998) Int. J. Primatol. 19, 73–81. [Google Scholar]
- 33.Hemingway, C. A. (1998) Int. J. Primatol. 19, 355–377. [Google Scholar]
- 34.Altmann, J. (2001) Baboon Mothers and Infants (Univ. of Chicago Press, Chicago).
- 35.Tilden, C. D. & Oftedal, O. T. (1997) Am. J. Primatol. 41, 195–211. [DOI] [PubMed] [Google Scholar]
- 36.Jolly, A., Dobson, A., Rasamimanana, H. M., Walker, J., O'Connor, S., Solberg, M. & Perel, V. (2002) Int. J. Primatol. 23, 327–353. [Google Scholar]
- 37.Altmann, J. & Alberts, S. C. (2005) Behav. Ecol. Sociobiol. 57, 490–501. [Google Scholar]
- 38.Bronikowski, A. M., Alberts, S. C., Altmann, J., Packer, C., Carey, K. D. & Tatar, M. (2002) Proc. Natl. Acad. Sci. USA 99, 9591–9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fedigan, L. M. & Pavelka, M. S. M. (2001) Int. J. Primatol. 22, 109–125. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.