Abstract
Previous studies have implicated extracellular carbonic anhydrases (CAs) in buffering the alkaline pH shifts that accompany neuronal activity in the rat and mouse hippocampus. CAs IV and XIV both have been proposed to mediate this extracellular buffering. To examine the relative importance of these two isozymes in this and other physiological functions attributed to extracellular CAs, we produced CA IV and CA XIV knockout (KO) mice by targeted mutagenesis and the doubly deficient CA IV/XIV KO mice by intercrossing the individual null mice. Although CA IV and CA XIV null mice both are viable, the CA IV nulls are produced in smaller numbers than predicted, indicating either fetal or postnatal losses, which preferentially affect females. CA IV/XIV double KO mice are also produced in fewer numbers than predicted and are smaller than WT mice, and many females die prematurely before and after weaning. Electrophysiological studies on hippocampal slices on these KO mice showed that either CA can mediate buffering after synaptic transmission in hippocampal slices in the absence of the other, but that eliminating both is nearly as effective as the CA inhibitor, benzolamide, in blocking the buffering seen in the WT mice. Thus, both CA IV and CA XIV contribute to extracellular buffering in the central nervous system, although CA IV appears to be more important in the hippocampus. These individual and double KO mice should be valuable tools in clarifying the relative contributions of each CA to other physiological functions where extracellular CAs have been implicated.
Keywords: interstitial pH, mouse hippocampus, targeted mutagenesis
Rapid shifts in extracellular (interstitial) pH (pHe) are known to accompany neuronal discharge (1, 2). These changes can affect neuronal function by their effect on ion gated channels (3). The magnitude of their influence depends on the size, rate, and spread of these changes in pHe. One key factor in modulating these changes is the buffering capacity of the extracellular fluid. The largest potential source of this buffering activity is the CO2:bicarbonate buffering system, which depends on the reversible hydration of CO2 in the reaction, CO2 + H2O = H+ + HCO-3. This reaction occurs spontaneously, but can be accelerated nearly 5,000 times by one or more of the enzymes called carbonic anhydrases (CAs).
The first evidence that CAs play a role in buffering pH shifts in extracellular fluid in brain came from in vivo studies showing that the CA inhibitor acetazolamide markedly amplified the magnitude and duration of the alkaline pH transients associated with neuronal activity in rat cerebellum (4). Later studies (5-7) in brain slices showed similar effects with CA inhibitors that do not easily cross membranes, implicating an extracellular CA activity in this buffering. The same effects were observed in the CA II-deficient mouse, ruling out the possibility that the high activity isozyme, CA II, which is very abundant in cytosol of oligodendrocytes and also present in astrocytes, was mediating this effect after its release during preparation of the brain slices (8).
Initially, this extracellular CA activity was assumed to be CA IV, which was at the time the only known CA isozyme associated with the extracellular surface and had been demonstrated on certain endothelial and epithelial membranes (9-11). Subsequently, other extracellular CAs were described (12), including CA IX, CA XII, and CA XIV, all of which are membrane CAs whose CA domain faces the extracellular space. Evidence that CA IV was indeed a major player in buffering the interstitial space in hippocampal slices was provided by the demonstration that treatment of hippocampal slices with phosphatidylinositol-specific phospholipase C treatment was almost as effective as treatment with the impermeant inhibitor, benzolamide, in blocking the extracellular buffering (13). However, some residual extracellular CA activity remained after this treatment. Once it was shown immunologically to be present on neurons and axons in mouse brain (14) CA XIV became a logical candidate for this other CA.
To clarify the individual contributions of CA IV and CA XIV in this and other physiological systems where extracellular CAs have been implicated, we produced and characterized CA IV and CA XIV knockout (KO) mouse strains by targeted mutagenesis. From these individual CA IV KO and CA XIV KO mice, we produced the doubly deficient CA IV/CA XIV KO mice by intercrossing. In this article, we describe the production and characterization of these strains and also present electrophysiological studies on hippocampal slices from each of these strains. The results suggest that both CA XIV and CA IV contribute to buffering the interstitial space in mouse hippocampus.
Materials and Methods
Antibodies against mouse CA IV or mouse CA XIV were raised in rabbits by using affinity pure recombinant CA IV or CA XIV as described (15). Rabbit anti-goat IgG-peroxidase conjugates were purchased from Sigma-Aldrich. Molecular weight standards were from Bio-Rad.
Targeted Mutagenesis of the Mouse CA 4 Gene. To generate CA IV KO mice, we constructed a replacement targeting vector by ligating a 3.6-kb KpnI-XhoI fragment, containing intron 1a of the CA 4 gene, on one side of the Neo gene of the targeting vector pPNT-Cass LoxA (16, 17) and a 4.5-kb XhoI-NotI genomic fragment, extending from exon 4 to intron 7, on the other side of the Neo gene. Deleted exons 1b, 2, and 3, and 9 aa of exon 4 encoded all three active-site His residues in the 146 aa immediately after the signal sequence. The resulting frameshift produces an early termination in exon 4. After expression of cre recombinase in the germ line of the male chimeric transgenic mice, the tACE-CRE-neor cassette (17) is excised from the genome, leaving only one LoxP site behind.
The targeting vector was linearized and introduced into the 129S1/Sv-+p-derived ES cell line, W9.5s (Yale University, New Haven, CT; Jackson Catalog 000090, 1 × 107 cells) by electroporation (230 V and 500 μF) in a Bio-Rad Gene Pulser. After positive/negative selection in 400 μg of G418 (GIBCO/Invitrogen, Carlsbad, CA) and 2 μM ganciclovir (Syntex, Palo Alto, CA), Southern blot analysis was performed on genomic DNA from resistant clones. The genomic DNA was digested with SpeI and analyzed with an external probe from intron 1. Two clones demonstrating homologous recombination (see Fig. 5, which is published as supporting information on the PNAS web site) were microinjected into blastocysts of C57BL/6J mice and transferred into pseudopregnant mice as described (16). Chimeric offspring were bred to C57BL/6J mice. The agouti F1 offspring were tested for the targeted allele by PCR and direct sequencing of the PCR product. Heterozygous matings of the F1 mice produced homozygous F2 mutant mice.
The CA IV KO offspring were genotyped by PCR analysis of tail genomic DNA. The PCR conditions included 10 cycles at 94°C for 15 s, 68°C for 1 min and 20 cycles of 94°C for 15 s, 68°C for 1 min with 20-s autoextension, using 5′-CACCCTTCATCCTCGTCGGCTATGACCAAAAGC and 5′-TGAGTTCAATTCCCAGCTCCCACATTGTGGC as forward primers for WT and mutant alleles, respectively, and 5′-TGTGGATCTTGATGGGTTGTTTGTACACAGTCC as reverse primer for both. Products obtained from the KO and WT alleles were 700 bp and 1.5 kb, respectively.
Targeted Mutagenesis of the Mouse CA 14 Gene. The CA 14 gene was isolated from an S129 genomic library. The targeting vector was designed to replace exon 4, 16 bp of intron 3, and 13 bp of intron 4 with the tACE-CRE-neor cassette. An ≈3-kb NotI/XhoI genomic fragment, extending from intron 1 to intron 4, was ligated at one end of the cassette and an ≈2.2-kb fragment encompassing intron 4 to intron 11 was ligated at the opposite end of the cassette. Deleted exon 4 encodes 47 aa of the CA XIV coding sequence, including two of three active-site His residues (His-94 and His-96). The resulting frameshift predicts early termination in exon 5. Introduction of the targeting vector into the ES cells and positive/negative selection were as described above. Southern blot analysis was performed on genomic DNA from resistant clones. The DNA was digested with HpaI and AhdI and probed with an external probe (950-bp fragment encompassing 210 bp at the 3′ end of exon 1 and 740 bp at the 5′ end of intron 1) (see Fig. 6, which is published as supporting information on the PNAS web site).
Cells from two independent homologous recombinant clones were microinjected into blastocysts and transferred into pseudopregnant mice, and chimeric offspring were bred to C57BL/6J mice. The agouti F1 offspring were tested for the targeted allele by PCR and direct sequencing of the amplified PCR product. Heterozygous matings of the F1 mice produced homozygous F2 mutant mice.
PCR conditions for genotyping CA XIV KO mice included 30 cycles at 92°C for 15 s, 68°C for 2 min with 2-s autoextension, with 5′-AGGCTCCTGCAGCTAAGGTGGGATCAGACG (forward primer) and 5′-TCCATCCTCTGAGCTCCACTGGACCGAACC (reverse primer). Products obtained from the KO and WT alleles were 200 and 350 bp, respectively. Their identities were confirmed by direct sequencing. In accordance with Mouse Nomenclature Rules and Guidelines from The Jackson Laboratories (www.informatics.jax.org/mgihome/nomen/table.shtml), the CA IV KO mouse line was designated CAIVdl1Sws. The CA XIV KO mouse line was designated CAXIVdl1Sws.
RT-PCR of CA IV and CA XIV Transcripts. One microgram of total RNA isolated from mouse tissues with a RNeasy kit (Qiagen, Valencia, CA) was reverse-transcribed with oligo(dT) primer in a 20-μl final volume and used as template for PCR amplification. The CA IV primers were 5′-CACCCTTCATCCTCGTCGGCTATGACCAAAAGC (forward primer) and 5′-TGTGGATCTTGATGGGTTGTTTGTACACAGTCC (reverse primer). A 500-bp PCR product was predicted from WT animals but none was expected from the CA IV KO mice.
The RT-PCR conditions for CA XIV transcripts were as described above. The primers were 5′-GCAGCTTTCCCTGCCCCCAACCCTGC (forward primer) and 5′-GCCTCTGTGGTAGCCCGAGCAGAGGTG (reverse primer). A 750-bp PCR product was predicted from CA XIV RNA from WT animals, but none from CA XIV KO mice.
Isolation of Tissue Membranes and Western Blot Analysis. Mouse tissues were homogenized in 3 volumes of homogenization buffer, 25 mM Tris·SO4 (pH 7.5) and 1 mM each of O- phenanthroline, benzamidine-HCl, and iodoacetamide, with a polytron homogenizer. Total tissue membranes were recovered by centrifugation at 500 × g for 15 min followed by centrifugation of postnuclear supernatant at 100,000 × g for 30 min at 4°C. The membrane pellets were resuspended in homogenization buffer by sonication. The protein concentration was determined by the microLowry procedure (18).
The tissue membranes (50 μg protein) were solubilized in nonreducing sample buffer. SDS/PAGE was carried out (19), after which the peptides were electrophoretically transferred to Immobilon membranes. The CAs were identified by incubating with antisera against mouse CA IV or mouse CA XIV followed by secondary antibodies conjugated with peroxidase. The blots were developed by using isoluminol substrate for peroxidase.
Preparation and Solutions for Studies of Brain pHe. Studies were performed on hippocampal slices prepared from adult WT and CA KO mice. Transverse slices of 300-μm thickness were cut on a Vibratome under ice-cold artificial cerebral spinal fluid (aCSF). Slices were then incubated in aCSF at room temperature for at least 1 h before electrophysiological experiments. The aCSF contained 124 mM NaCl, 3.0 mM KCl, 2.0 mM CaCl2, 1.5 mM MgCl2, 26 mM NaHCO3, 1.0 mM NaH2PO4, and 10 mM d-glucose and was gassed with 95% O2 and 5% CO2 (pH 7.4). To ensure pure antidromic activation of pyramidal neurons, the aCSF also contained 20 μM 6-cyano-7-nitro-nitroquinoxaline-2,3-dione (CNQX) and 50 μM 2-amino-5-phosphonovalerate (APV) to block ionotropic glutamate receptors and 100 μM picrotoxin to block GABA-A receptors. To detect the activity of extracellular CA, benzolamide (10 μM) was added to the aCSF during experiments (see below). CNQX and APV were obtained from Tocris Cookson (Ellisville, MO). Picrotoxin was purchased from Sigma-Aldrich. Benzolamide was a gift of E. R. Swenson (University of Washington, Seattle).
Stimulation and Recording. Double-barreled pH microelectrodes were fabricated as described by Chesler and Chan (20). In brief, a pair of bound, thin-walled glass capillaries [AM Systems (Carlsborg, WA) 6170] was heated, twisted 180°, and then drawn on a vertical pipette puller. The reference barrel was backfilled with 2 M NaCl, and the eventual pH-sensitive barrel was backfilled with a solution containing 75 mM NaCl and 75 mM NaH2PO4 (pH 7.4). The tip of the double-barreled pipette was then broken to a diameter of 3-5 μm. The pH barrel was rendered hydrophobic by repeated suction and ejection of 4% trimethylchlorosilane (Sigma) in xylene. A column of proton-selective mixture (Fluka 95291) was incorporated into the tip by suction. The slope response for pHe electrodes was 57-59 mV per decade change in H+ activity. Slow DC potentials on the reference barrels were subtracted from the voltage on the ion-selective barrels to yield the H+ signals. Evoked antidromic field potentials were recorded by the reference barrel.
Effect of Benzolamide on the CA Activities of Mouse CA IV and CA XIV. The affinity-purified mouse CA IV or mouse CA XIV equivalent to 1 unit of protein were mixed with 0-1.0 μM benzolamide in 10 mM Tris·SO4 (pH 7.5) before assaying for CA. The CA activity was determined by using the endpoint titration assay method described by Sundaram et al. (21). The concentration of benzolamide that inhibited 50% of the enzyme activity was named the inhibition constant, Ki.
Results
Generation of the CA IV KO Mice. The CA 4 gene was targeted in ES cells, and the mutant allele (Fig. 1A) was identified by Southern blot as described in Materials and Methods (see Fig. 5). Tail-positive chimeric mice produced from blastocyst injections confirmed germ-line transmission of the mutant allele. F1 and F2 offspring produced by mating heterozygotes were genotyped by PCR from genomic DNA, and homozygotes were identified (Fig. 1B). Although F1 heterozygote matings produced F2 homozygotes of S129sv × C57BL/6 hybrid strain background, the number of mice homozygous for the deletion was only 38% of the number expected from Mendelian ratios, i.e., 55 of expected 145 (see Table 1, which is published as supporting information on the PNAS web site). In addition, the sex ratio was skewed; only 31% of the homozygotes were female (38 male, 17 female). Thus, there was a reduced number of both male and female CA IV mice, but a greater deficiency of females. Regardless of gender, the CA IV KO mice that survived to weaning appeared healthy, grew, and were fertile when crossed with WT partners. However, matings between male and female homozygous CA IV KO mice produced small litters, and the pups produced did not survive, even when the male parent was removed immediately after birth. For this reason, CA IV KO mice used in physiological studies were produced from heterozygote crosses.
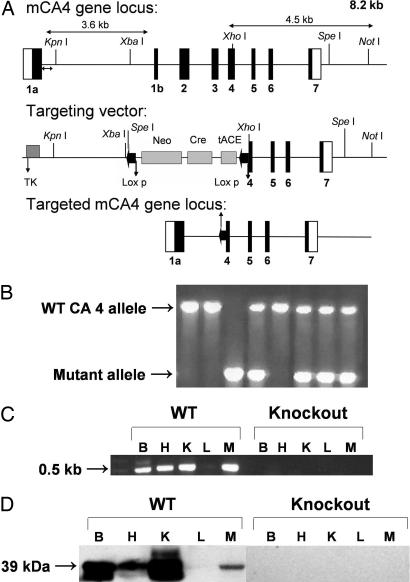
Fig. 1.
Generation, genotyping, and characterization of CA IV KO mice. (A) Structure of the CA IV gene (Top), the targeting construct (Middle), and the predicted structure of the disrupted CA IV gene after homologous recombination and excision of the Neo cassette (Bottom). The double arrow indicates the position of the external probe. Only the relevant restriction sites are shown. The numbered solid boxes represent exons. Neo and TK (thymidine kinase) refer to positive and negative selection markers, respectively. (B) Genotyping of CA IV KO colony. Genotyping by PCR was done on mouse tail genomic DNA. PCR products present in WT (1.5 kb) and mutant (0.7 kb) mice were both present in the heterozygotes. (C) CA IV transcripts in brain (B), heart (H), kidney (K), liver (L), and muscle (M) from WT and KO mice. Total RNA (≤ 1 μg) was reverse-transcribed and amplified by PCR. A 0.5-kb band was amplified from all tissues except liver from the WT animals but none from the KO mice. (D) Western blot analysis. The membrane proteins (50 μg) from brain (B), heart (H), kidney (K), liver (L), and muscle (M) of CA IV WT and KO mice were electrophoresed on a 12% SDS gel. After blotting, the membranes were probed with rabbit anti-mouse CA IV antibodies. A 39-kDa band of varying intensity was present in all tissues examined except liver from the WT animals. None was present in tissues from KO animals.
Analysis of KO Mice for CA IV Transcript and Protein. The presence of CA 4 transcripts was ascertained by RT-PCR on total RNA from brain, heart, kidney, liver, and muscle of either WT or the putative KO mice (Fig. 1C). The expected 0.5-kb fragment was amplified from total RNA from brain, heart, kidney, and muscle, but not liver, from the WT mouse, but not from RNA from any of the same tissues from homozygous KO mice. The absence of transcript in liver from WT mice is consistent with previous immunohistochemical evidence that hepatocytes in WT mice do not express CA IV, although they do express CA XIV (22). CA IV protein was analyzed by Western blot on membrane preparations from brain, heart, kidney, liver, and muscle from either WT or KO animals (Fig. 1D). A 39-kDa band was present in extracts of all tissues examined except liver from WT animals. No CA IV protein was detectable in KO mice in tissues that normally express CA IV.
Generation of CA XIV KO Mice. CA XIV KO mice were produced by targeted mutagenesis of the CA 14 gene in ES cells as described in Materials and Methods. The mutant allele (Fig. 2A) was identified in targeted ES cells by Southern blot (see Fig. 6). Offspring of chimeric mice produced from blastocyst injections were screened by PCR to identify germ-line transmission. Both the F1 mice and the F2 offspring produced by mating heterozygotes were genotyped by PCR from genomic DNA and homozygotes identified (Fig. 2B). Homozygous CA XIV KO mice for electrophysiological experiments were produced by heterozygote or homozygote matings. The numbers and genders of WT, heterozygous, and homozygous mice were nearly those expected from Mendelian ratios (see Table 1). The CA XIV KO mice appeared healthy, grew, and were fertile.
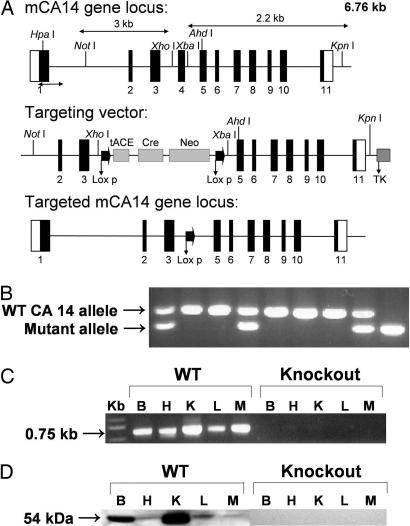
Fig. 2.
Generation, genotyping, and characterization of CA XIV KO mice. (A) Structure of the CA XIV gene (Top), the targeting construct (Middle), and the predicted structure of the disrupted CA XIV gene after homologous recombination and excision of the Neo cassette (Bottom). The double arrow indicates the position of the external probe. Relevant restriction sites are shown. The numbered solid boxes represent exons. Neo and TK (thymidine kinase) refers to positive and negative selection markers, respectively. (B) Genotyping of CA XIV KO colony. PCR was done on mouse tail genomic DNA. PCR products amplified from WT (350 bp) and mutant (200 bp) mice were both present in the heterozygotes. (C) CA XIV transcript analysis in brain (B), heart (H), kidney (K), liver (L), and muscle (M) from WT and KO mice. Total RNA (≤ 1 μg) was reverse-transcribed and amplified by PCR. A 0.75-kb band was seen in RNA from WT mice but none in RNA from KO animals. (D) Western blot analysis. Membrane proteins (50 μg) from brain (B), heart (H), kidney (K), liver (L), and muscle (M) of CA XIV WT and KO mice were electrophoresed on a 12% SDS gel. After blotting, the membranes were probed with rabbit anti-mouse CA XIV antibodies. A 54-kDa band was present in all tissues examined from WT animals. No CA XIV protein was present in any tissues from KO animals.
Analysis of CA XIV mRNA Transcript and Protein. To identify CA 14 transcripts, we performed RT-PCR on total RNA from brain, heart, kidney, liver, and muscle of either WT or KO animals (Fig. 2C). The predicted 0.75-kb fragment was amplified from RNA from tissues of WT mice, but none from the same tissues from CA XIV KO mice. To detect CA XIV protein in tissues from the KO mice, we performed Western blot analyses on membrane preparations from brain, heart, kidney, liver, and muscle from WT and KO animals. A 54-kDa band was detected in extracts of tissues from the WT mice but was missing in the same tissues from KO mice (Fig. 2D).
Generation of CA IV/CA XIV Double KO Mice. To produce mice lacking both CA IV and CA XIV, we had to breed CA IV-/- male mice with CA XIV-/- female mice over several generations to obtain breeding pairs of CA IV-/-/CA XIV-/- males and CA IV+/-/CA XIV-/- females. These breeding pairs produced successful pregnancies and live-born pups that the CA IV+/-/CA XIV-/- mothers nurtured through weaning. However, the number of CA IV/CA XIV double KO mice was only 38% of the number expected (23 instead of 60). The sex ratio was distorted with 74% of the CA IV/CA XIV double KO being male (17 of 23). The male double KO mice appeared healthy, grew, and were fertile, but both males and females were significantly smaller than WT mice. The males were used for the physiological studies described below. Many of the female double KO mice died early, and the few that survived were infertile.
Physiological Studies of Hippocampal Slices. Hippocampal slices were studied in a submersion-style recording chamber superfused with aCSF at 32°C. The CA1 pyramidal neurons were activated antidromically by stimuli delivered to their axons by a twisted pair of 50-μm-diameter platinum iridium wires placed on the alveus (Fig. 3A). A pH microelectrode was lowered into the CA1 area stratum pyramidale (Fig. 3B) to a depth (of ≈100 μm) where maximal amplitude of the antidromic field potential (Fig. 3C) was elicited by single supramaximal antidromic stimulus (200-μs duration). Further experiments were carried out only if the population spike amplitude exceeded 1 mV.
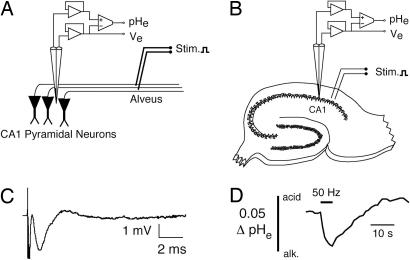
Fig. 3.
Stimulation and recording arrangement. (A) Schematized stimulation and recording arrangement. CA1 pyramidal neurons were activated by antidromic action potentials elicited by stimuli delivered to their axons in the alveus. (B) Placement of double-barreled pH microelectrode in the stratum radiatum of the CA1 pyramidal layer, with approximate location of stimulating electrode. (C) Example of a single antidromic population spike record via the reference barrel of a pH microelectrode. (D) Example of an extracellular alkaline pHe transient elicited by 50-Hz stimulation of the alveus for 5 s. Alkaline changes are downward in all records.
Detection of Extracellular CA Activity and Data Analysis on CA IV KO and CA XIV KO Mice. Activation of the hippocampal pyramidal neurons causes a stereotyped extracellular alkaline transient. This response was postulated to result from Ca2+-H+ exchange mediated by the plasmalemmal Ca2+-ATPase [reviewed by Chesler (23)]. These alkaline transients are buffered by extracellular CA-catalyzed hydration of CO2. The amplification of the alkalosis by the poorly permeant CA inhibitor benzolamide serves as an indicator of this extracellular CA activity. To test the effect of benzolamide on mouse hippocampal slices, a 5-s, 50-Hz, supramaximal antidromic stimulus train was used to elicit an alkaline transient (Fig. 3D). Two to three such responses were evoked (at 5-min intervals), and their amplitudes were averaged to provide a control amplitude for each slice. The superfusate was then changed to aCSF containing 10 μM benzolamide. After 15 min, a second series of alkaline responses was evoked, and their amplitudes were averaged. For each slice, the effect of benzolamide was quantified as percent amplification of the control response amplitude.
Evoked pHe Transients in WT vs. CA KO Mice. The same paradigm was carried out on hippocampal slices from WT, CA IV KO, CA XIV KO, and CA IV/CA XIV double KO animals. For WT, CA IV KO, CA XIV KO, and CA IV/CA XIV double KO preparations, multiple comparisons of alkaline shift amplitude, the effect of benzolamide, and the antidromic field potential amplitude were carried out by using four-way ANOVA with a Bonferroni post hoc test. Means were presented with standard error and n values for the number of slices.
In hippocampal slices from WT animals, a 5-s, 50-Hz antidromic stimulus train caused an alkaline transient with a mean amplitude of 0.04 ± 0.006 pH units (n = 16) in the stratum pyramidale (Fig. 4A). Similar results were noted in slices from the single KO animals. The mean alkaline transient was 0.06 ± 0.008 (n = 14) in slices from CA IV KO mice and 0.03 ± 0.006 (n = 22) in slices from CA XIV KO mice. There were no significant pairwise differences among the alkaline responses from WT, CA IV KO, and CA XIV KO slices (P > 0.05). There was also no pairwise difference in the amplitude of the evoked field potential recorded at the start or the end of the 50-Hz stimulus train, suggesting that excitability of the CA1 pyramidal neurons was comparable among preparations.
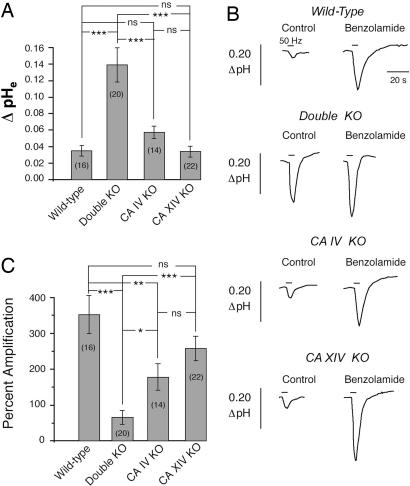
Fig. 4.
Comparison of evoked pHe responses. (A) Mean alkaline transient amplitude in hippocampal slices from WT and KO animals. (B) Effect of benzolamide on the alkaline transients recorded in hippocampal slices from WT and KO animals. Alkaline shifts are downward. (C) Composite effect of benzolamide on the amplitude of the alkaline transient in WT and KO preparations (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, P > 0.05).
In hippocampal slices from CA IV/CA XIV double KO animals, the alkaline transient averaged 0.14 ± 0.016 units pH (n = 20), which was significantly greater than the mean responses in slices from WT, CA IV KO, and CA XIV KO slices. This difference was not attributable to increased excitability of the double KO preparation, as there were no pairwise differences in the amplitude of the first or last field potential of the 50-Hz stimulus train. Statistics for the amplitude of the alkaline transient for all four preparations are summarized in Fig. 4A. These data suggest that both CA IV and CA XIV contribute to the extracellular buffering in the WT mouse and, at least, that either is sufficient when the other is eliminated. The effect of loss of both is very striking.
Effect of Benzolamide in WT vs. CA KO Mice. In aCSF containing 10 μM benzolamide, the amplitude of the evoked alkaline transient was increased in all preparations (Fig. 4 B and C). In slices from WT animals, benzolamide amplified the alkalosis by 352 ± 53% (n = 16). In the slices from CA XIV KO mice, the mean amplification of the response (258 ± 34%, n = 22) was not significantly different from WT. In the slices from CA IV KO mice, however, benzolamide had a significantly smaller effect compared with WT, with a mean amplification of 178 ± 37% (n = 14, P < 0.01). The alkaline transient of the slices from the double KO mice was amplified least by benzolamide, with a mean amplification of 65 ± 19% (n = 20) that was smaller compared with the effect on slices from WT (P < 0.001), CA XIV KO (P < 0.001), and CA IV KO (P < 0.05) mice. In all preparations, benzolamide had no effect on the amplitude of the antidromic field potentials.
The inhibition constant, Ki, for benzolamide with affinity-purified mouse CA IV and CA XIV was 0.3 ± 0.03 μM and 0.2 ± 0.02 μM, respectively. CA IV and CA XIV are very sensitive to benzolamide and both should be completely inhibited by the concentration used (10 μM) in these experiments.
Discussion
The fact that CA IV and CA XIV KO mice survived demonstrated that neither gene is essential for viability, although the CA IV KO mice of both sexes were produced in lower than expected numbers, and females were preferentially lost in gestation or during the early newborn period. The bases for the deviation from Mendelian ratios and the preferential loss of females have not yet been established. On the other hand, the CA XIV KO mice of both sexes appeared to have normal viability and fertility.
The double KO mice were also produced in lower than expected numbers. In addition, the mice were smaller than WT mice, and many females died before age 10 months. Nonetheless, we produced sufficient numbers of single and double KO mice for electophysiological studies to study the relative contributions of CA IV and CA XIV to buffering the extracellular space in the hippocampus. Previous studies of this region in the brain in the rat suggested a dominant role for CA IV in buffering the extracellular space. The most convincing evidence was the observation that treatment of hippocampal slices with phosphoinositol-specific phospholipase C eliminated most of the extracellular CA activity (13). The only CA known at that time that would be expected to be removed by this treatment was the glycosylphosphatidylinositol-anchored CA IV. In addition, another study recently demonstrated that nearly all of the extracellular CA on the surface of rat astrocytes was attributable to CA IV (24). On the other hand, immunocytochemical studies showed that CA XIV was present on some neurons and axons in brain tissue (14), leading Parkkila et al. (14) to suggest that CA XIV was also important.
The present studies on single and double KO mice provide evidence that CA XIV does in fact contribute to extracellular buffering in the mouse hippocampus. In the CA IV KO mouse, the activity of the remaining CA XIV was sufficient to keep the alkaline transient similar in amplitude to that of WT (Fig. 4A). This result suggests that CA XIV can contribute to pHe regulation. Moreover, KO of the remaining CA XIV activity, i.e., in the double KO preparation, increased the amplitude >2-fold compared with slices from the CA IV single KO mice (Fig. 4 A and B). This observation again argues that CA XIV can play a role in pHe regulation.
However, there was no statistical difference in the effect of the CA inhibitor, benzolamide, when comparing the CA XIV KO with WT. In these CA XIV KO mice, the benzolamide inhibited the remaining CA IV. Thus, in the absence of CA XIV, the CA IV expressed by itself appeared to have nearly the same capacity to regulate pHe as did CA IV plus CA XIV in the WT mouse.
In the CA IV KO mice, the benzolamide inhibited the remaining CA XIV (Fig. 4C). In these CA IV KO mice, there was a significant difference in amplification by benzolamide compared with the WT and double KO mice, which argues that CA XIV was clearly working to regulate pHe.
Taken together, these data are consistent with both CA IV and CA XIV contributing to pH regulation in the extracellular space in the hippocampus. They are also consistent with CA IV having a somewhat greater effect on pH regulation than CA XIV in this region of the brain. Of course, one could overestimate the importance of either of these isozymes in normal physiology from these studies if one or the other of the two genes (or both) is up-regulated in response to the KO of the other, compared with its level of expression in the WT mouse. However, comparisons of CA IV and CA XIV transcript levels by real-time PCR in the respective mice showed no evidence of up-regulation of either transcript when the other was absent (data not shown). We also found no significant difference in the Ki of benzolamide for CA IV or CA XIV.
Although the evidence indicates that both CA IV and CA XIV contribute to buffering the extracellular space in mouse hippocampus, different regions of the brain may rely more on one isozyme than another. An extreme example of this fact was pointed out recently when two laboratories showed independently that neural retina, one of the most highly active regions of the nervous system metabolically, expresses CA XIV abundantly instead of CA IV on the surface of Müller glial cells and astrocytes (25, 26). Both studies confirmed an earlier study showing that no CA IV was detected in retina (27). An example of the other extreme was suggested by the observation that CA IV is the dominant, if not the only, CA expressed on the surface of both isolated and cultured cortical astrocytes (24).
One caution in interpreting these results is that EST data (www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=unigene) suggest that two additional membrane CAs, CA XII and CA XV, are expressed in brain. Hilvo et al. (28) recently characterized CA XV and confirmed that brain was one of the mouse tissues in which CA XV mRNA was detected. Regional localization of CA XII and CA XV in brain has not yet been determined. Thus, it is unclear whether either could contribute to buffering the extracellular space in the hippocampus.
Another interesting question raised by these observations is whether the absence of either CA IV or CA XIV or both will produce cognitive impairment caused by loss of the CA-enhanced buffering of the extracellular space in some regions of the brain. This and other interesting questions about the relative roles of CA IV and CA XIV in the physiology of brain, eye, kidney, and muscle in which both enzymes are expressed remain challenges for future studies for which the single and double KO mice described here are well suited.
Supplementary Material
Acknowledgments
We thank Tracey Baird for editorial assistance in preparing this manuscript. This work was supported by National Institutes of Health Grants DK40163 (to W.S.S.) and NS032123 (to M.C.).
Author contributions: G.N.S., A.W., S.M., N.S., M.C., and W.S.S. designed research; G.N.S., B.U., A.W., T.B., S.M., and N.S. performed research; G.N.S., B.U., A.W., T.B., S.M., and N.S. contributed new reagents/analytic tools; G.N.S., A.W., S.M., N.S., M.C., and W.S.S. analyzed data; and G.N.S., A.W., M.C., and W.S.S. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: CA, carbonic anhydrase; KO, knockout; aCSF, artificial cerebral spinal fluid; pHe, extracellular pH.
References
- 1.Chesler, M. (1990) Prog. Neurobiol. 34, 401-427. [DOI] [PubMed] [Google Scholar]
- 2.Chesler, M. & Kaila, K. (1992) Trends Neurosci. 15, 396-402. [DOI] [PubMed] [Google Scholar]
- 3.Traynelis, S. F. (1998) in pH and Brain Functions, eds. Kaila, K. & Ransom, B. (Wiley-Liss, New York), pp. 417-446.
- 4.Kraig, R. P., Ferreira-Filho, C. R. & Nicholson, C. (1983) J. Neurophysiol. 49, 831-850. [DOI] [PubMed] [Google Scholar]
- 5.Chen, J. C. & Chesler, M. (1992) J. Neurophysiol. 67, 29-36. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J. C. & Chesler, M. (1992) Proc. Natl. Acad. Sci. USA 89, 7786-7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaila, K., Paalasmaa, P., Taira, T. & Voipio, J. (1992) NeuroReport 3, 105-108. [DOI] [PubMed] [Google Scholar]
- 8.Tong, C. K., Cammer, W. & Chesler, M. (2000) Glia 31, 125-130. [DOI] [PubMed] [Google Scholar]
- 9.Carter, N. D., Fryer, A., Grant, A. G., Hume, R., Strange, R. G. & Wistrand, P. J. (1990) Biochim. Biophys. Acta 1026, 113-116. [DOI] [PubMed] [Google Scholar]
- 10.Ghandour, M. S., Langley, O. K., Zhu, X. L., Waheed, A. & Sly, W. S. (1992) Proc. Natl. Acad. Sci. USA 89, 6823-6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waheed, A., Zhu, X. L., Sly, W. S., Wetzel, P. & Gros, G. (1992) Arch. Biochem. Biophys. 294, 550-556. [DOI] [PubMed] [Google Scholar]
- 12.Hewett-Emmett, D. (2000) in The Carbonic Anhydrases: New Horizons, eds. Chegwidden, W. R., Carter, N. D. & Edwards, Y. H. (Birkhauser, Basel), pp. 29-76.
- 13.Tong, C. K., Brion, L. P., Suarez, C. & Chesler, M. (2000) J. Neurosci. 20, 8247-8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parkkila, S., Parkkila, A. K., Rajaniemi, H., Shah, G. N., Grubb, J. H., Waheed, A. & Sly, W. S. (2001) Proc. Natl. Acad. Sci. USA 98, 1918-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu, X. L. & Sly, W. S. (1990) J. Biol. Chem. 265, 8795-8801. [PubMed] [Google Scholar]
- 16.Fleming, R. E., Ahmann, J. R., Migas, M. C., Waheed, A., Koeffler, H. P., Kawabata, H., Britton, R. S., Bacon, B. R. & Sly, W. S. (2002) Proc. Natl. Acad. Sci. USA 99, 10653-10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunting, M., Bernstein, K. E., Greer, J. M., Capecchi, M. R. & Thomas, K. R. (1999) Genes Dev. 13, 1524-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. (1951) J. Biol. Chem. 193, 265-275. [PubMed] [Google Scholar]
- 19.Laemmli, U. K. (1970) Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- 20.Chesler, M. & Chan, C. Y. (1988) Neuroscience 27, 941-948. [DOI] [PubMed] [Google Scholar]
- 21.Sundaram, V., Rumbolo, P., Grubb, J., Strisciuglio, P. & Sly, W. S. (1986) Am. J. Hum. Genet. 38, 125-136. [PMC free article] [PubMed] [Google Scholar]
- 22.Parkkila, S., Kivela, A. J., Kaunisto, K., Parkkila, A. K., Hakkola, J., Rajaniemi, H., Waheed, A. & Sly, W. S. (2002) BMC Gastroenterol. 2, 13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chesler, M. (2003) Physiol. Rev. 83, 1183-1221. [DOI] [PubMed] [Google Scholar]
- 24.Svichar, N., Esquenazi, S., Waheed, A., Sly, W. S. & Chesler, M. (2005) Glia, in press. [DOI] [PubMed]
- 25.Ochrietor, J. D., Clamp, M. F., Grubb, J. H., Shah, G. N., Waheed, A., Sly, W. S. & Linser, P. J. (2005) Exp. Eye Res. 81, 492-500. [DOI] [PubMed] [Google Scholar]
- 26.Nagelhus, E. A., Mathiisen, T. M., Bateman, A. C., Haug, F. M., Ottersen, O. P., Grubb, J. H., Waheed, A. & Sly, W. S. (2005) Proc. Natl. Acad. Sci. USA 102, 8030-8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hageman, G. S., Zhu, X. L., Waheed, A. & Sly, W. S. (1991) Proc. Natl. Acad. Sci. USA 88, 2716-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilvo, M., Tolvanen, M., Clark, A., Shen, B., Shah, G. N., Waheed, A., Halmi, P., Hanninen, M., Hamalainen, J. M., Vihinen, M., et al. (2005) Biochem. J., in press. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.