Abstract
Trichogramma are facultative gregarious egg parasitoids that attack a wide range of lepidopterous eggs. Because hosts in which parasitoids develop vary in terms of available food, the progeny produced by parasitoid females vary in size and fitness. While one might expect that the developmental rate and emergence rhythm should be similar for all individuals reared under the same environmental conditions, variations in the duration of development of individuals reared under uniform conditions have been found in several insect species. In the Hymenopteran egg parasitoid Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae) adults emerge at the beginning of the photoperiod on two consecutive days. Longer development may influence the fitness of adults and have an impact on mating opportunities. Size, longevity and daily and lifetime fecundity were measured for female T. evanescens that developed in nine and ten days. We observed a significant relationship between size and both longevity and lifetime fecundity. While early emerging females did not live longer and did not produce progeny with a different sex-ratio than females that emerged later, they were larger and produced more progeny than late females. We conclude that early emerging females have a higher fitness than late emerging females T. evanescens.
Keywords: emergence, fecundity, longevity, parasitoid, sex-ratio, size
Introduction
Trichogramma species are facultatively gregarious (Rabinovich 1971), polyphagous egg parasitoids that are often used in inundative biological control programs (Smith 1996) against a wide range of Lepidopterous eggs (Corrigan and Laing 1994). Trichogramma spp. are moderately synovigenic (Jervis et al. 2001) and females start to lay their eggs shortly after emergence (Bigler et al. 1987; Chassain and Boulétreau 1991). Hosts in which parasitoids develop are variable resources in terms of available food and, as a result, parasitoid adults vary in size with males being generally smaller than females (van den Assem et al. 1989). In a host of a given size, females grow larger than males and males emerge earlier than females (Charnov et al. 1981).
Several studies have found that the female's fitness increases with its size (King 1987; Boivin and Lagacé 1999) indicating that, for an adult parasitoid, being large has consequences on its reproductive success (van den Assem et al. 1989). While body size has no direct relation to fitness, it may be used as a primary proxy when its relationship with another primary proxy (for example fecundity, longevity or mating ability) is known (Roitberg et al. 2001). The size of a female may influence its longevity, its searching efficiency, its egg load and its capacity to locate hosts. The size of a male may influence its longevity, its capacity to locate females and the number of matings (Godfray 1994). In general, the size of Trichogramma species seems to be positively related to performance (McDougall and Mills 1997).
Insect developmental rates and emergence rhythms tend to be similar for all individuals of a population reared under the same environmental conditions (Saunders 1976; Beck 1991). However, variations in the developmental period of cohorts reared under the same conditions have been found in many insects (Shaffer 1983). In the species Trichogramma evanescens, emergence occur mostly at the beginning of the photoperiod and are spread over two days (nine and ten days at 24 ± 1 °C) with 89% of the males and 95% of the females emerging on the first day (Doyon and Boivin 2004). Because emergence is concentrated at the beginning of the photoperiod, the duration of immature development varies by a few hours between individuals depending on the time of egg deposition. When a female parasitizes a host late in the day, the immature may not be ready to emerge on the morning of the ninth day and have to delay its emergence until the next day. Individuals that delay emergence may experience either an increase or a decrease in fitness, depending on how host resources are used. An increase in fitness is expected if the supplementary developmental time can be used by the individual to either exploit more fully the host resource or to mature. However, a decrease in fitness is more likely if the individual is merely waiting for emergence while burning reserves to maintain its somatic functions. Individuals emerging later can also be penalized by encountering low-quality mates (Carvalho et al. 1998) or by experiencing lower mating opportunities (Waage and Ng 1984).
Emerging early in the day is certainly an advantage for short-lived diurnal species such as Trichogramma. Under laboratory conditions, T. evanescens females deposit 56% of their eggs during the first 24 hours (Boivin and Lagacé 1999) and survival in the field probably does not exceed 24–48 hours. It is, therefore, important to emerge at the beginning of a day when host location is possible. Females that are unable to emerge on the morning of the ninth day are thus facing a trade-off between emerging late in the day and missing oviposition opportunities or delaying emergence to the tenth day and possibly having a lower fitness.
In this paper, we compared the longevity, fecundity (daily and lifetime fecundity) and size of females T. evanescens that emerged after nine days of development (early females) versus females that emerged after ten days of development (late females).
Material and Methods
Biological material
The T. evanescens strain we used originated from Egypt, and was reared on Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs at 25 ± 1 °C, 40 ± 10% RH under a light dark photoperiod of 8L:16D. Individuals of E. kuehniella were reared at 25 ± 2 °C, 40 ± 10% RH and 8L:16D. The E. kuehniella eggs were less than 24 h old and were cold sterilized at −15 °C for 24 h.
Longevity, fecundity and size of female T. evanescens
Approximately 100 T. evanescens females were placed in contact with about 1000 E. kuehniella eggs for 30 min and after which the eggs were incubated at 24 ± 1 °C. Under these conditions, female T. evanescens emerge on the ninth (early females) and tenth (late females) days after oviposition (Doyon 2004). Early (n = 29) and late females (n = 26) were individually mated at emergence by a male less than 3 h old and were then placed in a 3.7 ml tube (without food) with approximately 250 eggs of E. kuehniella that were changed every 24 h during the life of the female. These eggs were incubated in the same rearing chamber, in 3.7 ml tubes, and at emergence the individuals were counted and the sex-ratio calculated. To determine the longevity of early (n = 30) and late females (n = 30), we monitored their survival every 4 h (±15 min) from 6:30 to 22:30. The death of females was assigned to the mid-point between two observations. Because overall body size and hind tibia length are highly correlated in Trichogramma species (Bai 1986), and because it is less sensitive to distortion than other body parts (Bai 1986), at the end of the experiment, the hind tibia length of all female T. evanescens was measured.
Statistical analyses
Linear regressions were used to obtain the relation between size and longevity, and size and lifetime fecundity. An analysis of covariance with size as co-variable was used to compare the lifetime fecundity of early and late females. The longevity and daily fecundity of early and late females T. evanescens were compared with t-tests. The Fisher's exact test was used to compare the daily sex-ratio of the progeny of the females.
Results
As expected, a significant relationship was found between size and longevity (Fig. 1) and size and lifetime fecundity (Fig. 2) for both early and late females. The mean longevity of early females (112.45 ± 0.25 h) was not significantly different from the longevity of late females (116.25 ± 0.25 h) (t = −0.0164; df = 58; p = 0.630). The longevities varied from a minimum of 43.5 h to a maximum of 162.5 h.
Figure 1.
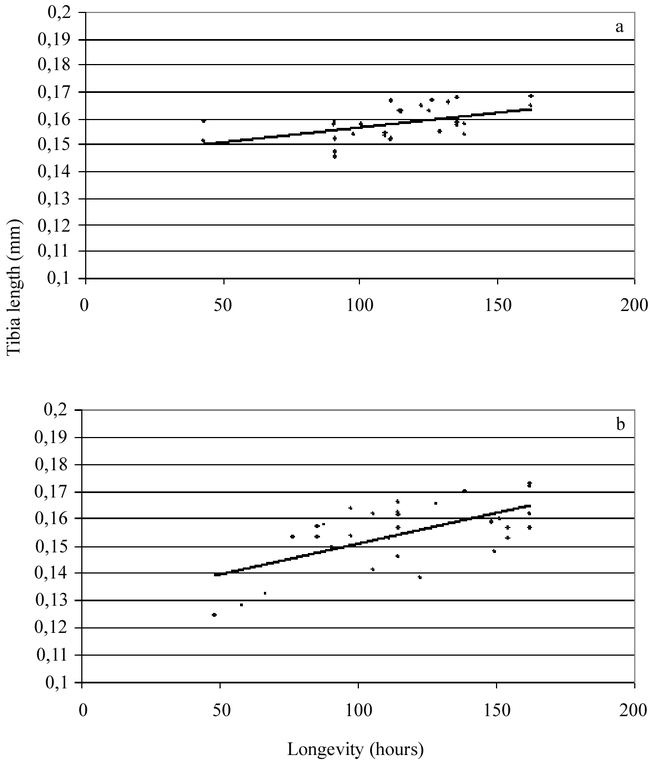
Longevity vs. tibia length of (a) early females (linear regression R = 0.516, d.f. = 29, p d′′ 0.01). (b) late females (linear regression R = 0.624, d.f. = 29, p d′′ 0.01).
Figure 2.
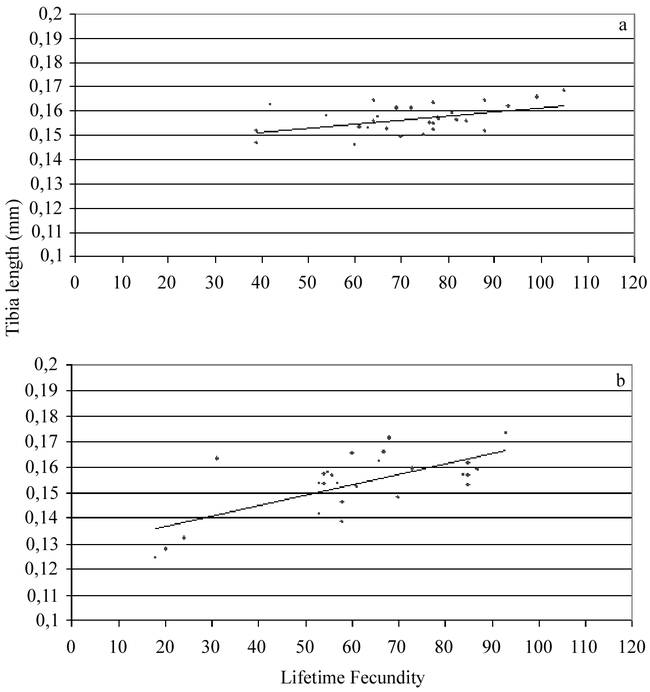
Lifetime fecundity vs. tibia length of (a) early females (linear regression R = 0.478, d.f. = 28, p d′′ 0.01). (b) late females (linear regression R = 0.676, d.f. = 25, p d′′ 0.01).
On the first four days of their life, early females had a higher daily fecundity than late females. From day 5 to day 7, there was no significant difference between the fecundity of early and late females (Table 1). The daily (Table 2) and lifetime sex-ratio of the progeny produced by early and late females did not differ (Fisher's exact test p = 0.4284; df = 1; p > 0.05). However, the sex-ratio of the progeny of females increased with the age of the female in both treatments (Table 2).
Table 1.
Daily fecundity of early and late female T. evanescens reared on E. kuehniella.
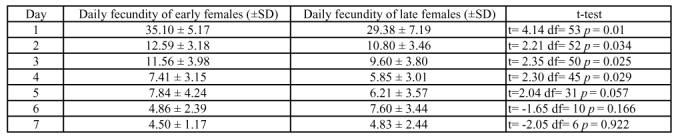
Table 2.
Sex-ratio of early and late females T. evanescens reared on E. kuehniella.
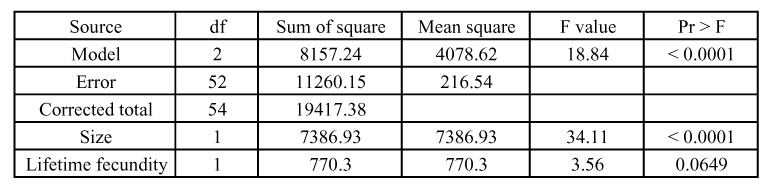
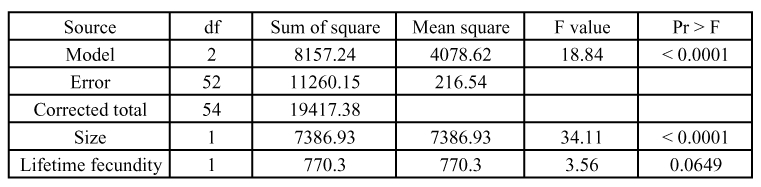
Because fecundity is correlated with size in many parasitoid species, the lifetime fecundity of females was analyzed with size as a co-variable. While the complete model showed highly significant differences between durations of development, the individual analysis indicated that lifetime fecundity showed no significant differences, although fecundity was higher for females with short developmental time (early females = 70.18 eggs, late females = 62.57 eggs). Size was highly significantly correlated with fecundity (Table 3). The mean hind tibia length was 0.160 ± 0.007 mm for early females and 0.154 ± 0.012 mm for late females. The smallest hind tibia length was 0.141 mm for early females and 0.124 mm for late females. The largest hind tibia length was 0.178 mm for early females and 0.173 (±0.012) mm for late females.
Table 3.
Analysis of variance of the lifetime fecundity of early and late females with size as a co-variable.
Discussion
In T. evanescens, a significant relationship was found between the size of females and both longevity and lifetime fecundity. These data confirm the results of Waage and Ng (1984), van den Assem (1989), Bennett and Hoffmann (1998), Boivin and Lagacé (1999), Sagarra et al. (2001) and Rivero and West (2002) who showed that large females parasitoids live longer and produce more progeny than small females, and therefore are at an advantage because they have a higher fitness. Therefore, if differences in size are observed between early and late females, we can predict that these differences will translate into differences in fitness.
Both longevity and the sex-ratio of progeny oviposited by early and late females did not vary significantly. In parasitic Hymenoptera, progeny sex-ratios are known to vary and to be determined at oviposition by arrhenotoky (Luck et al. 1992) where males develop from unfertilized eggs (haploid) and females from fertilized eggs (diploid) (Flanders 1965). Following mating, female parasitoids store sperm and can manipulate the sex-ratio of their progeny by controlling the access of sperm to the egg during oviposition (Flanders 1965; Suzuki et al. 1984). The gradual increase in sex-ratio that was observed was due to sperm depletion of the female (Boivin and Lagacé 1999, Boivin et al. 2004). Female T. evanescens can store up to 50 sperm in their spermatheca (Damiens unpublished data).
Early females were larger and more fecund than late females and they produced 18.8% more progeny. Because each egg deposited contributes to the reproductive success of females, early females had a higher fitness than late females. Thus, emerging early had a considerable effect on the size of females and their production of eggs. Although the effect of emergence day on fecundity was almost significant, most of that effect was due to the difference in size between early and late females. The late females were probably not ready to emerge at the beginning of the photoperiod on day 9, so they had to wait another 24 hours to emerge as adults. This increase in developmental time as pupae or sub-imago could have used part of their energy reserves which resulted in a smaller size and a lower fecundity. In Trichogramma parasitoids, oogenesis starts during the pre-pupal stage (Volkoff and Daumal 1994) and continues after adult emergence (Mills and Kuhlmann 2000). Trichogramma species have been reported as proovigenic by several authors (Pak and Oatman 1982; Waage and Ng 1984; Bai et al. 1992) but they have since been described as moderately synovigenic (Jervis et al. 2001). In many species, females emerge with an egg load that accounts for a fraction of their potential fecundity (Houseweart et al. 1983; Smith and Hubbes 1986; Bai and Smith 1993; Kuhlmann and Mills 1999). Our hypothesis was that females would spend some of their resources maintaining their somatic functions while waiting for emergence on the tenth day. However, exhausting resources, probably of their fat reserve, should not have influenced the size of the females when measured as hind tibia length. The results suggest that it was not the longer developmental time that produced small individuals but rather that the smaller individuals took longer to develop.
Superparasitism, which is of common occurrence in Trichogramma spp. in the laboratory (Yadav et al. 2001), could also be the cause of the increase in developmental time in late females. The eggs of E. kuehniella usually support the development of a single adult Trichogramma per egg. However, more than one parasitoid egg may be initially deposited per host (Klomp and Terrink 1978). Larval competition results in the death of some individuals and the survival of others (Corrigan et al. 1995). Superparasitism affects subsequent parasitoid development (Ahmad et al. 2002) and has been suggested as a factor leading to extended emergence periods in several Trichogramma species (Parra et al. 1988).
Trichogramma spp. are the most widely used parasitoid agents for the biological control of lepidopterous pests (Waage and Ng 1984). They are particularly used in inundative releases projects (Corrigan and Laing 1994). The negative impact of longer development on the size and fecundity of Trichogramma females suggests that the quality of the individuals produced in mass rearing could be improved by maximizing the proportion of the population emerging early.
Acknowledgments
We thank Josiane Vaillancourt for rearing T. evanescens, Danielle Thibodeau and Marie-Josée Gauvin for technical assistance and Shahrokh Khanizadeh for advice on statistics. This is contribution 335/2004.09.01R of the Centre de Recherche et de Développement en Horticulture, Agriculture et Agroalimentaire Canada, Saint-Jean-sur-Richelieu, Québec, Canada.
References
- Ahmad M, Ahmad MJ, Mishra RK, Sheel SK. Superparasitism by Trichogramma poliae in the eggs of Clostera cupreata (Lepidoptera: Notodontidae) and its effect on offspring. Journal of Tropical Forest Science. 2002;14:61–70. [Google Scholar]
- van den Assem J, van Iersel JJA, Los-den Hartogh RL. Is being large more important for female than for male parasitic wasps? Behavior. 1989;108:160–195. [Google Scholar]
- Bai B. 1986 Host effect on quality attributes of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) and host discrimination by Copidosoma truncatellum Dalman (Hymenoptera: Encyrtidae). M. Sc. Thesis, University of California at Riverside, Riverside, California, U.S.A. [Google Scholar]
- Bai B, Smith SM. Effect of host availability on reproduction and survival of the parasitoid wasp Trichogramma minutum. Ecological Entomology. 1993;18:279–286. [Google Scholar]
- Bai B, Luck RF, Forster L, Stephens B, Janssen JAM. The effect of host size on quality attributes of the egg parasitoid, Trichogramma pretiosum. Entomologia Experimentalis et Applicata. 1992;64:37–48. [Google Scholar]
- Beck SD. 1991 Thermoperiodism. In: Lee RE, Denlinger DL, editors. Insects at Low Temperature. 199–228.New York: Chapman & Hall. [Google Scholar]
- Bennett DM, Hoffmann AA. Effects of size and fluctuating asymmetry on field fitness of the parasitoid Trichogramma carverae (Hymenoptera: Trichogrammatidae) Journal of Animal Ecology. 1998;67:580–591. [Google Scholar]
- Bigler F, Meyer A, Bosshart S. Quality assessment in Trichogramma maidis Pintureau et Voegelé reared from eggs of the factitious hosts Ephestia kuehniella Zeller and Sitotroga cerealella Olivier. Journal of Applied Entomology. 1987;104:340–353. [Google Scholar]
- Boivin G, Lagacé M. Effet de la taille sur la fitness de Trichogramma evanescens (Hymenoptera: Trichogrammatidae) Annales de la Société Entomologique de France. 1999;35:371–378. [Google Scholar]
- Boivin G, Jacob S, and Damiens D. 2004 Spermatogeny as a life history index in parasitoid wasps. Oecologia. [DOI] [PubMed] [Google Scholar]
- Carvalho MC, Queiroz PCD, Ruszezyk A. Protandry and females size-fecundity variation in the tropical butterfly Brassolis sophorae. Oecologia. 1998;116:98–102. doi: 10.1007/s004420050567. [DOI] [PubMed] [Google Scholar]
- Charnov EL, Los-den Hartogh RL, Jones TW, Assem JVD. Sex ratio evolution in a variable environment. Nature. 1981;289:27–33. doi: 10.1038/289027a0. [DOI] [PubMed] [Google Scholar]
- Chassain C, Boulétreau M. Genetic variability in quantitative traits of host exploitation in Trichogramma (Hymenoptera: Trichogrammatidae) Genetica. 1991;83:195–202. [Google Scholar]
- Corrigan JE, Laing JE. Effects of the rearing host species and the host species attacked on performance by Trichogramma minutum Riley (Hymenoptera: Trichogrammatidae) Biological Control. 1994;23:755–760. [Google Scholar]
- Corrigan JE, Laing JE, Zubricky JS. Effects of parasitoid to host ratio and time of day of parasitism on development and emergence of Trichogramma minutum (Hymenoptera : Trichogrammatidae) parasitizing eggs of Ephestia kuehniella (Lepidoptera : Pyralidae) Annals of the Entomological Society of America. 1995;88:773–780. [Google Scholar]
- Doyon J. 2004 L'impact de la protandrie sur la capacité d'accouplement des mâles et l'effet de développement sur la valeur adaptative des femelles chez Trichogramma evanescens Westwood (Hymenoptera : Trichogrammatidae). M. Sc. Thesis, Université du Québec à Montréal, Montreal, Canada. [Google Scholar]
- Doyon J, Boivin G. Impact of protandry on mating capacity of males in Trichogramma evanescens Westwood (Hymenoptera : Trichogrammatidae) 2004 [Google Scholar]
- Flanders SE. On the sexuality and sex ratios of hymenopterous populations. American Naturalist. 1965;99:489–494. [Google Scholar]
- Godfray HCJ. 1994 Parasitoids: Behavioral and Evolutionary Ecology. Princeton, New Jersey: Princeton University Press. [Google Scholar]
- Houseweart MW, Jennings DT, Welty C, Southhard SG. Progeny production by Trichogramma minutum (Hymenoptera: Trichogrammatidae) utilizing eggs of Choristoneura fumiferana (Lepidoptera: Gelechiidae) The Canadian Entomologist. 1983;115:1245–1252. [Google Scholar]
- Jervis MA, Heimpel GE, Ferns PN, Harvey JA, Kidd NA. Life history strategies in parasitoid wasps: A comparative analysis of ovigeny. Journal of Animal Ecology. 2001;70:442–458. [Google Scholar]
- King BH. Offspring sex ratios in parasitoid wasps. Quarterly Review of Biology. 1987;62:367–396. [Google Scholar]
- Klomp H, Teerink BJ. The elimination of supernumerary larvae of the gregarious egg-parasitoid Trichogramma embryophagum (Hymenoptera: Trichogrammatidae) in eggs of the host Ephestia kuehniella (Lepidoptera: Pyralidae) Entomophaga. 1978;23:153–159. [Google Scholar]
- Kuhlmann U, Mills NJ. Comparative analysis of the reproductive attributes of three commercially-produced Trichogramma species and the influence of parasitoid size. Biocontrol Science and Technology. 1999;9:335–346. [Google Scholar]
- Luck RF, Stouthamer R, and Nunney L. 1992 Sex determination and sex ratio patters in parasitic Hymenoptera. In: Wrench DL and Ebbert M, editors. Evolution and diversity of sex ratio in haplodiploid insects and mites. 442–476.New York: Chapman & Hall. [Google Scholar]
- McDougall SJ, Mills NJ. Dispersal of Trichogramma platneri (Hymenoptera: Trichogrammatidae) from point-source releases in an apple orchard in California. Journal of Applied Entomology. 1997;121:205–209. [Google Scholar]
- Mills NJ, Kuhlmann U. The relationship between egg load and fecundity among Trichogramma parasitoids. Ecological Entomology. 2000;25:315–324. [Google Scholar]
- Pak GA, Oatman ER. Biology of Trichogramma brevicapillum. Entomologia Experimentalis et Applicata. 1982;32:61–67. [Google Scholar]
- Parra JRP, Zucchi RA, and Silveira Neto S. 1988 Perspectives of biological control using Trichogramma and/or Trichogrammatoidea in the state of Sào Paulo (Brazil). In: Trichogramma and other egg parasites, 43. 527–540.INRA. [Google Scholar]
- Rabinovisch JE. Population dynamics of Telenomus fariai (Hymenoptera: Scelionidae), a parasite of Chaga's disease vector. Parasite size and vital space. Revista de Biologia Tropical. 1971;19:109–120. [PubMed] [Google Scholar]
- Rivero A, West SA. The physiological costs of being small in parasitic wasp. Evolutionary Ecology Research. 2002;4:407–420. [Google Scholar]
- Roitberg BD, Boivin G, Vet L. Fitness, parasitoids, and biological control: An opinion. The Canadian Entomologist. 2001;133:429–438. [Google Scholar]
- Sagarra LA, Vincent C, Stewart RK. Body size as an indicator of parasitoid quality in male and female Anagyrus kamali (Hymenoptera : Encyrtidae) Bulletin of Entomological Research. 2001;91:363–367. doi: 10.1079/ber2001121. [DOI] [PubMed] [Google Scholar]
- Saunders DS. 1976 Insect Clocks. New York: Pergamont. [Google Scholar]
- Shaffer PL. Prediction of variation in developmental period of insects and mites reared at constant temperatures. Environmental Entomology. 1983;12:1012–1019. [Google Scholar]
- Smith SM. Biological control with Trichogramma: Advances, successes, and potential of their use. Annual Review of Entomology. 1996;41:375–406. doi: 10.1146/annurev.en.41.010196.002111. [DOI] [PubMed] [Google Scholar]
- Smith SM, Hubbes M. Strains of the egg parasitoids Trichogramma minutum Riley. II. Utilization for release against the spruce budworm. Journal of Applied Entomology. 1986;102:81–83. [Google Scholar]
- Suzuki Y, Tsuiji H, Sasakawa M. Sex allocation and the effects of superparasitism on secondary sex ratios in the gregarious parasitoid, Trichogramma chilonis (Hymenoptera: Trichogrammatidae) Animal Behavior. 1984;32:478–484. [Google Scholar]
- Volkoff AN, Daumal J. Ovarian cycle in immature and adult stages of Trichogramma cacoeciae and T. brassicae (Hymenoptera: Trichogrammatidae) Entomophaga. 1994;39:303–312. [Google Scholar]
- Waage JK, Ming NGS. The reproductive strategy of a parasitic wasp I. Optimal progeny and sex allocation in Trichogramma evanescens. Journal of Animal Ecology. 1984;53:401–415. [Google Scholar]
- Yadav RC, Singh SP, Jalali SK, Rao NS. Effect of host egg density on parasitism and adult emergence in Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae) in two systems. Journal of Biological Control. 2001;15:11–14. [Google Scholar]


