Abstract
The ectoparasitic mite, Varroa destructor, is the most destructive parasite of managed honeybee colonies worldwide. Since V. destructor transfers pathogens to honeybees, it may be adaptive for bees to respond to mite infestation by upregulating their immune responses. Mites, however, may overcome the host's immune responses by suppressing them, which could facilitate the mite's ability to feed on hemolymph. A humoral immune response of bees parasitized by V. destructor may be detected by studying the expression levels of antibacterial peptides, such as abaecin and defensin, known to be immune-responsive. Expression levels for these two antibacterial peptides changed non-linearly with respect to the number of mites parasitizing honeybee pupae. Bees exposed to low or moderate number of mites had fewer immune-related transcripts than pupae that were never parasitized or pupae with high mite loads. Although many of the pupae tested indicated the presence of bacteria, no correlation with mite numbers or immune-response levels existed. All bees tested negative for acute paralysis and Kashmir bee viruses known to be vectored by V. destructor.
Keywords: abaecin, antimicrobial peptide, Apis mellifera, defensin, innate immunity
Introduction
Apis mellifera honeybees have numerous parasites and pathogens. The most destructive is the ectoparasitic mite, Varroa destructor. Varroa infestations result in colony-level mortality if untreated with acaricides. A. mellifera is not the natural host for V. destructor. The host shift of V. destructor from Apis cerana to A. mellifera occurred when humans introduced A. mellifera into regions where A. cerana occurred. Mites quickly spread throughout most of the current range of A. mellifera (Oldroyd 1999). Adult female V. destructor mites feed on adult bees, while both adult and immature mites of both sexes feed on developing pupal bees. V. destructor has direct impact on developing and adult bees, including lowered body weights (Bowen-Walker and Gunn 2001; de Jong et al. 1982) and reduced longevity (de Jong and de Jong 1983, Kovac and Crailsheim 1988). These impacts translate into both lowered productivity (Murilhas 2002) and higher mortality at the colony level.
V. destructor are also known to be associated with honeybee pathogens and are confirmed in some cases to be vectors of disease. Several experimental studies indicate that mites transfer single-stranded RNA viruses between bees (Bowen-Walker et al. 1999; Chen et al. 2004). Mites also produce wounds through the exoskeleton of bees while feeding and these wound sites are known to harbor infections of Melissococcus pluton, a primary component of the brood disease European foulbrood (Kanbar and Engels 2003). Given the possibility that mites can transmit disease, it may be adaptive for bees to respond to mite presence by upregulating their immune responses. Alternatively, mites could benefit by inhibiting the immune responses of bees if bee responses incorporate peptides, enzymes, or cells that negatively impact the feeding of mites. Finally, bees that are parasitized by mites could be less able to mount an effective immune response due to physiological costs of both parasitism and the immune response itself.
Honeybees appear to mount a cellular immune response at wound sites caused by V. destructor (Kanbar and Engels 2003). Bees also possess a humoral immune response leading to an upregulation of several antimicrobial peptides in response to both wound infections (Casteels-Josson et al. 1994) and oral bacterial infections (Evans 2004). To explore whether the presence of V. destructor affects the humoral immune response, transcript levels for two antimicrobial peptides, abaecin and defensin, that show activity against bacteria were examined. Both show activity against gram-positive and gram-negative bacteria (Casteels et al. 1990, 1994). It was found that bees exposed to low or moderate numbers of mites sharply reduce their immune-peptide transcripts when compared to both heavily parasitized and unparasitized bees.
Materials and Methods
Collection of honeybees
Honeybee pupae were collected from an apiary at the USDA Honeybee Breeding, Genetics and Physiology Laboratory, Baton Rouge, Lousiana, in August 2002. All bees were of Apis mellifera ligustica stock, from a single breeder in northern California. Brood cells from a total of 7 colonies were uncapped individually and adult and nymphal mites in each cell counted. Bee pupae from cells containing zero, one, two, three, four, five, or six mites were held in a −80 °C freezer until RNA extraction. Pupal cells with 1–4 mites and 5–6 mites were considered moderately and highly infested respectively. Pupae were classified according to their developmental state as described in Bitondi et al. (1998) and weighed. Pupae were divided into three approximate age groups, brown-eyed, unpigmented cuticle (280 hours), brown-eyed, light pigmented cuticle (315 hours), and brown-eyed, medium pigmented cuticle (350 hours).
RNA extraction and cDNA synthesis
Total RNA was extracted from abdomens of individual pupae using the RNAqueous protocol (Ambion, www.ambion.com), after which RNA's were quantified by spectrophotometry. DNA was removed using 45-minute DNAse incubation at 37 °C (5 Units DNAse I in appropriate buffer; Boehringer Mannheim (www.roche.com) with the RNAse inhibitor RNAsin; Ambion). Next, 1st-strand cDNA's were generated from approximately 2 µg total RNA using a mix of 50 U Superscript II (Invitrogen, www.invitrogen.com), 2 nmol DNTP mix, and a composite of 2 nmol poly dT-18 and 0.1 nmol poly dT(12–18). Synthesis was carried out at 42 °C for 1 hour.
Quantitative PCR amplification
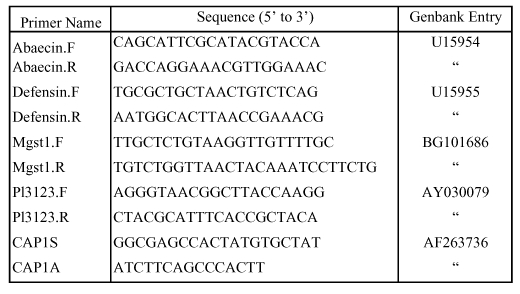
DNA products were amplified in 96-well microtiter trays using specific oligonucleotide primers and an Icycler Real-Time PCR thermal cycler (Bio-Rad, www.bio-rad.com). Fifty µl reactions consisted of 2 U Taq DNA polymerase with suggested buffer (Boehringer Mannheim), 0.2 µM fluorescein, 1 mM DNTP mix, 2 mM MgCl2, 0.2 µM of each primer and a final concentration of 2.5× SYBRGreen 1 (Applied Biosystems, appliedbiosystems.com). Oligonucleotide primers for PCR are described in Table 1. Abaecin and defensin primers were designed from precursor sequences for these genes (Casteels-Josson et al. 1994; Evans 2004). Primers pl3123.f and pl3123.r were designed from the 16s rRNA sequence of Paenibacillus larvae larvae (Heyndrickx 1996), a bacterial species responsible for American foulbrood disease. These primers also closely matched the 16s gene sequences of several other species of Paenibacillus, and these species could also be cross-amplified. We screened for presence of acute bee paralysis virus and Kashnmir bee virus in these samples using PCR primers that cross-amplify the capsid protein from these two species (primers Cap1S and Cap1A, Evans 2001). Transcript levels for a gene with low transcriptional variation across bees (microsomal glutathione-S-transferase; mGsT; Evans and Wheeler 2000) were used to normalize against variable mRNA levels. Expression of this gene strongly correlates with total RNA levels predicted by spectrophotometry.
Table 1.
Oligonucleotide primers and sequence identification for real-time quantitative RT-PCR.
The thermal program for all reactions was 95 °C for 3 min followed by 40 cycles of (95 °C for 30 s, 58 °C for 30 s, 72 °C for 1 min 30 s). To insure the PCR products were the predicted sizes, melt-curve analyses were conducted. Fluorescence was measured repeatedly during the 58 °C step using appropriate laser excitation and filtration (Evans 2004).
Data analyses
Fluorescence levels were normalized using average fluorescence from the fluorescin included in the reaction mix. Threshold cycles were defined when well fluorescence became greater than 10 times the mean standard deviation across all samples. Threshold cycle numbers for defensin and abaecin were then subtracted from the MGsT threshold for each sample. This value was then scaled as a power of 1.8, the de facto reaction efficiency, to produce an estimate of relative cDNA abundance. Analyses of variance were carried out using source honeybee colony and mite number as factors and the controlled threshold cycle for abaecin or defensin as a response. Separate analyses of variance contrasted the presence or absence of bacteria against mite number, abaecin or defensin transcript levels as nominal (mite number) or continuous variables, respectively. We also used pupal age as a covariate in testing for factors involved with immune-gene transcript levels.
Results
Body mass
No direct impact of mite numbers on the body mass of pupae was observed. Mite-free pupae weighed 126.3 mg, on average (least-squares mean, SE = 2.32, n = 24), those with 1–4 mites averaged 124.5 mg (SE = 0.0016, n = 40), those with 5–6 mites averaged 126.5 mg (SE = 0.0017, n = 28). Body weight also did not vary significantly by pupal age, nor was there an interaction between pupal age and mite load with respect to body weight (2-way ANOVA, p = 0.8 for mite load × age interaction). There was significant body-weight variation at the level of colonies (ANOVA df = 7, F Ratio = 5.0, p < 0.0001). Colony-level mean body mass for six colonies with five or more sampled pupae ranged from 119 mg (SE = 2.2 mg) to 130 mg (SE 1.2 mg).
Antibiotic peptide expression
Both abaecin and defensin transcript levels varied significantly as a function of mite presence (Fig. 1). Generally pupae infested with 1–4 adult and immature mites had lower levels of antimicrobial transcripts than did pupae with either no mites or heavy infections of 5–6 mites. This trend differed slightly for the two antimicrobial peptides. For abaecin, expression levels with a single mite were significantly lower than were those with no mites. However, for defensin this difference was not significant, although bees with 2, 3, and 4 mites showed significant inhibition. There was no significant relationship between pupal body weight and antimicrobial peptide expression (Fig. 2). Expression of abaecin and defensin did not vary as a function of pupal age (mixed-model ANOVA with mite number and age as factors, p = 0.8, n = 12, 57, and 25 bees in the age classes 280 h, 315 h, and 350 h, respectively).
Figure 1.
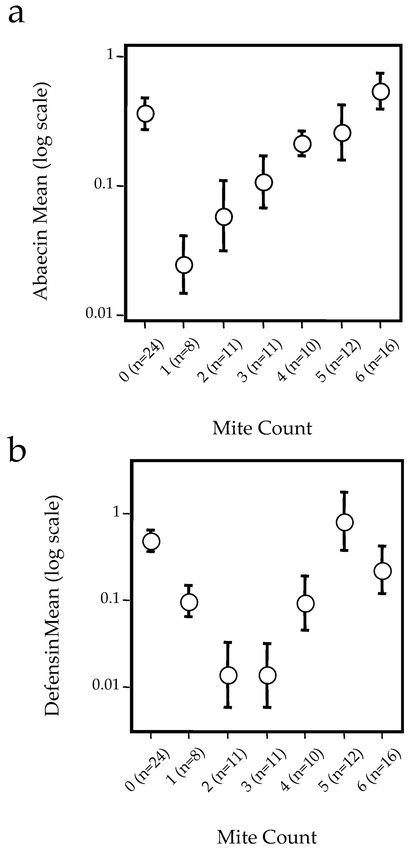
Expression levels (mean ± SE) for pupae exposed to different numbers of mites for antibacterial genes abaecin (a) and defensin (b).
Figure 2.
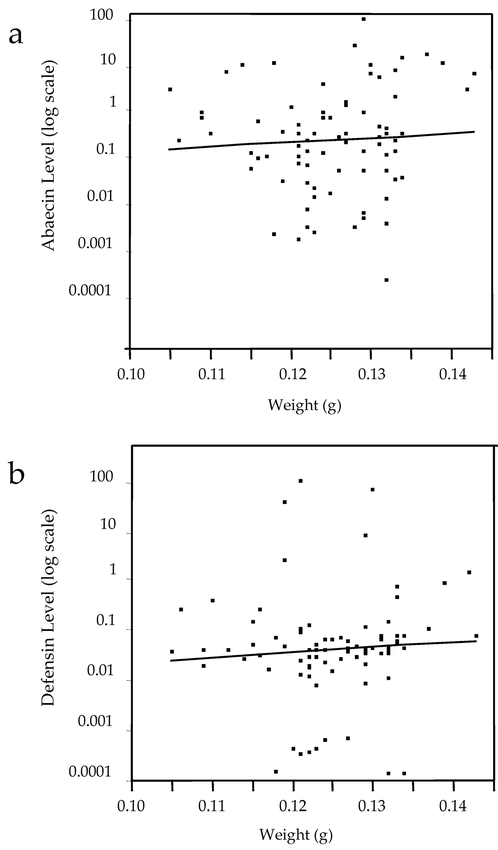
Variation between bee body weight and antimicrobial peptide transcript levels for abaecin (a) and defensin (b).
Transcript levels for abaecin and defensin were significantly correlated among individual bees, (correlation coefficient = 0.33, p < 0.0005). Abaecin levels did not differ between the 7 colonies analyzed (Fig. 3a). Defensin levels were similar in 6 colonies, while transcripts for this gene appeared to be essentially absent in most pupae in colony “C” (Fig. 3b). The mean level of defensin found in pupae of this colony was > 100-fold less than that found in the remaining colonies (mean levels of = 0.0019, SE = 0.003 versus 0.398, SE = 0.16).
Figure 3.
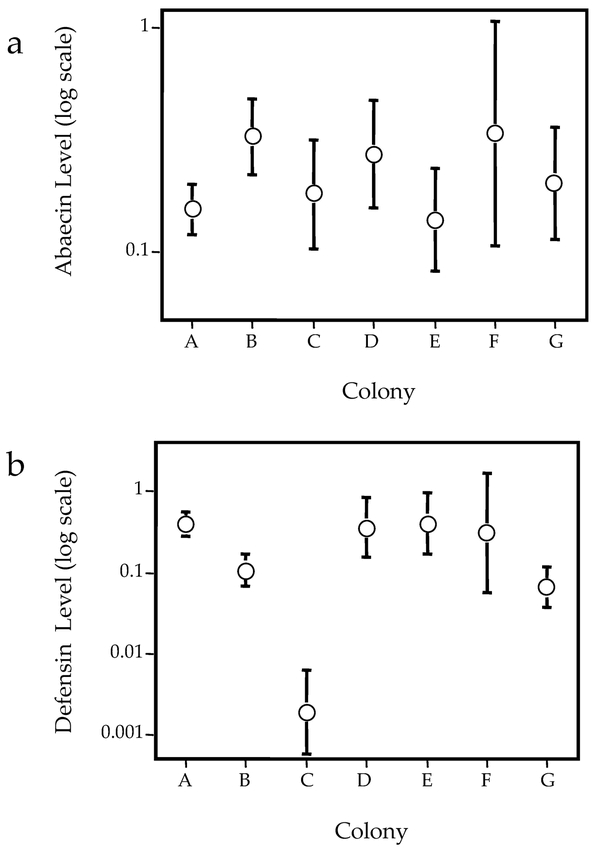
Abaecin (a) and defensin (b) transcript (mean ± SE) for pupae in 7 different honeybee colonies.
Presence of potential pathogens
Pupae screened for bacteria in the genus Paenibacillus showed low 16S rRNA transcript levels when compared to larvae orally inoculated in vitro with P. l. larvae (Evans 2004). Overall 58% (n = 94) of screened pupae indicated the presence of Paenibacillus sp. but levels of bacteria were not correlated with mite number. Bacteria were present in 62% of the pupae with no mites (n = 24), 57% of pupae with a moderate number of mites (1 to 4 mites, n = 40) and 54% of pupae with high numbers of mites (5 or 6 mites, n = 28). Bacterial levels did not vary as a function of pupal age. Transcript levels of both immuno-peptides (abaecin and defensin) did not change as a function of bacterial presence. No signs of clinical American foulbrood or of viral disease were present in these samples, nor were there signs of transcripts from acute bee paralysis or Kashmir bee viruses.
Discussion
Honeybee pupae collected when parasitized by moderate numbers of V. destructor showed a significant down-regulation of immune-related transcripts. It is possible that mites directly reduce bee immune responses when they begin to feed, perhaps as a means of ensuring that feeding sites are maintained. Interestingly, the apparent suppression of immune-gene transcripts disappeared when bee pupa were faced with higher mite loads. Several non-exclusive mechanisms might explain this idiosyncratic relationship between mite parasitism and immune responses.
First, immune-gene suppression by V. destructor could be ephemeral, such that older pupae collected with mite foundresses, daughters, and sons eventually restart their immune response. Mite load in our study reflects the number of invading foundresses and nymphal offspring found within each cell. Accordingly, pupae with heavy mite loads may have been challenged for a longer time, allowing a stronger initiation of an immune response. This scenario is weakened, however, because there was no correlation between pupal age and immune-gene activity regardless of mite load. We would expect a correlation if bees could counteract mite suppression of their immune systems over time. Alternatively, high numbers of mites may be required to generate the specific cues or stress levels sufficient for upregulation of immune-related genes. The presence of immunity-inducing pathogens could have been especially high in the heavily parasitized bees used in this study, a result suggested in the context of virus transmission by Chen et al. (2004). No difference in pathogen load was found, although only a subset of possible pathogens important for developing bees (Morse and Flottum 1997) were surveyed. It would be interesting to search more exhaustively for correlates between immune-gene activity and the presence of one or more pathogens.
We have focused on the humoral immune response, and it will be interesting to determine the impact of V. destructor on the cellular immune response (Tzou et al. 2002). Kanbar and Engels (2003) observed a cellular immune response at wound sites on honeybee pupae where V. destructor have fed. They noted an aggregation of haemocytes in the center of the wounds. This observation suggests that haemocytes are involved both with deterring subsequent infections and with healing wound sites. Future studies could explore both the localization of humoral and cellular immune responses involved with wound sites, and the dynamics by which these immune responses change in response to the introduction of pathogens. This and future studies will help define the role V. destructor plays in vectoring diseases as well as mechanisms used by bees to limit the impact of this novel pest.
Acknowledgments
We thank Ahline Angeles and Molly McCoy for assistance in collecting pupal samples and Dawn Lopez for laboratory assistance. Supported in part by USDA-NRI grant # 2002-02546 to JDE. This work was completed in cooperation with the Louisiana Agricultural Experiment Station.
References
- Bitondi MMG, Mora IM, Simoes ZLP, Figueiredo VLC. The Apis mellifera pupal melanization program is affected by treatment with a juvenile hormone analogue. Journal of Insect Physiology. 1998;44:499–507. doi: 10.1016/s0022-1910(97)00113-3. [DOI] [PubMed] [Google Scholar]
- Bowen-Walker PL, Gunn A. The effect of the ectoparasitic mite, Varroa destructor on adult worker honeybee (Apis mellifera) emergence weights, water, protein, carbohydrate, and lipid levels. Entomologia Experimentalis et Applicata. 2001;101:207–217. [Google Scholar]
- Bowen-Walker PL, Martin SJ, Gunn A. The transmission of deformed wing virus between honeybees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni Oud. Journal of Invertebrate Pathology. 1999;73:101–106. doi: 10.1006/jipa.1998.4807. [DOI] [PubMed] [Google Scholar]
- Casteels-Josson K, Zhang W, Capaci T, Casteels P, Tempst P. Acute transcriptional response of the honeybee peptide-antibiotics gene repertoire and required post-translational conversion of the precursor structures. Journal of Biological Chemistry. 1994;269:28569–28575. [PubMed] [Google Scholar]
- Casteels P, Ampe C, Riviere L, Van Damme J, Elicone C, Fleming M, Jacobs F, Tempst P. Isolation and characterization of abaecin, a major antibacterial response peptide in the honeybee (Apis mellifera) European Journal of Biochemistry. 1990;187:381–386. doi: 10.1111/j.1432-1033.1990.tb15315.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pettis JS, Evans JD, Kramer M, Feldlaufer MF. Transmission of Kashmir bee virus by the ectoparasitic mite Varroa destructor Anderson and Trueman. Apidologie. 2004;35:441–448. [Google Scholar]
- De Jong D, De Jong PH. Longevity of Africanized honeybees (Hymenoptera: Apidae) infested by Varroa jacobsoni (Parasitiformes: Varroidae) Journal of Economic Entomology. 1983;76:766–768. [Google Scholar]
- De Jong D, De Jong PH, Goncalves LS. Weight loss and other damage to developing worker honeybees from infestation with Varroa jacobsoni. Journal of Apicultural Research. 1982;21:165–167. [Google Scholar]
- Evans JD. Transcriptional immune response by honeybee larvae during invasion by the bacterial pathogen, Paenibacillus larvae. Journal of Invertebrate Pathology. 2004;85:105–111. doi: 10.1016/j.jip.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Evans JD. Genetic evidence for coinfection of honeybees by acute bee paralysis and Kashmir bee viruses. Journal of Invertebrate Pathology. 2001;78:189–193. doi: 10.1006/jipa.2001.5066. [DOI] [PubMed] [Google Scholar]
- Evans JD, Wheeler DE. 2000 Expression profiles during honeybee caste determination. Genome Biology. 2research. 0001.1–0001.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyndrickx M, Vandemeulebroecke K, Hoste B, Janssen P, Kersters K, DeVos P, Logan NA, Ali N, and Berkeley RCW. 1996 Reclassification of Paenibacillus (formerly Bacillus) pulvifaciens (Nakamura 1984). Ash et al. 1994, a later subjective synonym of Paenibacillus (formerly Bacillus) larvae (White 1906) Ash et al. 1994, as a subspecies of P. larvae, with emended descriptions of P. larvae as P. larvae subsp. larvae and P. larvae subsp. pulvifaciens. International Journal of Systematic Bacteriology. 46:270–279. [DOI] [PubMed] [Google Scholar]
- Kanbar G, Engels W. Ultrastructure and bacterial infection of wounds in honeybee (Apis mellifera) pupae punctured by Varroa mites. Parasitology Research. 2003;90:349–354. doi: 10.1007/s00436-003-0827-4. [DOI] [PubMed] [Google Scholar]
- Kovac H, Crailsheim K. Lifespan of Apis-mellifera-carnica Pollm. infested by Varroa-Jacobsoni Oud. in relation to season and extent of infestation. Journal of Apicultural Research. 1988;27:230–238. [Google Scholar]
- Morse RA, Flottum K. 1997 Honeybee Pests Predators and Diseases. A.I. Root Co., Medina, Ohio. [Google Scholar]
- Murilhas AM. Varroa destructor infestation impact on Apis mellifera carnica capped worker brood production, bee population and honey storage in a Mediterranean climate. Apidologie. 2002;33:271–281. [Google Scholar]
- Oldroyd BP. Coevolution while you wait: Varroa jacobsoni, a new parasite of western honeybees. Trends in Ecology and Evolution. 1999;14:312–315. doi: 10.1016/s0169-5347(99)01613-4. [DOI] [PubMed] [Google Scholar]
- Tzou P, De Gregorio E, Lemaitre B. How Drosophila combats microbial infection: A model to study innate immunity and host-pathogen interactions. Current Opinion in Microbiology. 2002;5:102–110. doi: 10.1016/s1369-5274(02)00294-1. [DOI] [PubMed] [Google Scholar]


