Abstract
Background
During the 2000–2001 influenza vaccination season, a new adverse event associated with the influenza vaccine, called oculorespiratory syndrome (ORS), was identified in Canada. We examined the risk of recurrence of ORS for individuals affected in 2000–2001 who were revaccinated in the community setting in 2001–2002.
Methods
We conducted a telephone survey in May 2002 in which participants were asked whether they had been revaccinated in the 2001–2002 immunization season and whether they had experienced any recurrence of ORS or any other adverse event. Eligible individuals (n = 609) included residents of British Columbia aged 18 years or more who had reported any type of adverse event associated with the influenza vaccine in 2000 and who had participated in a survey in September 2001 that characterized their adverse event experience.
Results
The response rate to this survey was 92% (561 of 609 people approached). Of the 561 participants, 202 were revaccinated in 2001. Among the 202 revaccinated, 122 individuals had previously reported ORS in 2000: 40 had described their ORS experience in 2000 as mild (present but not bothersome), 44 as moderate (interrupting daily activities), 35 as severe (preventing daily activities) and 3 did not provide specific details. Six of the 122 individuals experienced a recurrence of ORS following revaccination. The estimated risk of recurrence for ORS following revaccination in the community setting was 5% (95% confidence interval 2.2%–10.5%). Four of the 6 individuals described their ORS recurrence in 2001 as milder than the ORS they had experienced in 2000.
Interpretation
We found a low risk of recurrence of ORS for individuals previously affected in 2000 when they were revaccinated in 2001, including those whose ORS in 2000 had prevented daily activities but was not clinically severe. Health care providers should be confident in the safety of recommending revaccination of these individuals. As with all vaccines, however, a detailed risk–benefit assessment should be undertaken before revaccination of people whose previous adverse event experience may have included collapse, respiratory difficulty (including throat tightness) and/or chest discomfort requiring emergency intervention.
During the 2000–2001 influenza immunization campaign, a new adverse event associated with the influenza vaccine, called oculorespiratory syndrome (ORS), was identified in Canada. ORS was defined in 2000–2001 as bilateral red eyes or respiratory symptoms (cough, wheeze, chest tightness, difficulty breathing or sore throat) or facial edema beginning between 2 and 24 hours following influenza vaccination with full resolution within 48 hours.1,2,3,4,5 The risk of ORS was greater for women, individuals aged 40–59 years and those vaccinated for the first time against influenza in 2000.4,5
In all, 2450 adverse events associated with the influenza vaccine were reported nationally in 2000–2001: 1735 individuals reported ocular or respiratory symptoms and 960 met the 2000–2001 ORS case definition. Ninety-six percent (925) of the 960 ORS cases were linked to one Canadian manufacturer's vaccine (Fluviral S/F produced by Shire Biologics).1,4 This vaccine comprised only 3.8 million (31%) of the more than 12 million doses of influenza vaccine distributed in Canada that season. Conversely, 1% of ORS cases (12) were reported in association with the 2 other influenza vaccines distributed in Canada (Vaxigrip and Fluzone produced by Aventis Pasteur).1,4 Product-related investigations found no contamination in the implicated product, and all vaccines were completely inactivated. Electron microscopy, however, found an excess of aggregated unsplit influenza virions in the implicated vaccine.1,4 A similar excess of adverse events with a cluster around a third manufacturer's vaccine was noted during 1995–1996 in Europe.6 Electron microscopy demonstrated similar morphologic aberrations in that vaccine.7
In preparation for the 2001–2002 vaccination campaign, Shire Biologics adjusted its manufacturing process to reduce the aggregate content. A randomized, double-blind placebo-controlled crossover trial was used to assess the risk of recurrence of ORS for previously affected individuals using this reformulated vaccine. This trial was halted early when only 65 of 150 intended participants had received an injection. Of the 35 who had received the reformulated vaccine, 11 reported symptoms qualifying as ORS (31.4%, 95% confidence interval [CI] 18.2%–48.6%) compared with 2 of 30 people (6.7%) who had received placebo (vaccine-attributable recurrence risk for ORS 24.8%, 95% CI 7%–42.5%).8 As a result, the National Advisory Committee on Immunization advised caution and the preferential use of the Aventis Pasteur product in the revaccination of previously affected individuals, particularly those whose ORS had interrupted or prevented daily activities.1
In September 2001, the BC Centre for Disease Control (BCCDC) conducted a telephone survey of British Columbia residents aged 18 years or more who had reported any adverse event associated with the influenza vaccine during the 2000–2001 immunization campaign. The purpose was to assess the clinical profile, severity and impact of the syndrome systematically. The 609 respondents comprised 25% of the 2450 individuals with adverse events reported nationally in 2000.1 In order to clarify the risk of recurrence for ORS within the setting of regular program activities and with larger numbers than were included in the clinical trial, the BCCDC conducted a second follow-up survey of this cohort in May 2002 that is described here. The main objective was to estimate the risk of ORS recurrence following revaccination in 2001–2002 for individuals previously affected in 2000–2001 and to determine whether this risk of recurrence varied with the severity of the ORS experience in 2000–2001. The secondary objective was to assess willingness to be revaccinated in 2002–2003.
Methods
Eligible individuals (n = 609) included residents of BC aged 18 years of age or more who had reported any type of adverse event associated with the influenza vaccine in 2000 and who had participated in the survey in September 2001 that characterized this adverse event experience. Seven nurse interviewers were trained to conduct a standard telephone survey using a prepiloted questionnaire. The nurses were blind to the adverse event category of the person they were calling. Telephone interviews were conducted during May 2002. The research ethics board of the University of British Columbia approved this survey.
The National Advisory Committee on Immunization revised the case definition for ORS for 2001–2002 to include the same clinical features as in 2000, but with onset at any time within 24 hours of influenza vaccination and with no restriction on duration.1,4 For this study, the revised 2001 case definition for ORS was used to categorize the adverse event experience associated with the influenza vaccine of participants in both 2000 and 2001, and to derive risk of recurrence. In the presentation of results, ORS refers to this revised 2001 definition.
Severity of ORS in 2000 was assessed during the September 2001 survey using subjective and objective scales. Subjective measures included a 3-choice Likert scale (mild: present but not bothersome; moderate: interfered with daily activities; severe: prevented daily activities) as well as a numeric score. The numeric score ranged from zero (no symptoms) to 10 (“felt life-threatening”). Objective measures of severity were assessed in terms of missed work, need for physician visit, prescribed therapy, emergency department visit or overnight stay in hospital. The severity of the adverse event experience in 2001 was compared with that in 2000 on a 3-choice Likert scale (worse, the same, milder), and willingness to be reimmunized in 2002 was measured on a 5-choice Likert scale.
χ2 was the test of significance (p < 0.05) for categorical variables, and the appropriate parametric or nonparametric test was used for numeric variables (t-test or Mann-Whitney respectively). The 95% confidence intervals for risk of recurrence were calculated by exact binomial distribution.
Results
Of the 609 participants in the original September 2001 survey, 561 (92%) responded to the May 2002 survey. The 561 respondents did not differ from the 48 nonrespondents regarding their demographic or clinical features. Of the 561 participants in the May 2002 survey, 398 had experienced ORS in 2000 that met the revised 2001 case definition.
Revaccination in 2001
Among the 561 participants, 202 (36%) were revaccinated in 2001. BC received primarily the Vaxigrip vaccine for its public campaign in 2001–2002 (> 80% of all doses distributed); the 202 vaccinated participants included 11 from the region that received Fluviral S/F.
Of the 398 participants who had experienced ORS in 2000, 122 were revaccinated in 2001 (31%) compared with 80 of 163 individuals (49%) who had experienced a non-ORS event (p < 0.001). The rate of revaccination did not vary with respect to sex, smoking status, or history of allergies or lung disease. Revaccination in 2001 was significantly associated with age, influenza immunization history before 2000, indication for vaccination (as specified in 2000) and adverse event severity in 2000 (Table 1). The median numeric score of ORS severity in 2000 for vaccinated individuals was 5.0 and for nonvaccinated individuals was 6.0 (p < 0.001).
Table 1
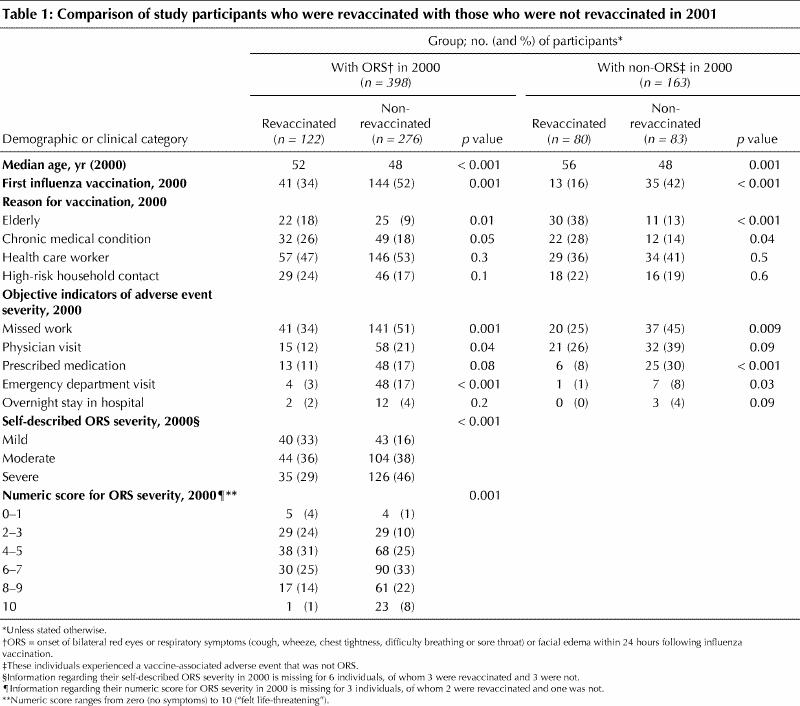
The 122 individuals who experienced ORS in 2000 and were revaccinated included the following: 40 of the 83 whose original experience had been described as mild (48%), 44 of 148, as moderate (30%); 35 of 161, as severe (22%) (p < 0.001) and 3 for whom a category was not specified. The rate of revaccination also varied with the numeric score for ORS severity (p < 0.001); of the 24 individuals who had ranked the severity of their ORS in 2000 as 10, only one (4%) was revaccinated versus 17 of 78 (22%) who had ranked their ORS severity as 8 or 9, 30 of 120 (25%), as 6 or 7, 38 of 106 (36%), as 4 or 5, and 34 of 67 (51%) as 1–3 (Table 1). Two individuals had not provided a numeric rank for the severity of their ORS experience. The median numeric score for the 35 revaccinated individuals who described their experience in 2000 as severe was 7.0 versus 8.0 for the 126 individuals who described their ORS as severe but who were not revaccinated (p = 0.004).
The rate of revaccination by objective indicator of ORS severity is shown in Fig. 1. Among the individuals with ORS who were revaccinated, 15 had required a physician visit in 2000, 13 were prescribed medication, 4 were seen in the emergency department and 2 were hospitalized overnight.
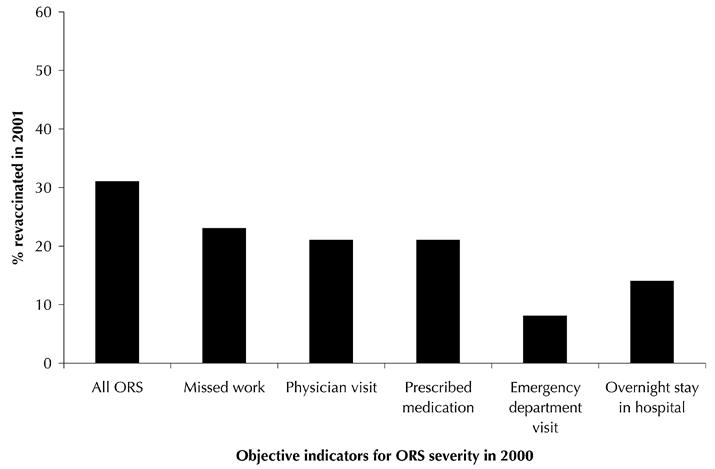
Fig. 1: Percentage of study participants revaccinated in 2001 by objective indicators of ORS severity in 2000. ORS = onset of bilateral red eyes or respiratory symptoms (cough, wheeze, chest tightness, difficulty breathing or sore throat) or facial edema within 24 hours following influenza vaccination.
Adverse event experience following revaccination in 2001
Individuals who had previously reported ORS were no more likely than those who reported a non-ORS event in 2000 to report any adverse event following revaccination in 2001 (13% v. 16%, p = 0.5). The frequency of reported symptoms by adverse event category is shown in Table 2.
Table 2
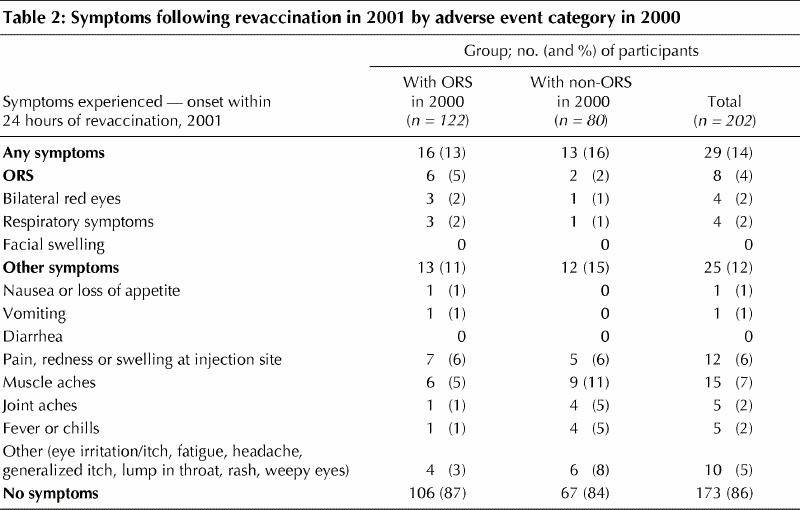
Eight of the 202 individuals revaccinated in 2001 (4%, 95% CI 1.9%–7.6%) described ORS following revaccination. All 8 reported only one category of symptom each: 4 described bilateral red eyes only, 4 described respiratory symptoms only and none described facial edema. One additional person described eye irritation without eye redness, and a further additional person described the sensation of a “lump in the throat” in the 24 hours following vaccination.
Six of the 122 people with ORS in 2000, who are among the 8 described above, experienced a recurrence after revaccination in 2001 providing an ORS recurrence rate of 5% (95% CI 2.2%–10.5%). Two people experienced ORS following revaccination in 2001 who were part of the group of 80 who reported a non-ORS event in 2000 (2%, 95% CI 0.4%–8.6%). Both had experienced oculorespiratory symptoms in 2000 but with onset beyond 24 hours. All those affected in 2001 had received Vaxigrip in keeping with National Advisory Committee on Immunization recommendation.1
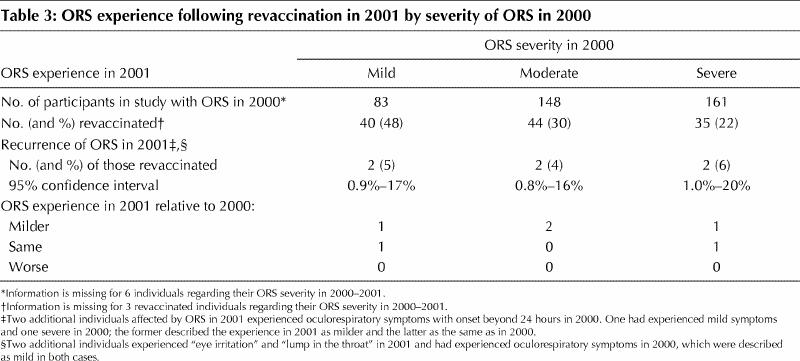
None of the 6 participants with a second experience of ORS following revaccination in 2001 described their experience as worse than in 2000. Four (67%) described their experience as milder. Two (33%) considered their experience as being of the same severity as in 2000: one had originally described the experience as mild (numeric score of 2) and one as severe (numeric score of 8). (Table 3)
Table 3
Willingness to be revaccinated in 2002
Among the 561 participants in our survey, 257 (46%) indicated that they were unlikely or very unlikely to be revaccinated during the upcoming 2002–2003 campaign. Of the 202 respondents who had already been revaccinated in 2001, 184 (91%) indicated that they were likely or very likely to be vaccinated again in 2002 versus 52 of 359 (14%) who had not already been revaccinated (p < 0.001).
Among the 398 people who had reported ORS in 2000, 146 (37%) indicated that they were likely or very likely to be revaccinated in 2002 compared with 90 of the 163 (55%) who had reported a non-ORS event in 2000 (p = 0.001). Of the 6 revaccinated individuals who experienced ORS, 4 indicated they were very likely to be reimmunized, one was undecided and one indicated that he or she was very unlikely to be reimmunized in 2002. The last-mentioned individual experienced moderate ORS in 2000 that was described as milder in 2001.
Interpretation
This report summarizes the revaccination experience of 122 people affected by ORS — including 40 whose symptoms in 2000 were mild, 44 whose symptoms interrupted daily activities and 35 whose symptoms prevented daily activities. We found a low rate of recurrence of ORS following revaccination (5%, 95% CI 2.2%–10.5%) that did not vary across these categories. Underrepresented among the reimmunized in this survey are those whose ORS experience may have been clinically severe. Revaccinated participants included only one who experienced ORS that was ranked 10 on the numeric scale, 4 who required a visit to an emergency department and 2 who required an overnight stay in hospital.
The recurrence rate identified in the initial clinical trial was a concern and led to caution regarding the revaccination of previously affected individuals.1 In retrospect, of the 11 people who experienced recurrence of ORS in the clinical trial, most described their symptoms in 2001 as having been mild and most described these as milder than in 2000.1,8 This is in keeping with the reassuring findings from our survey. Despite the almost exclusive use of Vaxigrip in our survey versus Fluviral S/F in the clinical trial, the low risk we have found is also likely to apply to Fluviral S/F. A separate survey conducted in Quebec found no statistically significant difference in the risk of ORS using the 2001 formulation of each vaccine.9
Participants in our survey were queried 6 months after receipt of their vaccine. This may raise concerns that the rate we have found underestimates the true risk of recurrence. Recall at 6 months is likely to be substantially less than within several hours of vaccination. The September 2001 survey was also conducted nearly one year following vaccination in 2000. Validity checks demonstrated that 84% of respondents to that survey were consistent with their original adverse event report on ORS status (κ statistic 0.7) (BC Centre for Disease Control, unpublished data, 2002). The easy recollection of symptoms in September 2001 nearly one year following vaccination hinted at the impressive nature of the experience in 2000 (BC Centre for Disease Control, unpublished data, 2002). The low rate of adverse events cited by this same cohort in May 2002 is therefore significant. It corroborates the vivid experience of those affected by ORS in 2000 at the same time that it provides reassuring results about their benign experience in 2001 and the safety of reimmunizing them in subsequent years. Our survey provides a practical estimate of the risk of ORS recurrence that has both clinical and programmatic relevance.
Based on these results and those of other studies,1,8 previous cautionary recommendations have been updated.10 Health care providers should be confident in recommending revaccination of individuals who have previously experienced ORS, including ORS that interrupted or prevented daily activities but was not clinically severe. Strong recommendation by a health care provider is among the most important determinants of influenza vaccine acceptance.11,12,13 Our survey found a low rate of ORS recurrence that in most cases would not deter immunization — 4 of the 6 individuals who experienced recurrence in 2001 said that they would still be revaccinated in 2002. Revaccination should proceed as soon as possible to offset the negative impact of ORS in 2000; 91% of individuals with ORS who were revaccinated in 2001 would be vaccinated again in 2002 compared with 14% who have not yet been reimmunized.
Although the immunologic mechanism for ORS has yet to be elaborated, skin-test studies have shown that people who experienced ORS are at no greater risk than others of anaphylactic reaction.14 Instead, a distinct but as yet undetermined immunologic mechanism is likely involved. All influenza vaccines in 2000 contained completely inactivated virus.4 ORS, however, shares clinical features with influenza — in addition to respiratory symptoms, 20% of individuals with influenza also experience ocular symptoms including conjunctivitis.15 This may provide an important clue to the immunologic pathway for ORS.
Our survey included few participants who experienced ORS that might be considered clinically severe. Further systematic evaluation of these individuals in a controlled setting is warranted. As with all vaccines, a detailed risk–benefit assessment should be undertaken before reimmunization of individuals whose previous adverse event experience may have included collapse, respiratory difficulty (including throat tightness) and/or chest discomfort requiring emergency intervention. This assessment should include determination of whether symptoms may have had some other cause.
Enhanced surveillance efforts for ORS should continue across Canada in order to confirm the low rate of recurrence we have found and to provide additional reassurance. Perhaps most importantly, manufacturing and licensing safeguards should be considered to limit the proportion of unsplit or aggregated virions in influenza vaccines on an annual basis. Influenza is a major cause of morbidity and mortality in Canada each year, and the influenza vaccine remains our best defence against it.
Acknowledgments
We are grateful to Ms. Valencia Remple for study coordination, to the nurses of West Coast Clinical Research for conducting the interviews and to Ms. Marie Way and Mr. Joel King for data entry. We also acknowledge Dr. David Scheifele, Director, Vaccine Evaluation Centre for review of the manuscript and Dr. Patricia Daly, Medical Health Officer, Vancouver Coastal Health Authority, for additional commentary.
Footnotes
Fast-tracked article
This article has been peer reviewed.
Contributors: Dr. Skowronski contributed substantially to the conception and design of the study and to the analysis and interpretation of the data. She also drafted the article and performed revisions. Ms. Strauss contributed substantially to the conception and design of the study and to aquisition of the data. She also revised the article for important intellectual content. Dr. Kendall contributed substantially to the conception and design of the study and revised the article for important intellectual content. Dr. Duval contributed substantially to the interpretation of the data and revised the draft article for important intellectual content. Dr. De Serres contributed substantially to the conception and design of the study and interpretation of the data and revised the article for important intellectual content. All authors gave final approval of the version to be published.
Competing interests: None declared for Ms. Strauss, Dr. Kendall and Dr. Duval. Drs. Skowronski and De Serres have received a speaker's fee and travel expenses for a pertussis presentation from Aventis Pasteur.
Correspondence to: Dr. Danuta M. Skowronski, UBC Centre for Disease Control, 655 West 12th Ave., Vancouver BC V5Z 4R4; fax 604 660-0197; danuta.skowronski@bccdc.ca
References
- 1.National Advisory Committee on Immunization. Supplementary statement for the 2001–2002 season: influenza vaccination of persons who experienced oculo-respiratory syndrome following previous influenza vaccination. Can Commun Dis Rep 2001;27(ACS-7):1-7. [PubMed]
- 2.Bureau of Infectious Diseases. Oculo-respiratory syndrome in association with the influenza vaccine: Canada, October–November 2000. Can Commun Dis Rep 2000;26(23):1-2. [PubMed]
- 3.National Advisory Committee on Immunization. Supplementary statement on influenza vaccination: continued use of Fluviral influenza vaccine in the 2000–2001 season. Can Commun Dis Rep 2001;27(ACS-1):1-3. [PubMed]
- 4.National Advisory Committee on Immunization. Statement on influenza vaccination for the 2001–2002 season. Can Commun Dis Rep 2001;27(ACS-4):13-8.11227821
- 5.Boulianne N, De Serres G, Duval B, Shadmani R, Rochette L. Clinical manifestations and incidence of oculo-respiratory syndrome following influenza vaccination — Quebec, 2000. Can Commun Dis Rep 2001;27(10):85-90. [PubMed]
- 6.Spila-Alegiani S, Salmaso S, Rota MC, Tozzi AE, Raschetti R, the Italian SVEVA group. Reactogenicity in the elderly of nine commercial influenza vaccines: results from the Italian SVEVA study. Vaccine 1999;17:1898-904. [DOI] [PubMed]
- 7.Tumova B, Schramlova J, Vitkova E, Sulova V, Radovnicky V. Is the release control of influenza vaccine sufficient? [poster P5-14, abstract p 82]. Options for the control of influenza III meeting; 1996 May 4-9; Cairns, North Queensland, Australia.
- 8.Skowronski DM, De Serres G, Warrington R, Russell ML, Davies D, Duval B, et al. Oculo-respiratory syndrome following influenza vaccine: recurrence risk with re-formulated vaccine for 2001–2002 [abstract S42]. Annual Conference on Vaccine Research; 2002 May 6; Baltimore (MD).
- 9.De Serres G, Boulianne N, Duval B, Rochette L, Grenier JL, Roussel R, et al. Oculo-respiratory syndrome after influenza vaccination: similar risk with two 2001–2002 influenza vaccines [abstract S44]. Fifth Annual Conference on Vaccine Research; 2002 May 6; Baltimore (MD).
- 10.National Advisory Committee on Immunization. Supplementary statement for the 2002–2003 season: update on oculo-respiratory syndrome in association with influenza vaccination. Can Commun Dis Rep 2002;28(ACS-6):1-8. [PubMed]
- 11.Bovier PA, Chamot E, Bouvier Gallacchi M, Loutan L. Importance of patients' perceptions and general practitioners' recommendations in understanding missed opportunities for immunisations in Swiss adults. Vaccine 2001; 19 (32):4760-7. [DOI] [PubMed]
- 12.Nichol KL, MacDonald R, Hauge M. Factors associated with influenza and pneumococcal vaccination behavior among high-risk adults. J Gen Intern Med 1996; 11(11):673-7. [DOI] [PubMed]
- 13.Ashby-Hughes B, Nickerson N. Provider endorsement: the strongest cue in prompting high-risk adults to receive influenza and pneumococcal immunizations. Clin Excell NursePract 1999;3(2):97-104. [PubMed]
- 14.Skowronski DM, De Serres G, Hebert J, Stark D, Warrington R, Macnabb J, et al. Skin testing to evaluate oculo-respiratory syndrome (ORS) associated with influenza vaccination during the 2000–2001 season. Vaccine 2002;20: 2713-9. [DOI] [PubMed]
- 15.Nicholson KG. Human influenza. In: Textbook of influenza. London: Blackwell Science; 1998. p. 219-64.


