Abstract
The rare, autosomal recessive Roberts syndrome (RBS) is characterized by tetraphocomelia, profound growth deficiency of prenatal onset, craniofacial anomalies, microcephaly, and mental deficiency. SC phocomelia (SC) has a milder phenotype, with a lesser degree of limb reduction and with survival to adulthood. Since heterochromatin repulsion (HR) is characteristic for both disorders and is not complemented in somatic-cell hybrids, it has been hypothesized that the disorders are allelic. Recently, mutations in ESCO2 (establishment of cohesion 1 homolog 2) on 8p21.1 have been reported in RBS. To determine whether ESCO2 mutations are also responsible for SC, we studied three families with SC and two families in which variable degrees of limb and craniofacial abnormalities, detected by fetal ultrasound, led to pregnancy terminations. All cases were positive for HR. We identified seven novel mutations in exons 3–8 of ESCO2. In two families, affected individuals were homozygous—for a 5-nucleotide deletion in one family and a splice-site mutation in the other. In three nonconsanguineous families, probands were compound heterozygous for a single-nucleotide insertion or deletion, a nonsense mutation, or a splice-site mutation. Abnormal splice products were characterized at the RNA level. Since only protein-truncating mutations were identified, regardless of clinical severity, we conclude that genotype does not predict phenotype. Having established that RBS and SC are caused by mutations in the same gene, we delineated the clinical phenotype of the tetraphocomelia spectrum that is associated with HR and ESCO2 mutations and differentiated it from other types of phocomelia that are negative for HR.
Roberts syndrome (RBS [MIM 268300]) is named after the 1919 report of affected siblings by John B. Roberts, although at least one earlier well-documented case, first reported by Virchow in 1898, existed (Urban et al. 1997). RBS is a rare autosomal recessive disorder characterized by hypomelia, varying from tetraphocomelia to a lesser degree of limb deficiency that is more severe in the upper limbs; profound growth deficiency of prenatal onset; and craniofacial anomalies including microcephaly, cleft lip and palate, wide-set eyes, hypoplastic nasal alae, shallow orbits, micrognathia, frontal encephalocele, and mild-to-severe mental deficiency (Roberts 1919; Herrmann and Opitz 1977; Van Den Berg and Francke 1993a). Severely affected infants may be stillborn or die shortly after birth. In contrast, SC phocomelia syndrome (SC [MIM 269000]), first described by Herrmann et al. (1969), has a milder phenotype, with a lesser degree of symmetric limb reduction, and additionally includes flexion contractures of various joints, midfacial hemangioma, hypoplastic cartilage of ears and nose, scant silvery-blond hair, and cloudy corneae. Although microcephaly is present in SC, mental retardation may be mild, and survival into adulthood is common.
To distinguish these two disorders, a rating scale for clinical severity was initially developed in which R and S scores were assigned to patients reported to have RBS and SC, and R:S ratios were computed (Herrmann and Opitz 1977). This RS rating system, as redesigned (Van Den Berg and Francke 1993a), consists of six criteria: growth retardation, phocomelia of the arms, phocomelia of the legs, survival, and palatal and craniofacial abnormalities. Scores >0.5 indicate a more severe clinical phenotype consistent with RBS, and scores <−0.5 are consistent with a diagnosis of SC. Individuals with scores between 0.5 and −0.5 have multiple abnormalities that vary in severity. The presence of severely and mildly affected individuals in the same sibship raised the suspicion that the two disorders are allelic, representing a spectrum of severity. Furthermore, both are characterized by heterochromatin repulsion (HR) or premature centromere separation (PCS) in mitotic cells (Judge 1973; Freeman et al. 1974; Tomkins et al. 1979). Somatic-cell complementation studies revealed that cells positive for HR (HR+) from individuals with RBS and from those with SC do not complement each other, further supporting the notion that the same gene is affected in the two disorders (McDaniel et al. 2000).
Cell biologic studies documented abnormalities in metaphase duration, anaphase progression, and nuclear morphology and increased frequency of aneuploid cells and micronuclei in RBS (Tomkins and Sisken 1984). These characteristics, as well as the HR phenomenon, are recessive, since they are not found in heterozygous cells and are complemented by the Chinese hamster genome in somatic-cell hybrids (Krassikoff et al. 1986). As part of the development of a selection system for identification of complementing cDNAs, Van Den Berg and Francke (1993b) reported that RBS cells are hypersensitive to gamma radiation, mitomycin C, G418, and hygromycin but not to colcemid or streptonigrin.
Although the reduced growth rate and viability of cultured RBS cells may indicate a mechanism for reduced intrauterine/postnatal growth and specific limb and craniofacial abnormalities, the in vitro complementation studies did not lead to the identification of the RBS gene. Chromosome-banding studies failed to discover specific structural abnormalities, such as deletions or translocations, that could have hinted at the site of the responsible gene. Recent microcell-mediated gene-transfer experiments, however, identified the proximal 8p region as hosting a gene capable of complementing the HR and hygromycin-sensitivity phenotypes (McDaniel et al. 2005). The finding of 8p21 marker homozygosity in five unrelated RBS samples supported the notion that the HR-complementing locus on 8p may indeed be the RBS gene. Because of the rarity of the disorder and the fact that most cases are sporadic, linkage studies have not been performed until recently, when Vega et al. (2005) reported a genomewide homozygosity mapping study of seven consanguineous RBS-affected families from two isolated villages in Colombia, confirming linkage to 8p21.2-p12 between markers D8S258 and D8S505, with a maximum LOD score of 13.4 at D8S1839. By using a positional candidate-gene approach, they identified mutations in a transcript located >250 kb centromeric to D8S1839 (UCSC Genome Browser). Extending mutation analyses to mostly consanguineous RBSaffected families from Turkey, Italy, and Canada, Vega et al. (2005) reported eight different mutations in a gene they called “ESCO2” (establishment of cohesion 1 homolog 2) in 18 affected individuals from 15 families.
ESCO2 had been described elsewhere as EFO2 (establishment factor ortholog 2), a human ortholog of the yeast Eco1/Ctf7 acetyltransferase gene that needs to be expressed in S phase for the establishment of sister-chromatid cohesion (Bellows et al. 2003). Whereas Eco1 in yeast and deco in Drosophila are single-copy genes, Bellows et al. (2003) identified four human transcripts that contain the highly conserved Eco1/Ctf7p core domain and that are derived from loci on different human chromosomes. They characterized EFO1 on 18q11.2 in detail and EFO2 on 8p21 only to the extent of the carboxy-terminal 334 aa that include the evolutionarily conserved domains. Eco and ESCO genes have a conserved H2C2 zinc finger motif and an acetyltransferase domain but no other sequence similarities (Hou and Zou 2005; Vega et al. 2005). EFO2/ESCO2 comprises 11 exons spanning 30.3 kb, with the start codon in exon 2 and the stop codon in exon 11. The 1,806-nt ORF predicts a protein of 601 aa with a unique N-terminus.
To determine whether mutations in ESCO2 are also responsible for the SC phenotype and to search for genotype-phenotype correlations, we performed mutation analysis on six affected individuals from five families—three with SC who survived to adulthood, two with intermediate phenotypes, and one with classic RBS. Affected individuals from all five families were HR+. The study was institutional review board approved, and informed consent was obtained. DNA was extracted from peripheral blood lymphocytes, lymphoblastoid cell lines (LCLs), fibroblasts, amniocyte cultures, and archival paraffin-embedded tissues. We designed PCR primers that amplify each of the 11 exons, including the intron-exon boundaries, on the basis of GenBank RefSeq AY882862 (table 1). We purified the amplified PCR products after gel electrophoresis (Qiaquick [Qiagen]) and sequenced them bidirectionally using BigDye Terminator chemistry and an ABI 3100 sequencer (Applied Biosystems).
Table 1.
ESCO2 Primers and Conditions for Genomic PCR and RT-PCR[Note]
|
Primer Sequence(5′→3′) |
||||
| Region | Forward | Reverse | Size(bp) | Temperature(°C) |
| Genomic: | ||||
| Exon 2 | AAAGGGGGTATAATTTTGATAAAGC | AGGCTAGTGGAAGAACGAAAA | 238 | 58 |
| Exon 3-1 | GACGCAAAATAATCTTATCAATGG | ACAGTGGAGAGCGCAGATTT | 245 | 59 |
| Exon 3-2 | GTGCGCTCAAAACAACTGAA | TCCACCCTGGAGTGTAACTTG | 452 | 58 |
| Exon 3-3 | AGCAAAATCGAGTGATCTATAAGCC | CTGAAGTTTTGTTATACAGCCATTTT | 426 | 58 |
| Exon 4 | AGATCTGCTGGGATTCATTCA | AAAGAAAGACCCAGAAATTACAAGAA | 266 | 59 |
| Exon 5 | CCTCCATGGAATTACCTTTGC | TGAAAAAGAGACCCAGAGGAA | 264 | 59 |
| Exon 6 | GGCTGCTTTCTTTCCCTTGT | AGCTTTTCTGCAAAGGGCTA | 238 | 59 |
| Exon 7 | GGTGAAATTTGAATGAATGGTT | GGTGGTAGAGGTGGTGGAAA | 302 | 58 |
| Exon 8 | GCCTTTTGTCTTCTCCACATC | GCAAAGGCAGTTTGCACTTC | 262 | 59 |
| Exon 9 | CAATAGTGAAATAATTTTGGAGGTG | GAGTGTCATTAATGGTAGCAATCTT | 400 | 57 |
| Exon 10 | GCAGTGTGAACTCATCTGTGG | CGAGAGGTTCTGGGAAAGC | 293 | 59 |
| Exon 11 | TTGTGCTCATGTTAATTAGAATGTTA | GGAGCTCTTTGAACTTATCCAGA | 251 | 58 |
| cDNA: | ||||
| Exon 6/7 | CAGTTTTCTGTGGGATCTGTCA | TGCTGCATTTCATCTTCAGG | 191 | 58 |
| Exon 5/7 | CCAATCTTCAGTGCATCTTCA | GGCAGAACCAACACGATTTT | 347 | 59 |
Note.— PCR was performed with 100 ng of genomic DNA, 25 mM dNTPs, 10 pmol of each primer, and Taq polymerase (Invitrogen).
We discovered seven novel inactivating mutations that account for all mutant alleles in these families (fig. 1). Here, we provide documentation of all mutations, including sequences of the abnormal transcripts resulting from splice-site mutations, and a comparative analysis of the phenotypes of new cases and cases reported elsewhere.
Figure 1.
Gene structure of the ESCO2 gene, with location of identified mutations. Top, Eight mutations in 15 families with RBS reported by Vega et al. (2005). Bottom, Seven mutations found in four families with SC and one family with RBS (present study). UTRs are shown as white boxes, and the coding regions of exons 2–11, as gray boxes. Shown is the location of the functional domains—the C2H2 zinc finger-like domain (diagonal stripes) and the acetyltransferase domain (horizontal stripes).
Family 1.—This is one of the original families described as having “SC pseudothalidomide syndrome” by Herrmann et al. (1969, family C). In an LCL from the proband of this family (LCL639), established in our laboratory (Van Den Berg and Francke 1993b), we found two mutations: c.751_752insA, causing a frameshift and premature stop codon (p.K253fsX26), and c.1269G→A, causing a nonsense mutation (p.W423X) that results in a predicted protein missing the acetyltransferase domain. Although the parents were not available for study, we assume that the proband was compound heterozygous for two different mutations in exons 3 and 8 (table 2 and fig. 2).
Table 2.
ESCO2 Mutations Identified
| Familyand Mutation | Exonor Intron | MutationType | mRNA Change | Amino AcidChange |
| Family 1: | ||||
| c.751_752insA | Exon 3 | Frameshift | … | p.K253fsX26 |
| c.1269G→A | Exon 8 | Nonsense | … | p.W423X |
| Family 2: | ||||
| c.604C→T | Exon 3 | Nonsense | … | p.Q202X |
| c.752delA | Exon 3 | Frameshift | … | p.K253fsX12 |
| Family 3: | ||||
| c.1131+1G→A | Intron 6 | 5′ splice site | r.1014_1131del118 | p.R338fsX17 |
| Family 4: | ||||
| c.307_311delAGAAA | Exon 3 | Frameshift | … | p.I102fsX1 |
| Family 5: | ||||
| c.752delA | Exon 3 | Frameshift | … | p.K253fsX12 |
| c.1132−7A→G | Intron 6 | 3′ splice site | r.1131_1132insTTATAG | p.I377_378insLX |
Figure 2.
Pedigrees and ESCO2 mutations. Sites of mutations are indicated by arrows above the tracings. For compound heterozygotes (families 1, 2, and 5), both mutations are shown. In family 3, the mother is a heterozygote, whereas the son is homozygous for the same mutation. In family 4, the homozygous mutant sequence is shown in comparison with a normal reference sequence. Sequences of RT-PCR products reveal skipping of exon 6 (in family 3) and inclusion of 6 nt from intron 6 (in family 5). “P” with an arrow indicates the proband. A blackened symbol indicates affected with RBS or SC; unblackened symbol, unaffected; dot in center, obligate carrier; unblackened triangle, spontaneous abortion; blackened triangle with slash, affected fetus and induced abortion; double line between symbols, known consanguinity.
The proband (patient 3 of Herrmann et al. [1969], with an updated description by Feingold [1992]) was born to nonconsanguinous white parents (table 3). Symmetrical limb defects included bilateral absence of the radius and ulna, small thumbs, clinodactyly of hypoplastic fifth fingers, absence of the fibulae, bilateral syndactyly of the fourth and fifth toes, and wide gaps between the first and second toes. His hair was fine and silvery-blond but became brown and less sparse with age. He also had widely spaced eyes with downslanting palpebral fissures and bluish sclerae, a small tip of the nose, hypoplastic nasal alae, anteversion of the nostrils, and a capillary hemangioma of the upper lip, nose, and forehead. The auricles were posteriorly angulated, and the lower portion of the helices and the lobules were absent. He had a normal neurologic status and started to walk at age 13 mo. He developed seizures at age 9 mo and required anticonvulsant therapy throughout his life. Cognitively, he was in the moderately retarded range. A cavernous hemangioma of his right optic nerve was removed surgically, which resulted in blindness of that eye and severe ptosis. At age 14 years, he developed moyamoya disease (spontaneous occlusion of the circle of Willis), which caused left hemiparesis and additional minor strokes that led to loss of ambulation. He died at age 23 years from complications of a myocardial infarct.
Table 3.
Characteristic Clinical Findings in the Five Study Families[Note]
|
Family 1 |
Family 2 |
Family 4 |
||||||
| Characteristic | Proband | Sibling | Proband | Sibling | Family 3 | Fetus A | Fetus B | Family 5 |
| Clinical diagnosisa | SC | RBS | SC | SC | SC | RBS/SC | RBS/SC | RBS |
| Sex | M | M | F | F | M | F | F | F |
| Gestation (wk) | 43 | 35 | 40 | 40 | 40 | 25 | 22 | 20 |
| Birth weight (kg) | 2.1 | .85 | 1.82 | .36 | .34 | D+E | ||
| Age at death | 23 years | SB | 34 years | 43 years | …b | TOP at 25 wk | TOP at 22 wk | TOP at 20 wk |
| Growth retardation | 139 cm | + | 140 cm | + | Short stature | + | − | + |
| Mental retardation | Moderate | NA | IQ 66 | IQ 78 | Borderline | NA | NA | NA |
| Microcephaly (OFC) | −4 SD | − | −4 SD | + | −4 SD | + | ||
| Craniofacial hemangioma | + | + | + | − | − | NA | NA | NA |
| Proptosis of eyes | − | + | − | − | + | + | + | + |
| Hypertelorism | + | − | − | + | + | + | ||
| Corneal clouding | − | − | − | + | NA | NA | NA | |
| Hypoplastic nasal alae | + | + | + | + | + | + | NA | |
| Cleft palate | − | + | − | − | − | − | − | NA |
| Cleft lip | − | Unilateral | − | − | − | − | − | − |
| Prominent maxilla | + | + | + | + | + | − | NA | |
| Micrognathia | − | + | − | + | − | + | − | NA |
| Phocomelia (no. of limbs) | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Humerus | Normal | Hypoplastic | Hypoplastic | Humerora-dial fusion | Hypoplastic | + | ||
| Ulna | Absent | Hypoplastic | Hypoplastic | Hypoplastic | Absent | Hypoplastic | Absent | |
| Radius | Absent | Absent | Absent | Hypoplastic | Absent | Absent | Absent | |
| No. of carpal bones | 3 | 2 | 1 | 0 | 0 | |||
| No. of fingers | 5 | 3 | 4 | 4 | 5 | 4 | 5 | 3 |
| Thumbs | Hypoplastic | Absent | Absent | Absent | Hypoplastic | Absent | Hypoplastic | |
| Femur | − | − | NA | Hypoplastic | Hypoplastic | Normal | ||
| Patella | Hypoplastic | NA | Absent | NA | NA | NA | ||
| Tibia | − | Hypoplastic | NA | Bowed | Hypoplastic | Normal | ||
| Fibula | Absent | + | Hypoplastic | NA | Absent | Absent | Hypoplastic | |
| No. of toes | 5 | 5 | NA | 5 | 5 | 5 | 5 | |
| Syndactyly | 4th, 5th toes | − | − | − | − | + | − | |
| Flexion contractures | + | + | + | + | + | + | + | NA |
| Arterial occlusion | + | − | + | − | ||||
| HR | + | + | + | + | + | + | ||
| RS ratingc | −1 | 1.2 | −.83 | −.83 | −.17 | .2 | −.8 | 1.5 |
Note.— Plus sign (+) = presence of a characteristic; minus sign (−) = absence of characteristic; D+E = dilatation and extraction; NA = information not available because of leg amputation, D+E, or fetal age; OFC = occipital-frontal circumference; SB = stillbirth; TOP = termination of pregnancy.
Bold italics indicate an ESCO2 mutation was documented.
Alive at age 31 years.
Van Den Berg and Francke (1993a).
The proband had a severely affected, premature stillborn brother (patient 4 of Herrmann et al. [1969]). This brother had an enlarged head, widely patent anterior fontanel, protruding eyes, unilateral cleft lip and palate, deformed nose, micrognathia, and symmetrical limb deformities, with only three digits on each hand, flexion contractures of the knee joints, short tibiofibular areas, and deformed ankle joints (on the basis of the pathology report by Herrmann et al. [1969]).
Family 2.—In a nonconsanguineous white family of German descent, two adult sisters received a diagnosis of SC (Parry et al. 1986). Different cell types of the proband, peripheral blood lymphocytes, Epstein-Barr virus–transformed lymphoblasts (LCL121), skin fibroblasts, and metastatic melanoma cells had been previously studied in our laboratory and were reported as HR+ (Krassikoff et al. 1986; Parry et al. 1986; Van Den Berg and Francke 1993b). We screened a cell line of the proband (AG4344 [Coriell Cell Repository]) and found two different mutations: c.604C→T, a nonsense mutation causing a premature stop codon (p.Q202X), and the single-nucleotide deletion c.752delA, causing a frameshift with a predicted truncated protein (p.K253fsX12). The proband appeared to be a compound heterozygote with two different mutations in exon 3 (table 2 and fig. 2).
The proband was ascertained at age 32 years because of malignant melanoma of the left scapular region. She was short and microcephalic, with a broad high forehead, a faint heart-shaped nevus flammeus above the nasal bridge, a beak-shaped nose, a high nasal bridge, hypoplasia of the cartilage of tip, and nasal alae (fig. 3B and table 3). The upper limbs were symmetrically malformed, with short bowed forearms, fixed radial deviation of the hands, absent thumbs, brachymesophalangy, and camptodactyly. The lower limbs appeared short, with hypoplastic patellae and mild talipes equinovarus. The short, flat feet had a gap between the short, proximally placed hallux and second toe. On roentgenograms, there was thoracic kyphoscoliosis with small vertebrae, short and thin humeri and ulnae, absent radii, first metacarpals, thumb phalanges, and middle phalanges of both fifth fingers. There was a proximal (Y-shaped) synostosis of the fourth and fifth metacarpals bilaterally. The middle phalanges of the second and fourth fingers and the first phalanx of the fifth fingers were hypoplastic. The patellae were hypoplastic and irregularly shaped. The distal tibiae and fibulae were hypoplastic and abnormally modeled. Toes 3, 4, and 5 lacked middle phalanges bilaterally. Neurologically, she had congenital cranial nerve paralysis. Her full-scale IQ was 66 (verbal IQ 70; performance IQ 67). She died of metastatic malignant melanoma at age 34 years. A full-term pregnancy at age 24 years had resulted in an unaffected daughter.
Figure 3.
Phenotypes of affected individuals of families 1 and 2. A, Proband of family 1 at age 14 mo. Left, Phocomelia of the upper extremities. Lower extremities are less affected. Upper middle, Lateral view of the head, demonstrating hypoplastic nasal alae and low-set posteriorly rotated simple ears. Lower middle, View with radius and ulna absent, small thumb, and clinodactyly of the fifth finger (figs. 12, 15, and 13 in Herrmann et al. 1969, p. 86; reprinted with permission of the March of Dimes). Upper right, Silvery-blond hair, widely spaced narrow eyes, and hypoplastic nasal alae. Lower right, Proband at age 15 years, with beaked nose, prominent maxilla, ptosis, and blindness of the right eye due to surgical removal of a cavernous hemangioma (figs. 1 and 2 in Feingold 1992, p. 898; reprinted with permission of Wiley-Liss, a subsidiary of John Wiley & Sons). B, Proband of family 2, with high forehead, wide nasal bridge, drooping eyelids, hypoplastic nasal alae, beaked nose, wide short philtrum, thin upper lip, and bilateral facial nerve paralysis. Dorsal view of the hands shows absent thumbs and highly symmetrical contractures of fingers. C, Affected sister of family 2, with similar facial appearance as that of the proband and micrognathia. Palmar view of the hands shows absent thumbs and highly symmetrical contractures. (Panels B and C include fig. 1A and fig. 2A and 2D in Parry et al. 1986, pp. 655 and 658; reprinted with permission of Wiley-Liss, a subsidiary of John Wiley & Sons.)
The proband’s sister was similarly affected (fig. 3C and table 3). Born with severe knee contractures that resulted in amputation of the lower limbs, she died at age 43 years, after a massive stroke (Parry et al. 1986; case 68 in Van Den Berg and Francke 1993a).
Family 3.—The male proband (case 100 in Van Den Berg and Francke 1993a) appeared to be homozygous for a splice-site mutation (c.1131+1G→A) in intron 6. Only the mutant sequence was present in amplicons of genomic DNA. Since this mutation abolishes the donor splice site, we performed transcript amplification and sequencing, using RNA extracted from an LCL. The RNA was treated with DNaseI, and RT-PCR was performed with primer pairs designed for exon-to-exon amplification (table 1). The purified PCR amplicons were sequenced and revealed the skipping of exon 6 (118 nt) (r.1014_1131del118) causing a frameshift in the mutant mRNA and premature termination of translation (p.R338fsX17) (table 2 and fig. 2). We found no evidence of the presence of a normal transcript. The mutation was also present in the mother, but the father was unavailable, and no information regarding consanguinity could be obtained. We assume that the proband is homozygous for this mutation, although the possibility that he is hemizygous and carries a deletion of this region on the other allele has not been formally excluded.
The proband was born to an 18-year-old African American mother (G2P0Ab1) and a father reported to be healthy and young but uninvolved with the family. Orthopedic examination at age 6 wk noted mild microcephaly, marked shortness of the limbs, clubbing of both hands, and contractures at the knees, with a 90° flexion and bilateral popliteal pterygium. At age 6 years, he was found to have microcephaly, with a head circumference of 46 cm (−4 SD), intact hard and soft palate, ankyloglossia, hypoplastic midface with hypoplastic alae nasi, and maxillary dental protrusion. Radiographs showed a normal but small skull, normal vertebral bodies, humeroradial fusion, and hypoplastic radii and ulnae. His hands showed four metacarpals, with two of them fused, and a single carpal bone. Phalanges of thumbs and fifth fingers were hypoplastic. The pubic rami of the pelvis were absent. Symmetrical abnormalities of the lower limbs included anterior bowing and absent patellae and fibulae. The feet were normal. When seen at age 18 years, the proband was short, with adult sexual characteristics, microcephaly, midface hypoplasia, maxillary protuberance, hypoplastic nasal bridge and nasal alae, shallow orbits, corneal clouding, and small ears. His cognitive function was in the borderline retarded range. At his current age of 31 years, he lives with his mother, is mobile and is able to use both upper limbs and to take care of himself. He is reported to be otherwise healthy.
Family 4.—The parents were Hispanic second cousins and had two affected and three unaffected daughters. In both affected sibs, we found a homozygous 5-nt deletion (c.307_311delAAAGA), causing a frameshift that leads to a truncated protein (p.I102fsX1) (table 2 and fig. 2).
In the first affected sib (fetus A), fetal ultrasound at 23 wk gestation (by dates) demonstrated short femora (length consistent with 17 wk) and hypoechoic femoral shafts. Lower legs and forearms could not be identified on ultrasound scan. An amniocyte culture showed a 46,XX karyotype with trisomy 7 mosaicism. Postmortem findings, after pregnancy termination at 25 wk (by dates; 20 wk by composite ultrasound), included prominent eyes, flattened nasal tip, and micrognathia (fig. 4A). The palate was deeply ridged and high-arched without clefting. The upper limbs were extremely short, with only four digits bilaterally and syndactyly (fig. 5). The short lower limbs, held in a frog-like position, had five digits bilaterally. The clitoris appeared enlarged, and internal organs showed no obvious anomalies. Symmetrical skeletal deficiencies were evident on radiographs (fig. 4A). Distal humeri were narrow, and radii and ulnae were absent. There were three metacarpals and four digits, with the thumbs missing. On the left side, the proximal phalanges of the two lateral digits were partially fused. The femora appeared normal. The tibiae were extremely reduced in length, and possibly fractured, and fibulae were absent. Metatarsals and toe phalanges, as well as the skull, spine, and ribs, appeared normal.
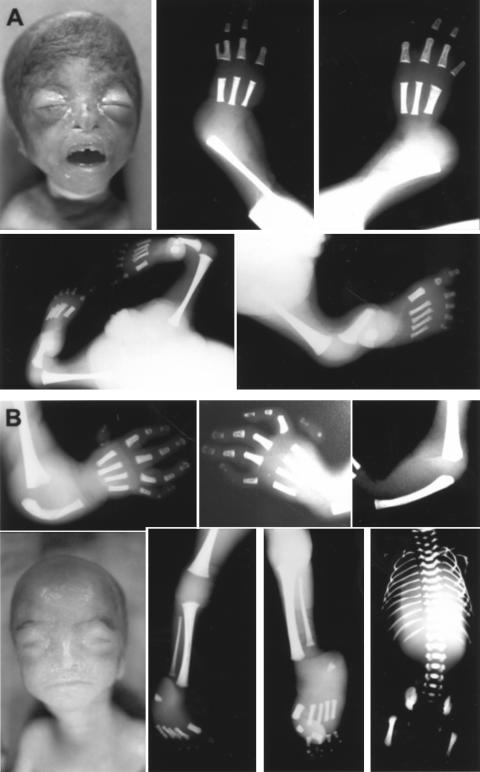
Figure 4.
Postmortem photographs and skeletal radiographs of affected sibs in family 4. A, Fetus A, with widely spaced protruding eyes, hypoplastic nasal alae, and beaked nose. Top, Radiographs of the left and right arm with missing radius and ulna, three metacarpals and four fingers each, and fusion of two proximal phalanges on the left. Bottom, Radiographs of the pelvis and legs contracted at the knees and the right leg extended. For description, see main text. B, Fetus B. Top, Radiographs of the left and right arm, with humerus present, single hypoplastic forearm bone, and five fingers with very hypoplastic appendage-like proximally placed thumbs. Bottom left, Similar appearance to fetus A, with widely spaced protruding eyes and beaked nose. Bottom middle, Lower legs with tibia and hypoplastic fibula present and five digits bilaterally. Bottom right, Normal appearance of vertebrae, ribs, and pelvis. See figure 5 for additional photographs.
Figure 5.
Comparison of the two affected fetuses in family 4. Although the facial features are similar, proptosis of the eyes and micrognathia may be more pronounced in fetus A. Fetus A has more-severe limb reduction, with dramatically foreshortened arms, missing thumbs, and syndactyly of the two most lateral digits. The legs are distinctly shorter and show severe contractures at the knees. The clitoris is enlarged. The rudimentary thumbs of fetus B are displaced and appear to lack articulation. In both fetuses, the relative degree of limb reduction is concordant in the upper and lower extremities, and the lateral symmetry of the malformations is striking. R = right; L = left.
In a subsequent pregnancy of this 35-year-old woman, an ultrasound examination at 19 wk demonstrated a similarly but less-severely affected offspring (fetus B) with short single-bone forearms bilaterally. Femur length was normal, but measurements of tibiae—and more so of fibulae—were reduced. Another ultrasound examination at 21 wk gestation confirmed single forearm bone and hypoplastic fibulae. When chromosome analysis of amniocytes showed a 46,XX karyotype with HR, a diagnosis of RBS was confirmed, and the pregnancy was terminated. Postmortem external examination showed a 348-g female fetus with a crown-rump length of 18 cm and a foot length of 3.1 cm (fig. 5). Head and neck were apparently normal. The upper limbs were markedly shortened, with proximally placed hypoplastic thumbs. The thorax, back, and abdomen were normal. The legs demonstrated severely clubbed feet, with five toes on each foot. Skeletal roentgenograms showed symmetrical reduction defects, including bilateral single-bone forearms, proximally placed hypoplastic thumbs, hypoplastic fibulae, and clubbed feet (fig. 4B).
Family 5.—In this nonconsanguineous white family, we discovered two heterozygous mutations in the proband (c.752delA and c.1132−7A→G) and traced each of them to a parent. The first mutation, c.752delA, caused a frameshift leading to a premature stop codon and a predicted truncated protein (p.K253fsX12). Interestingly, the same mutation was also found in family 2 with SC. The second mutation, c.1132−7A→G, was predicted to affect pre-mRNA splicing. The A→G substitution activates a cryptic splice site, which, in the human splice-site prediction program, yields a score of 0.97, whereas the score is only 0.41 for the natural splice site (Brunak et al. 1991; Hebsgaard et al. 1996; NetGene2 Server).
We used RNA extracted from a fibroblast culture for RT-PCR with PCR primers designed for exon-toexon amplification, followed by direct sequencing. At the transcript level, we detected an addition of 6 nt (r.1131_1132insTTATAG) to the 5′ end of exon 7. This inframe insertion of a leucine and a termination codon leads to premature termination of translation (p.I377_378insLX). Both mutations were present in amniocytes and fibroblast cultures established after pregnancy termination, and, in blood samples from the parents, one mutation each was found (table 2 and fig. 2).
An ultrasound examination at 18 wk gestation demonstrated a small-for-date fetus with a large frontal encephalocele, with fetal brain extruding into the amniotic cavity. The face appeared abnormal, with widely spaced orbits, bulging eyes, and flat midface. No clefting was noted. All four proximal and distal limbs were extremely reduced. Flipper-like hands at the shoulders had only three digits, but the feet, attached to the pelvis, appeared normal. Amniotic fluid alpha-fetoprotein was elevated, and an acetylcholinesterase assay had a positive result, consistent with an open neural tube defect. A fetal echocardiogram showed normal cardiac anatomy. Chromosome spreads from cultured fetal cells were HR+. The pregnancy was terminated by dilatation and extraction at 20 wk.
Comments.—The data reported here provide conclusive proof that RBS and SC are caused by mutations in the same gene, ESCO2, which encodes a putative acetyltransferase. ESCO2 is a human ortholog of a yeast gene involved in establishing sister-chromatid cohesion at the time of DNA replication. Mutations in the fly ortholog deco disrupt chromatid cohesion at the centromeres (Williams et al. 2003). On the basis of earlier clinical, cytogenetic, and somatic-cell complementation studies, it had been suspected that RBS and SC are allelic and may represent a single disorder with a wide range of clinical severity. For autosomal recessive disorders, as exemplified by the thalassemias, the expected molecular basis for such wide clinical variability would be allelic heterogeneity with different types of mutations and compound heterozygosity for null and hypomorphic alleles. Surprisingly, in our case collection, which ranges from mild adult to lethal presentations, we found exclusively inactivating nonsense and frameshift mutations that cause premature termination codons throughout the cDNA sequence. The only recurring mutation, c.752delA, was shared by an adult with SC and a fetus with severe RBS. Both affected individuals were compound heterozygotes, and the mutations on the other allele were inactivating as well, leading to a stop codon at position 202 in one and position 378 in the other. Vega et al. (2005) reported one nonsense and six frameshift mutations in exons 3–9 and one missense mutation (W539G) in exon 10. We identified two nonsense, three frameshift, and two splice-site mutations, which also caused frameshifts in exons 3–8 (fig. 1 and table 2). All affected individuals in the 15 families reported by Vega et al. (2005) had classic RBS, whereas six of the eight patients in our families had a milder phenotype, more consistent with SC, and four survived to adulthood. Apparently, neither the type nor the location of the ESCO2 mutations predicts the severity of the phenotype. The near absence of missense mutations may suggest that they cause a different phenotype or are selected against in heterozygotes.
HR was seen with all 15 ESCO2 mutations detected so far—eight by Vega et al. (2005) and seven in the present study. No mutation was found in one family with absent HR (authors' unpublished data). We propose that HR and ESCO2 mutations are tightly linked, causally related, and serve to distinguish this particular type of phocomelia from other phocomelia syndromes with pre- and postnatal growth deficiency. This phenotype can now be delineated with confidence on the basis of documented ESCO2 mutations. Including the entire RBS/SC clinical range of severity (table 3), the most characteristic findings are the wide flat face, hypertelorism, bulging eyes, beaked nose, hypoplastic nasal alae, midface capillary hemangioma, and, in severe cases, cleft lip and palate. The similarities of the facial gestalt of the individuals shown in figures 3 and 4 are quite striking, given their different ages. The characteristic RBS/SC limb-deficiency pattern affects all four limbs, is always symmetrical, and is usually more severe in the upper limbs. The striking symmetry is evident in figures 3B, 3C, 4, and 5. The greater degree of limb deficiency in fetus A, compared with fetus B, affects all four limbs equally. In both cases, however, the feet contained five metatarsals and five digits. This is significant because even in severe RBS, in which the long bones are absent, rather normally shaped feet are attached to the pelvis, as in Virchow’s 1898 case (Urban et al. 1997) and the proband of our family 5. Minor abnormalities, such as short halluces and wide spaces between the first and second toes, may be present. The symmetry affects not only bone structure but connective tissue as well, as demonstrated by knee contractures, resulting in a frog-like position of the legs (fetus A in fig. 5), and the finger contractures shown in figure 3B and 3C. Unrelated to the degree of limb deficiency, microcephaly is severe (−4 SD), and cognitive function is below normal.
We revisited the sources of the widely cited conclusion that only 80% of RBS/SC cases show the HR phenomenon and that HR+ and HR− cases are clinically indistinguishable. In their survey, Van Den Berg and Francke (1993a) stated that 38 of 48 RS cases that had been screened for HR were reported as HR+, but they expected this to be an underestimate. In fact, several cases with a normal karyotype report were not specifically examined for the presence of HR, because the phenomenon had not yet been described or was not widely known at the time (e.g., cases 21, 36, 46, 65, and the twins 51 and 52). Case 56, listed as HR−, was reported to be HR+ in the original article. Case 57, examined for HR and reported to be HR−, had a clinically distinct phenotype (normal size at birth, normal intelligence, and a more extensive skeletal dysplasia, including dysplastic clavicles and pelvic bones, fused ribs, and stenotic ear canals). Case 65 had asymmetric involvement of the upper limbs and facial features consistent with Cornelia de Lange syndrome (CdLS [MIM 122470]). Cases 77 and 78—featuring more-severe involvement of the lower limbs than of the upper, with one sibling lacking all toes and the other having two rudimentary toes—are inconsistent with the RBS/SC type of tetraphocomelia. Therefore, the absence of HR confirms that cases 77 and 78 have a different type of phocomelia. Thus, a critical review of the original literature leads us to conclude that the 10 cases in Van Den Berg and Francke (1993a) cannot be confirmed as RBS with absent HR. The same conclusion holds for the recent case from Korea described as RBS without HR (Hwang et al. 2002). The proband had cleft palate and absent radius, ulna, and fibula but five digits on the hands and feet and normal head size and intelligence. We therefore propose that HR is pathognomonic for the RBS/SC multiple-malformation syndrome that is caused by mutations in ESCO2. Since HR is detectable in chorionic villous cells, pregnancies at risk can be monitored. In addition, after detection of both mutant ESCO2 alleles, preimplantation genetic diagnosis becomes a feasible option (M. Hughes, personal communication).
In countries where pregnancies are routinely monitored by ultrasound, new RBS/SC cases are usually detected prenatally in families with unsuspecting parents, and genetic counseling is requested. If HR is present and/or ESCO2 mutations are found, our data suggest that the prognosis for normal mental development is poor, regardless of the degree of limb-reduction defects. In addition, survivors beyond childhood may be prone to acute arterial vascular occlusions (e.g., moyamoya disease and myocardial infarction in family 1 and massive stroke in family 2). Furthermore, lagging chromosomes resulting in aneuploid cells, typically observed in RBS/SC cell cultures, could predispose to malignancies; however, so far, only two cases have been reported (melanoma in family 2 and rhabdomyosarcoma in a 23-mo-old child with HR+ RBS [Wenger et al. 1988]).
Although Vega et al. (2005) stated, “RBS is the first human disorder identified to our knowledge in which a chromatid cohesion defect is associated with developmental abnormalities” (p. 469), we wish to call attention to CdLS. This rare, usually sporadic, multiple-malformation disorder includes features that overlap with RBS/SC, such as prenatal-onset growth retardation, short stature, mental retardation, microcephaly, brachycephaly, micrognathia, cleft lip/palate, elbow contractures, small hands and feet, and, in ∼25% of cases, asymmetric phocomelia and oligodactyly of the upper extremities. Although there is wide phenotypic variability, CdLS is easily distinguished by a characteristic facies. Last year, it was found that CdLS is caused by heterozygous mutations in NIBPL (Nipped-B-like), which encodes a human ortholog of yeast Scc2-type sister-chromatid cohesin proteins and the Nipped-B developmental regulator in Drosophila (Krantz et al. 2004; Tonkin et al. 2004). Nipped-B mutants have premature separation of chromatids, and the Xenopus Scc ortholog is required to load cohesins onto chromatin (reviewed by Strachan [2005]). Although the mammalian orthologs NIBPL and ESCO2 (Hou and Zou 2005) are probably part of complexes with additional functions in chromatin looping and DNA replication and repair, and although the pathways that lead to the developmental defects have still to be unraveled, RBS/SC and CdLS represent the first-known members of a new class of chromatid cohesion defect disorders.
Acknowledgments
We thank the families, for participating in this study; Athena Cherry, Xu Li, and Jennifer Winters, for cytogenetic studies and amniocyte cultures; Geoffrey A. Machin, for an autopsy report; and Cathy Christian, Elise Obolensky, and Ana Stenzel, for genetic counseling and providing records. B.S. was supported by a fellowship from the Deutsche Forschungsgemeinschaft (SCHU-1567/1-1).
Web Resources
The accession number and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for RefSeq AY882862)
- NetGene2 Server, http://www.cbs.dtu.dk/services/NetGene2/ (for splice-site prediction progam)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for RBS, SC, and CdLS)
- UCSC Genome Browser, http://genome.ucsc.edu/ (for ESCO2 mapping data)
References
- Bellows AM, Kenna MA, Cassimeris L, Skibbens RV (2003) Human EFO1p exhibits acetyltransferase activity and is a unique combination of linker histone and Ctf7p/Eco1p chromatid cohesion establishment domains. Nucleic Acids Res 31:6334–6343 10.1093/nar/gkg811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunak S, Engelbrecht J, Knudsen S (1991) Prediction of human mRNA donor and acceptor sites from the DNA sequence. J Mol Biol 220:49–65 10.1016/0022-2836(91)90380-O [DOI] [PubMed] [Google Scholar]
- Feingold M (1992) History of C-patient with SC-Roberts/pseudothalidamide syndrome. Am J Med Genet 43:898–899 10.1002/ajmg.1320430532 [DOI] [PubMed] [Google Scholar]
- Freeman MV, Williams DW, Schimke RN, Temtamy SA, Vachier E, German J (1974) The Roberts syndrome. Clin Genet 5:1–16 [DOI] [PubMed] [Google Scholar]
- Hebsgaard SM, Korning PG, Tolstrup N, Engelbrecht J, Rouze P, Brunak S (1996) Splice site prediction in Arabidopsis thaliana pre-mRNA by combining local and global sequence information. Nucleic Acids Res 24:3439–3452 10.1093/nar/24.17.3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J, Feingold M, Tuffli G, Opitz J (1969) A familial dysmorphogenetic syndrome of limb deformities, characteristic facial appearance and associated anomalies: the “pseudothalidomide” or “SC-syndrome.” In: Birth defects: original article series. Vol. 5. March of Dimes, New York, pp 81–89 [Google Scholar]
- Herrmann J, Opitz JM (1977) The SC phocomelia and the Roberts syndrome: nosologic aspects. Eur J Pediatr 125:117–134 10.1007/BF00489985 [DOI] [PubMed] [Google Scholar]
- Hou F, Zou H (2005) Two human orthologues of Eco1/Ctf7 acetyltransferases are both required for proper sister-chromatid cohesion. Mol Biol Cell 16:3908–3918 10.1091/mbc.E04-12-1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Lee DK, Lee SI, Lee HS (2002) Roberts syndrome, normal cell division, and normal intelligence. J Craniofac Surg 13:390–394 10.1097/00001665-200205000-00005 [DOI] [PubMed] [Google Scholar]
- Judge C (1973) A sibship with the pseudothalidomide syndrome and an association with Rh incompatibility. Med J Aust 2:280–281 [DOI] [PubMed] [Google Scholar]
- Krantz ID, McCallum J, DeScipio C, Kaur M, Gillis LA, Yaeger D, Jukofsky L, Wasserman N, Bottani A, Morris CA, Nowaczyk MJ, Toriello H, Bamshad MJ, Carey JC, Rappaport E, Kawauchi S, Lander AD, Calof AL, Li HH, Devoto M, Jackson LG (2004) Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat Genet 36:631–635 10.1038/ng1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassikoff NE, Cowan JM, Parry DM, Francke U (1986) Chromatid repulsion associated with Roberts/SC phocomelia syndrome is reduced in malignant cells and not expressed in interspecies somatic-cell hybrids. Am J Hum Genet 39:618–630 [PMC free article] [PubMed] [Google Scholar]
- McDaniel LD, Prueitt R, Probst LC, Wilson KS, Tomkins D, Wilson GN, Schultz RA (2000) Novel assay for Roberts syndrome assigns variable phenotypes to one complementation group. Am J Med Genet 93:223–229 [DOI] [PubMed] [Google Scholar]
- McDaniel LD, Tomkins DJ, Stanbridge EJ, Somerville MJ, Friedberg EC, Schultz RA (2005) Mapping of a single locus capable of complementing the defective heterochromatin phenotype of Roberts syndrome cells. Am J Hum Genet 77:132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry DM, Mulvihill JJ, Tsai SE, Kaiser-Kupfer MI, Cowan JM (1986) SC phocomelia syndrome, premature centromere separation, and congenital cranial nerve paralysis in two sisters, one with malignant melanoma. Am J Med Genet 24:653–672 10.1002/ajmg.1320240410 [DOI] [PubMed] [Google Scholar]
- Roberts J (1919) A child with double cleft of lip and palate, protrusion of the intermaxillary portion of the upper jaw and imperfect development of the bones of the four extremeties. Ann Surg 70:252–253 [Google Scholar]
- Strachan T (2005) Cornelia de Lange syndrome and the link between chromosomal function, DNA repair and developmental gene regulation. Curr Opin Genet Dev 15:258–264 10.1016/j.gde.2005.04.005 [DOI] [PubMed] [Google Scholar]
- Tomkins D, Hunter A, Roberts M (1979) Cytogenetic findings in Roberts-SC phocomelia syndrome(s). Am J Med Genet 4:17–26 10.1002/ajmg.1320040104 [DOI] [PubMed] [Google Scholar]
- Tomkins DJ, Sisken JE (1984) Abnormalities in the cell-division cycle in Roberts syndrome fibroblasts: a cellular basis for the phenotypic characteristics? Am J Hum Genet 36:1332–1340 [PMC free article] [PubMed] [Google Scholar]
- Tonkin ET, Wang TJ, Lisgo S, Bamshad MJ, Strachan T (2004) NIPBL, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly Nipped-B, is mutated in Cornelia de Lange syndrome. Nat Genet 36:636–641 10.1038/ng1363 [DOI] [PubMed] [Google Scholar]
- Urban M, Rogalla P, Tinschert S, Krietsch P (1997) Tetraphocomelia and bilateral cleft lip in a historical case of Roberts syndrome [Virchow, 1898]. Am J Med Genet 72:307–314 [DOI] [PubMed] [Google Scholar]
- Van Den Berg DJ, Francke U (1993a) Roberts syndrome: a review of 100 cases and a new rating system for severity. Am J Med Genet 47:1104–1123 10.1002/ajmg.1320470735 [DOI] [PubMed] [Google Scholar]
- ——— (1993b) Sensitivity of Roberts syndrome cells to gamma radiation, mitomycin C, and protein synthesis inhibitors. Somat Cell Mol Genet 19:377–392 10.1007/BF01232749 [DOI] [PubMed] [Google Scholar]
- Vega H, Waisfisz Q, Gordillo M, Sakai N, Yanagihara I, Yamada M, van Gosliga D, Kayserili H, Xu C, Ozono K, Jabs EW, Inui K, Joenje H (2005) Roberts syndrome is caused by mutations in ESCO2, a human homolog of yeast ECO1 that is essential for the establishment of sister chromatid cohesion. Nat Genet 37:468–470 10.1038/ng1548 [DOI] [PubMed] [Google Scholar]
- Wenger SL, Blatt J, Steele MW, Lloyd DA, Bellinger M, Phebus CK, Horn M, Jaffe R (1988) Rhabdomyosarcoma in Roberts syndrome. Cancer Genet Cytogenet 31:285–289 10.1016/0165-4608(88)90230-0 [DOI] [PubMed] [Google Scholar]
- Williasms BC, Garrett-Engele CM, Li Z, Williams EV, Rosenman ED, Goldberg ML (2003) Two putative acetyltransferases, san and deco, are required for establishing sister chromatid cohesion in Drosophila. Curr Biol 13:2025–2036 10.1016/j.cub.2003.11.018 [DOI] [PubMed] [Google Scholar]