Abstract
According to classical concepts of physiologic control, healthy systems are self-regulated to reduce variability and maintain physiologic constancy. Contrary to the predictions of homeostasis, however, the output of a wide variety of systems, such as the normal human heartbeat, fluctuates in a complex manner, even under resting conditions. Scaling techniques adapted from statistical physics reveal the presence of long-range, power-law correlations, as part of multifractal cascades operating over a wide range of time scales. These scaling properties suggest that the nonlinear regulatory systems are operating far from equilibrium, and that maintaining constancy is not the goal of physiologic control. In contrast, for subjects at high risk of sudden death (including those with heart failure), fractal organization, along with certain nonlinear interactions, breaks down. Application of fractal analysis may provide new approaches to assessing cardiac risk and forecasting sudden cardiac death, as well as to monitoring the aging process. Similar approaches show promise in assessing other regulatory systems, such as human gait control in health and disease. Elucidating the fractal and nonlinear mechanisms involved in physiologic control and complex signaling networks is emerging as a major challenge in the postgenomic era.
A hallmark of physiologic systems is their extraordinary complexity. The nonstationarity and nonlinearity of signals (Fig. 1) generated by living organisms defy traditional mechanistic approaches based on homeostasis and conventional biostatistical methodologies. Recognition that physiologic time series contain “hidden information” has fueled growing interest in applying concepts and techniques from statistical physics, including chaos theory, to a wide range of biomedical problems from molecular to organismic levels (1, 2).
Fig 1.
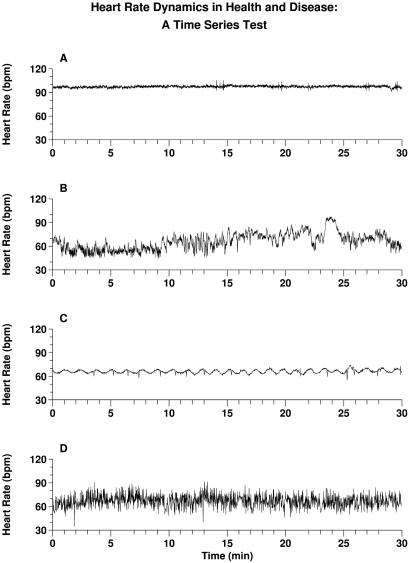
Representative heart rate recordings in health and disease, presented as four unknowns. One record is normal; the other three represent severe pathologies. Can you identify which is normal? Answers: A and C are from patients in sinus rhythm with severe congestive heart failure. D is from a subject with a cardiac arrhythmia, atrial fibrillation, which produces an erratic heart rate. The healthy record, B, far from a homeostatic constant state, is notable for its visually apparent nonstationarity and “patchiness.” These features are related to fractal and nonlinear properties. Their breakdown in disease may be associated with the emergence of excessive regularity (A) and (C), or uncorrelated randomness (D). Of note in C is the presence of strongly periodic oscillations (≈1/min), which are associated with Cheyne-Stokes breathing, a pathologic type of cyclic respiratory pattern. Quantifying and modeling the complexity of healthy variability, and detecting more subtle alterations with disease and aging, present major challenges in contemporary biomedicine.
This presentation describes one area of investigation that has engaged our collaborative attention, namely, fractal analysis of physiologic time series in health and disease. The discussion will focus primarily on certain features of the human heartbeat, one important example of complex physiologic fluctuations. The dynamics of another physiologic control system—human gait—is also briefly discussed. Recognizing that this topic represents only one selected aspect of the broad and rapidly expanding applications of complexity theory to biomedicine (Table 1), readers are referred to a number of useful reviews and references therein (1, 3–10).
Table 1.
Nonlinear/complexity mechanisms and phenomena in physiology: partial list of possible contenders
| Abrupt changes |
| Bifurcations |
| Intermittency/bursting |
| Bistability/multistability |
| Phase transitions |
| Hysteresis |
| Nonlinear oscillations |
| Limit cycles |
| Phase-resetting |
| Entrainment |
| Pacemaker annihilation |
| Scale-invariance |
| Fractal and multifractal scaling |
| Long-range correlations |
| Self-organized criticality |
| Diffusion limited aggregation |
| Alternans phenomena |
| Nonlinear waves: spirals; scrolls; solitons |
| Complex periodic cycles and quasiperiodicities |
| Stochastic resonance and related noise-modulated mechanisms |
| Time irreversibility |
| Complex networks |
| Deterministic chaos |
| Emergent properties |
Modified from ref. 13.
A motivating problem for our work is depicted in Fig. 1, which presents a dynamical self-test. Shown are 30-min heart rate time series from four subjects. Only one is from a healthy individual; the other three are from patients with life-threatening forms of heart disease. The problem is to identify the normal record. The (perhaps nonintuitive) answer to this “test” is given in the figure caption. Beyond its obvious diagnostic import, the problem of classifying temporal assays of integrated cardiac physiology has implications for understanding and modeling basic signaling and regulatory networks. These representative time series in health and disease illustrate two major themes of this review (11–16): (i) The output of healthy systems, under certain critical parameter conditions, reveals a type of complex variability associated with long-range (fractal) correlations, along with distinct classes of nonlinear interactions. (ii) Multiscale, nonlinear complexity appears to degrade in characteristic ways with aging and disease, reducing the adaptive capacity of the individual. Further, these “syndromes” of fractal/nonlinear breakdown may be quantified, with potential applications to diagnostic and prognostic assessment.
We begin, therefore, by defining the fractal concept. We then describe certain aspects of fractal scaling in health, followed by illustrations of the breakdown of fractal complexity in aging and disease. To catalyze progress in the applications of fractals, and complexity science in general, to biomedicine, we conclude with a plea for open-source data and algorithms.
Fractal Anatomies and Self-Similar Dynamics
The concept of a fractal is most often associated with irregular geometric objects that display self-similarity (refs. 3, 13, and 17–19; ref. 19 available at www.physionet.org/tutorials/fmnc/). Fractal forms are composed of subunits (and sub-sub-units, etc.) that resemble the structure of the overall object (Fig. 2 Left). In an idealized model, this property holds on all scales. The real world, however, necessarily imposes upper and lower bounds over which such scale-invariant behavior applies. Many non-Euclidean structures in nature, such as branching trees, wrinkly coastlines, and the rough surfaces of mountains, are fractal. A number of complex anatomic structures also display fractal-like geometry (3, 12, 13, 18, 20). Examples include arterial and venous trees and the branching of certain cardiac muscle bundles, as well as other networks such as the tracheobronchial tree and the His-Purkinje conduction system. These self-similar cardiopulmonary structures subserve at least one fundamental physiologic function: rapid and efficient transport over complex, spatially distributed networks. Fractal geometry also appears to underlie important aspects of cardiac mechanical function (21). A variety of other organ systems contain fractal-like structures that facilitate information dissemination (nervous system), nutrient absorption (bowel), as well as distribution, collection, and transport (biliary ducts, renal calyces, choroidal plexus, and placental chorionic villae). With aging and disease, fractal anatomic structures may show degradation in their structural complexity (11, 12). Examples include loss of dendritic arbor in aging cortical neurons (11) and vascular “pruning” in primary pulmonary hypertension.
Fig 2.
Schematic representations of self-similar structures and self-similar fluctuations. The tree-like, spatial fractal (Left) has self-similar branchings, such that the small-scale structure resembles the large-scale form. A fractal temporal process, such as healthy heart rate regulation (Right), may generate fluctuations on different time scales that are statistically self-similar. Adapted from ref. 13.
The fractal concept can be applied not just to irregular geometric forms that lack a characteristic (single) scale of length, but also to certain complex processes that lack a single scale of time (17, 19, 22, 23). Fractal processes generate irregular fluctuations across multiple time scales, analogous to scale-invariant objects that have a branching or wrinkly structure across multiple length scales. A qualitative appreciation for the self-similar nature of fractal processes can be obtained by plotting their fluctuations at different temporal resolutions. For example, Fig. 2 Right displays a heart rate time series from a healthy subject on three different time scales. Each graph has an irregular appearance, reminiscent of a mountain range. The irregularity seen on different scales is not readily distinguishable, suggesting statistical self-similarity. A more rigorous representation of the temporal self-similarity of the healthy heartbeat is provided by wavelet analysis (Fig. 3; refs. 24 and 25).
Fig 3.
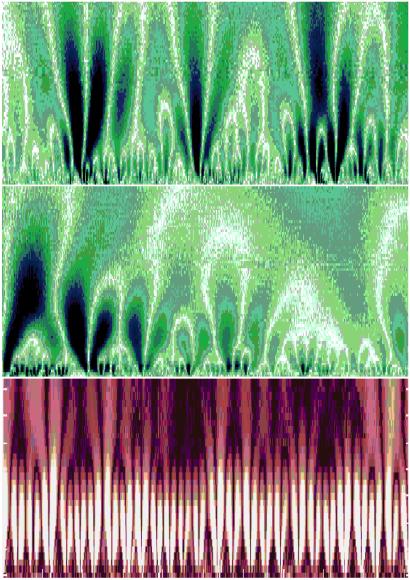
(Top) Color-coded wavelet analysis of a heart rate time series in health. The x axis represents time (≈1700 beats), and the y axis indicates the wavelet scale, extending from about 5 to 300 s, with large time scales at the top. The brighter colors indicate larger values of the wavelet amplitudes, corresponding to large heartbeat fluctuations. White tracks represent the wavelet transform maxima lines—the structure of these maxima lines shows the evolution of the heartbeat fluctuations with scale and time. This wavelet decomposition reveals a tree-like, self-similar hierarchy to the healthy cardiac dynamics. (Middle) Magnification of the central portion of the top panel, with 200 beats on the x axis and wavelet scale corresponding to about 5 to 75 s on the y axis, shows similar branching patterns. (Bottom) In contrast, wavelet decomposition of heartbeat intervals (≈1500 beats) from a patient with obstructive sleep apnea, a common pathologic condition, shows the loss of complex, multiscale hierarchy, with emergent, single-scale (periodic) behavior. The wavelet scale (along the y axis) extends from about 5 to 200 s. The red background is used to provide contrast with the fractal cascades under healthy conditions, shown in the Upper panels. Adapted from ref. 24.
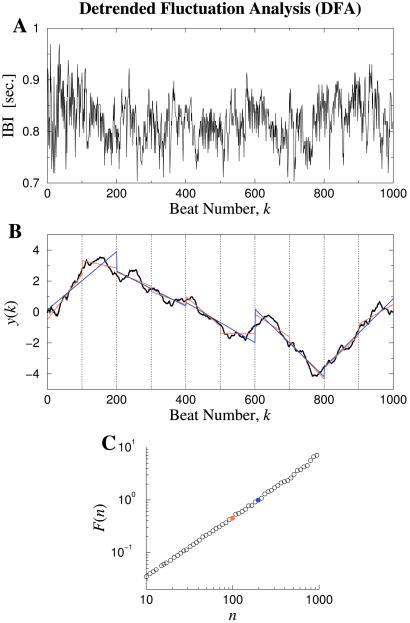
An important methodologic challenge is how to detect and quantify the scaling and correlation properties of physiologic time series, which are typically not only irregular, but also nonstationary (i.e., their statistical properties change with time). To help deal with the ubiquitous biologic “complication” of nonstationarity, we have introduced a modified rms analysis of a random walk—termed detrended fluctuation analysis (DFA; refs. 19 and 26–29). To illustrate the DFA algorithm briefly, consider the cardiac interbeat series shown in Fig. 4A. First, the original time series is integrated, and then divided into boxes of equal length, n. For each box of length n, a least squares line (representing the trend in that box) is fit to the data (Fig. 4B). For a given box size n, the characteristic size of the fluctuations, denoted by F(n), is then calculated as the rms deviation between y(k) and its trend in each box. This computation is repeated over all time scales (box sizes). Typically, F(n) will increase with box size n. A linear relationship on a log-log graph indicates the presence of scaling (self-similarity), such that fluctuations in small boxes are related to the fluctuations in larger boxes in a power-law fashion. The slope of the line relating log F(n) to log n determines the fractal scaling exponent, α (Fig. 4C). This exponent provides a measure of the “roughness” of the original time series: the larger the value of α, the smoother the time series. In this context, 1/f-like noise (α = 1) can be interpreted as a “compromise” between the complete unpredictability of white noise (α = 0.5) and the much smoother “landscape” of Brownian noise (α = 1.5; ref. 19).§
Fig 4.
Illustration of the DFA algorithm to test for scale-invariance and long-range correlations. (A) Interbeat interval (IBI) time series from a healthy young adult. (B) The solid black curve is the integrated time series, y(k). The vertical dotted lines indicate boxes of size n = 100 beats. The red straight line segments represent the “trend” estimated in each box by a linear least-squares fit. The blue straight line segments represent linear fits for box size n = 200. Note that the typical deviation from the y(k) curve to the red lines is smaller than the deviation to the blue lines. (C) The rms deviations, F(n), in B are plotted against the box size, n, in a double logarithmic plot. The red circle is the data point for F(100), and the blue circle is the data point for F(200). A straight-line graph indicates power-law scaling. The slope of the line, α, relates to the presence and type of two-point correlations. In this case, α ≃ 1.0, consistent with 1/f noise and long-range correlations; α = 0.5 indicates white noise with uncorrelated randomness; α = 1.5 indicates Brownian noise. See Figs. 5–7 and text. Adapted from ref. 19.
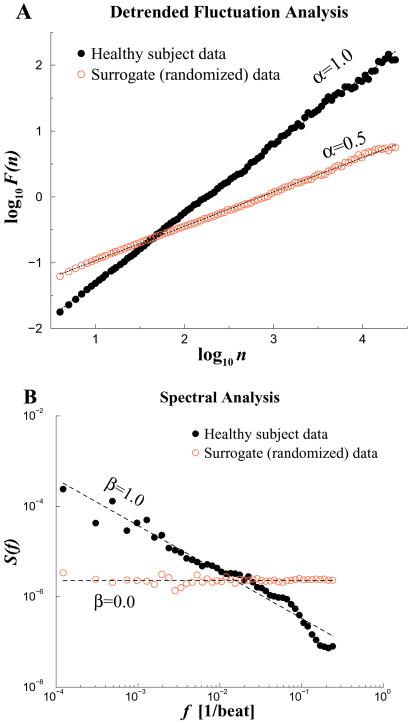
To test whether heartbeat time series exhibit fractal behavior and to determine their correlation properties, we can apply the DFA algorithm and other scaling techniques to long-term heart rate recordings. Fig. 5A compares the scaling analysis of the interbeat interval time series from a representative healthy young adult with its randomized time series as a control. For the healthy subject, DFA shows scaling behavior with exponent α ≃ 1 over nearly three decades, indicative of long-range correlations extending to thousands of heartbeats. As expected, the randomized “control” data set shows a trivial exponent α = 0.5, indicating a white noise output associated with the breakdown of correlations. Power spectrum analysis (Fig. 5B) is consistent with the DFA results. However, the β exponent derived from the power spectrum is less accurate because the stationarity requirements for Fourier analysis, are, as usual, not satisfied in this case of real-world data.¶ Systematic analysis has confirmed the scaling behavior and long-range correlation properties of the healthy human heartbeat (14, 22, 23).
Fig 5.
Fractal scaling analyses for two 24-hr interbeat interval time series. The solid black circles represent data from a healthy subject, whereas the open red circles are for the artificial time series generated by randomizing the sequential order of data points in the original time series. (A) Plot of log F(n) vs. log n by the DFA analysis. (B) Fourier power spectrum analysis. The spectra have been smoothed (binned) to reduce scatter. DFA and Fourier scaling exponents, α ≃ 1.0 and β ≃ 1.0, respectively, are consistent with long-range correlations (1/f noise). After randomization, α ≃ 0.5 and β ≃ 0, consistent with loss of correlation properties (white noise).
Altered Fractal Dynamics of the Heartbeat
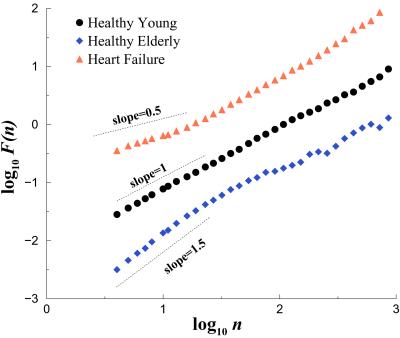
The presence of long-range (fractal) correlations in cardiovascular fluctuations in health has implications for understanding and modeling neuroautonomic regulation. Of particular note, this type of scaling behavior is not accounted for by traditional homeostatic mechanisms of control, whose goal is to maintain a constant, “steady-state” output (2, 13, 19, 22, 30). A related consideration is whether pathologic states and aging are associated with distinctive alterations in these scaling properties, which could be of practical diagnostic and prognostic use. We have found that datasets from patients with congestive heart failure are particularly useful in assessing correlations under markedly pathologic conditions, because this life-threatening condition is associated with profound abnormalities in both the sympathetic and parasympathetic control mechanisms that regulate beat-to-beat variability. Fig. 6 compares scaling analysis of representative 24-hr interbeat interval time series from a healthy subject and a patient with congestive heart failure. For large time scales (asymptotic behavior), data from the healthy subject (consistent with data in Figs. 4 and 5), show long-range correlations extending over nearly three decades, with α ≃ 1 (i.e., 1/f noise), whereas for the heart failure data set, α ≃ 1.3 (closer to Brownian noise). Analysis of scaling behavior in a variety of life-threatening cardiac pathologies indicates significant alterations in short and long-range heartbeat correlation properties, suggesting possible clinical applications (22, 27, 31–34). Subsequent studies have also revealed significant differences in scaling exponents associated with circadian variations in health and disease (35), as well as during different phases of sleep (36).
Fig 6.
Scaling analysis of heartbeat time series in health, aging, and disease. Plot of log F(n) vs. log n for data from a healthy young adult, a healthy elderly subject, and a subject with congestive heart failure. Compared with the healthy young subject, the heart failure and healthy elderly subjects show alterations in both short and longer range correlation properties. To facilitate assessment of these scaling differences, the plots are vertically offset from each other. Adapted from ref. 19.
We have applied similar analyses to assess the effects of physiologic aging on heartbeat correlation behavior. For a group of healthy elderly subjects (68–81 years), the cardiac interbeat interval time series suggested two scaling regions. Over short time scales, fluctuations resembled a random walk process (Brownian noise, α ≃ 1.5), whereas over the longer range, they resembled white noise (α ≃ 0.5). Both short-range and long-range exponents were significantly different in the elderly subjects compared with young adults (37). Further, the alterations of scaling behavior associated with physiologic aging exhibited different patterns compared with the changes associated with heart failure (Fig. 6). We have speculated that the degradation of short and longer range correlation properties with aging may be associated with the loss of integrated physiologic responsiveness, thereby increasing susceptibility to injury and illness in the elderly (11, 16).
Beyond 1/f Noise: Multifractal Scaling in Heartbeat Dynamics
The DFA algorithm, and related two-point correlation methods, such as Fourier transform and Hurst analyses, measure only one exponent characterizing a given signal. These methods, therefore, are most appropriate for the analysis of monofractal signals. Monofractals are homogeneous in the sense that they have the same scaling properties, characterized by only one singularity exponent throughout the entire signal (38–40). In contrast, an interesting class of signals is multifractal, requiring a larger, and theoretically infinite, number of indices to characterize their scaling properties. Multifractal signals are intrinsically more complex and inhomogeneous than monofractals (Fig. 7). The visually apparent “patchiness” of healthy heartbeat time series (Fig. 1B) suggests that different parts of the signal may have different scaling properties. In addition, evidence that heartbeat dynamics exhibit nonlinear properties (41–44) indicates the need to study higher order correlations. To obtain additional information about the singular properties of such signals, we recently adapted the wavelet modulus maxima method to physiologic time series (14). Our findings suggest that not only are heart rate time series of healthy humans multifractal signals (14), but that this type of complex variability is not simply attributable to physical activity (45). Furthermore, heart rate time series from patients with severe heart failure show a breakdown of multifractal scaling (ref. 14; Fig. 7).
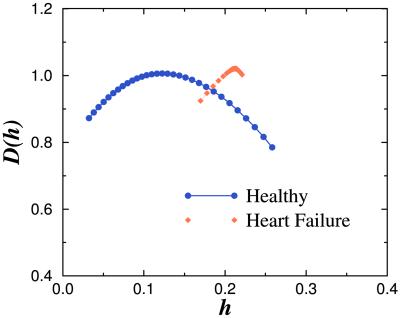
Fig 7.
Singularity spectra of heart rate signals in health and disease. The function D(h) measures the fractal dimension of the subset of the signal that is characterized by a local Hurst exponent with value h. (The local Hurst exponent h is related to the exponent α of the DFA method by the relationship α = 1 + h.) Note the broad range of values of h with non-zero fractal dimensions for the healthy heartbeat, indicating multifractal dynamics. In contrast, data from a representative subject with severe heart failure shows a much narrower range of values of h with non-zero fractal dimensions, indicating loss of multifractal complexity with a life-threatening disease. Adapted from ref. 14.
The detection of multifractal scaling in heart rate dynamics is of interest for a number of reasons (14, 38, 39, 45). Previous analyses by our group and others have focused only on the quantification of a single exponent (i.e., monofractal behavior) to characterize the 1/f-like scaling of healthy interbeat intervals and other physiologic time series over a wide range of time scales (20, 22, 23, 46, 47). Subsequent findings suggest that the healthy heartbeat is even more complex than suspected, because it requires multiple exponents for its characterization. The apparent loss of multifractal complexity in a life-threatening pathologic condition—namely heart failure—confirms and extends the monofractal (DFA and Fourier transform) analyses described above. These findings also pose a challenge to contemporary efforts aimed at developing realistic models of heart rate control and other processes under neuroautonomic regulation (14, 30, 48). No precedent exists in physiology to account for dynamics with such multiscale, nonlinear complexity, which in physical systems has been connected with turbulence and related phenomena (14, 49, 50). Multifractality in heartbeat dynamics raises the intriguing possibility that the nonlinear control mechanisms involve coupled cascades of feedback loops in a system operating far from equilibrium (14).
Fractal Scaling in Other Signals: Human Gait Dynamics
Concepts and methods described above to detect and quantify scaling behavior of heartbeat time series can be applied to other complex physiologic signals. An illustrative example is the analysis of the step-to-step (stride interval) fluctuations in human walking rhythm. The stride interval is analogous to the cardiac interbeat interval, and, like the heartbeat, traditionally it has been thought to be quite regular under healthy conditions. However, as shown in Fig. 8 Top, subtle but complex fluctuations are apparent in healthy gait dynamics. Whereas this “noise” had been previously observed, until recently these fluctuations had not been characterized. Our goal has been to analyze step-to-step fluctuations in gait to help understand the neural control of locomotion in health and disease (refs. 15 and 51–53; for ref. 52, text and data available at http://www.physionet.org/publications/); for ref. 53, data available at http://www.physionet.org/physiobank/database/gaitndd/?M=A).
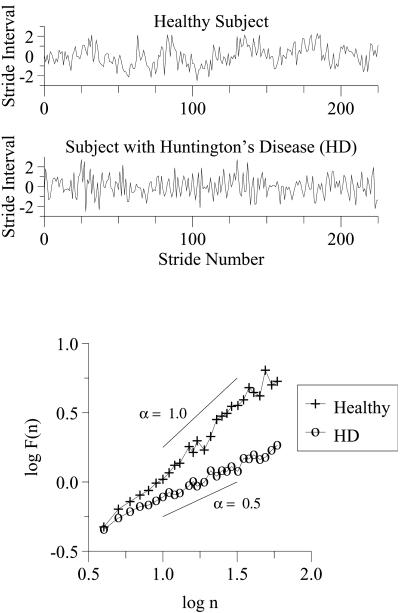
Fig 8.
Stride interval fluctuations in health and disease. For illustrative purposes, each time series has been normalized by subtracting its mean and dividing by its standard deviation. The breakdown in long-range correlations with Huntington's disease is indicated by the change in scaling exponent, α, from close to 1 in health to about 0.5 with severe pathology. Logarithmic values are given to base 10. Adapted from ref. 15.
By using scaling analysis techniques described above (Fig. 4), we have found that (i) for healthy adults, fluctuations in the stride interval during walking are not random, but display long-range correlations, extending over thousands of steps, consistent with a fractal gait rhythm (51) and (ii) these correlation properties evolve during childhood (52) and degrade both with physiologic aging and with certain degenerative neurologic diseases, including Huntington's disease and Parkinson's disease (15). Furthermore, in subjects with Huntington's disease, the scaling exponent, α, is strongly related to the degree of functional impairment (15).
These findings are of interest from both basic and bedside perspectives. The long-range correlations in stride dynamics are not accounted for by traditional central pattern generator models of rhythmic motor behavior (51, 54). The observation that aging and neurologic disease diminish stride interval correlation properties parallels the effects of age and pathology on heartbeat dynamics described above, and may also generalize to modeling other neuroregulatory processes. From a practical viewpoint, measurement of short and longer-range scaling properties, along with other temporal parameters of gait, may aid in the quantitative assessment of neurologic status and the efficacy of pharmacologic therapy and other interventions designed to maintain or restore gait stability (refs. 19, 53, 55, and 56; for ref. 55, data available at http://www.physionet.org/physiobank/database/umwdb/).
Fractal Physiology: Implications for the Dynamics of Health and Disease
A defining feature of healthy function is adaptability, the capacity to respond to unpredictable stimuli and stresses. Functional plasticity requires a broad range of integrated outputs. Fractal physiology, exemplified by long-range correlations in the human heartbeat, as well as in gait dynamics, may be adaptive from at least two perspectives (12–14, 19): (i) long-range correlations serve as a (self-) organizing mechanism for highly complex processes that generate fluctuations across a wide range of time scales; and (ii) the absence of a characteristic scale inhibits the emergence of highly periodic behaviors (mode-locking), which would greatly narrow functional responsiveness. This latter conjecture is supported by findings from life-threatening conditions such as heart failure, where the breakdown of fractal correlations is often accompanied by the emergence of a dominant mode (e.g., the Cheyne-Stokes breathing frequency; Fig. 1C). Transitions to strongly periodic dynamics are observed in many other pathologies, including Parkinson's disease (tremor), obstructive sleep apnea (ref. 41; Fig. 3), sudden cardiac death, epilepsy, and fetal distress syndromes, to name but a few (12–14, 57).
The paradoxical appearance of highly ordered dynamics with pathologic states (often termed “disorders”) exemplifies the concept of complexity loss in disease and aging (11, 13). We have defined physiologic complexity as relating, at least in part, to the presence of long-range (fractal) correlations, along with distinct classes of nonlinear interactions (13, 16). The antithesis of a scale-free system is one dominated by a characteristic frequency. Physiologic systems manifesting only one (or a few) dominant scale(s) become especially easy to recognize and characterize for clinicians because they necessarily display strongly periodic dynamics—repeating their behavior over a sustained time in a highly predictable (syndromic) pattern (Fig. 1 A and C; refs. 12 and 13).
Whereas fractals are irregular, not all irregular spatial or temporal structures are fractal. A key feature of the class of fractals observed in healthy physiology appears to be the distinctive type of long-range order described above (13, 19). This power-law correlation property can extend over many scales of space or time (Fig. 5). For complex physiologic processes, scale-invariance is the mechanism underlying a “memory” effect: the value of some variable, e.g., heart rate or stride interval at a given time, is related not just to immediately preceding values, but to fluctuations in the remote past (19, 51). Certain pathologies are marked by a breakdown of this organization, sometimes producing an uncorrelated randomness similar to “white noise,” and apparently distinct from deterministic chaos (13, 19). Examples include the erratic ventricular response in atrial fibrillation over relatively short time scales (Fig. 1D) and stride interval fluctuations in Huntington's disease (Fig. 8).
In summary, the breakdown of fractal physiologic complexity may be associated with excessive order (pathologic periodicity), on the one hand, or uncorrelated randomness, on the other (13, 16, 19). A unifying theme underlying both routes to pathology is the degradation of correlated, multiscale dynamics.
Future Directions: Addressing the Problem of Access to Physiologic Data
A major impediment to the dynamical analysis of physiologic signals has been the unavailability of large, well-characterized databases and algorithms necessary to promote multidisciplinary efforts to find “hidden information” in such recordings. In September, 1999, under the sponsorship of the National Center for Research Resources of the National Institutes of Health, we and our colleagues inaugurated the Research Resource for Complex Physiologic Signals (refs. 58 and 59; ref. 58 available at http://circ.ahajournals.org/cgi/content/full/101/23/e215). The “PhysioNet” Resource has three interdependent components: (i) PhysioBank is a large and growing archive of well-characterized digital recordings of physiologic signals. Currently available databases include multiparameter cardiopulmonary, neural, and other biomedical signals from healthy subjects and from those with a variety of conditions with major public health implications, such as life-threatening cardiac arrhythmias, congestive heart failure, sleep apnea, neurologic disorders, and aging. (ii) PhysioToolkit is a library of open-source software for physiologic signal processing and analysis, detection of physiologically significant events by using both classical techniques, and novel methods based on statistical physics and nonlinear dynamics (e.g., DFA and multifractal analysis). (iii) PhysioNet (from which the Resource derives its name) is an on-line forum for dissemination and exchange of recorded biomedical signals and open-source software for analyzing them.
PhysioNet recently initiated a series of unique web-based competitions (in the spirit of Fig. 1). Researchers are confronted with a major clinical problem (e.g., diagnosing sleep apnea from a single-lead electrocardiogram, or forecasting the occurrence of cardiac arrhythmias), and are challenged to develop new diagnostic methods based on “real-world” physiologic data made available over the Internet. This modality is intended to foster friendly competition and collaboration, helping to create interdisciplinary “laboratories without walls.” The initial experience has been very encouraging: PhysioNet challenges have been creatively addressed by researchers who would, otherwise, never have had access to these kinds of annotated biomedical datasets, including investigators with expertise in physics, computer science, bioengineering, and mathematics.
PhysioNet also provides a platform for investigators to publish not only the text of peer-reviewed articles, but also the supportive original data and algorithms (“Pub-Med Plus” initiative). This capability facilitates reanalysis of data with new techniques as they become available, permitting ongoing “data-leveraging” and “data-mining,” as well as validation studies. We anticipate that future explorations will focus productively not only on scale-invariance and its alterations with aging and disease, but also on the rich and remarkable array of other behaviors related to complexity and nonlinear dynamics in physiology and medicine listed in Table 1. Many more manifestations of physiologic complexity likely remain to be discovered. Just as GenBank and related databases have greatly accelerated progress in molecular biology, open-source datasets of carefully annotated dynamical signals, along with analytic software, promise to catalyze efforts to understand and model integrative physiologic control and complex signaling networks in the postgenomic era (2, 58). In a related way, open-source data and algorithms are likely to be essential not only in the development of new diagnostic and prognostic measures, but also in the discovery of novel therapeutic interventions.
Acknowledgments
We gratefully acknowledge support from the National Institutes of Health/National Center for Research Resources (P41-RR13622), National Institute of Child Health and Human Development (HD39838), National Institute on Aging (AG14100), the G. Harold and Leila Y. Mathers Charitable Foundation, the Fetzer Institute, the Centers for Disease Control and Prevention (H75-CCH119124), and the National Heart, Lung, and Blood Institute's Programs for Genomic Applications (U01 HL66582).
Abbreviations
DFA, detrended fluctuation analysis
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Self-Organized Complexity in the Physical, Biological, and Social Sciences,” held March 23–24, 2001, at the Arnold and Mabel Beckman Center of the National Academies of Science and Engineering in Irvine, CA.
A detailed description of the DFA algorithm with source code is available at www.physionet.org.
One alternative method to reduce the effects of nonstationarity in heart rate time series is to study the first difference of the original time series. In that case, the interbeat interval increments in health exhibit long-range anti-correlations, such that increases (decreases) in heart rate are likely to be followed by decreases (increases) over multiple time scales (22, 30).
References
- 1.Walleczek J., (1999) Nonlinear Dynamics, Self-Organization, and Biomedicine (Cambridge Univ. Press, Cambridge, U.K.).
- 2.Goldberger A. L. (2001) Am. J. Respir. Crit. Care Med. 163, 1289-1290. [DOI] [PubMed] [Google Scholar]
- 3.Bassingthwaighte J. B., Liebovitch, L. S. & West, B.J., (1994) Fractal Physiology (Oxford Univ. Press, New York).
- 4.Winfree A. T. (1994) Nature (London) 371, 233-236. [DOI] [PubMed] [Google Scholar]
- 5.Glass L. & Mackey, M. C., (1988) From Clocks to Chaos: the Rhythms of Life (Princeton Univ. Press, Princeton).
- 6.Goldbeter A., (1996) Biomedical Oscillations and Cellular Rhythms: The Molecular Basis of Periodic and Chaotic Behavior (Cambridge Univ. Press, Cambridge, U.K.).
- 7.West G. B., Brown, J. H. & Enquist, B. J. (1999) Science 284, 1677-1679. [DOI] [PubMed] [Google Scholar]
- 8.Glass L. (2001) Nature (London) 410, 277-284. [DOI] [PubMed] [Google Scholar]
- 9.Strogatz S. H. (2001) Nature (London) 410, 268-276. [DOI] [PubMed] [Google Scholar]
- 10.Belair J., Glass, L., an der Heiden, U. & Milton, J., (1995) Dynamical Disease: Mathematical Analysis of Human Illness (Am. Inst. Phys. Press, New York).
- 11.Lipsitz L. A. & Goldberger, A. L. (1992) J. Am. Med. Assoc. 267, 1806-1809. [PubMed] [Google Scholar]
- 12.Goldberger A. L. (1997) Perspect. Biol. Med. 40, 543-561. [DOI] [PubMed] [Google Scholar]
- 13.Goldberger A. L. (1996) Lancet 347, 1312-1314. [DOI] [PubMed] [Google Scholar]
- 14.Ivanov P. Ch., Amaral, L. A. N., Goldberger, A. L., Havlin, S., Rosenblum, M. G., Struzik, Z. & Stanley, H. E. (1999) Nature (London) 399, 461-465. [DOI] [PubMed] [Google Scholar]
- 15.Hausdorff J. M., Mitchell, S. L., Firtion, R., Peng, C.-K., Cudkowicz, M. E., Wei, J. Y. & Goldberger, A. L. (1997) J. Appl. Physiol. 82, 262-269. [DOI] [PubMed] [Google Scholar]
- 16.Goldberger, A. L., Peng, C.-K. & Lipsitz, L. A. (2002) Neurobiol. Aging, in press. [DOI] [PubMed]
- 17.Mandelbrot B. B., (1982) The Fractal Geometry of Nature. (Freeman, New York).
- 18.Weibel E. R. (1991) Am. J. Physiol. (Lung Cell. Mol. Physiol. 5) 261, L361-L369. [Google Scholar]
- 19.Peng C.-K., Hausdorff, J. M. & Goldberger, A. L. (2000) in Nonlinear Dynamics, Self-Organization, and Biomedicine, ed. Walleczek, J. (Cambridge Univ. Press, Cambridge, U.K.), pp. 66–96.
- 20.Abboud S., Berenfeld, O. & Sadeh, D. (1991) Circ. Res. 68, 1751-1760. [DOI] [PubMed] [Google Scholar]
- 21.Peskin C. S. & McQueen, D. M. (1994) Am. J. Physiol. (Heart Circ. Physiol. 35) 266, H319-H328. [DOI] [PubMed] [Google Scholar]
- 22.Peng C.-K., Mietus, J., Hausdorff, J. M., Havlin, S., Stanley, H. E. & Goldberger, A. L. (1993) Phys. Rev. Lett. 70, 1343-1346. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto Y. & Hughson, R. L. (1994) Am. J. Physiol. 266, R40-R49. [DOI] [PubMed] [Google Scholar]
- 24.Ivanov P. Ch., Rosenblum, M. G., Peng, C.-K., Mietus, J. E., Havlin, S., Stanley, H. E. & Goldberger, A. L. (1998) Physica A 249, 587-593. [DOI] [PubMed] [Google Scholar]
- 25.Ivanov P. Ch., Goldberger, A. L., Havlin, S., Peng, C.-K., Rosenblum, M. G. & Stanley, H. E. (1999) in Wavelets in Physics, ed. van den Berg, J. C. (Cambridge Univ. Press, Cambridge, U.K.), pp. 391–419.
- 26.Peng C.-K., Buldyrev, S. V., Havlin, S., Simons, M., Stanley, H. E. & Goldberger, A. L. (1994) Phys. Rev. E 49, 1685-1689. [DOI] [PubMed] [Google Scholar]
- 27.Peng C.-K., Havlin, S., Stanley, H. E. & Goldberger, A. L. (1995) Chaos 5, 82-87. [DOI] [PubMed] [Google Scholar]
- 28.Taqqu M. S., Teverovsky, V. & Willinger, W. (1995) Fractals 3, 785-798. [Google Scholar]
- 29.Hu K., Ivanov, P. Ch., Chen, Z., Carpena, P. & Stanley, H. E. (2001) Phys. Rev. E 64, 011114. (19). [DOI] [PubMed] [Google Scholar]
- 30.Ivanov P. Ch., Amaral, L. A. N., Goldberger, A. L. & Stanley, H. E. (1998) Europhys. Lett. 43, 363-368. [DOI] [PubMed] [Google Scholar]
- 31.Ho K. K. L., Moody, G. B., Peng, C.-K., Mietus, J. E., Larson, M. G., Levy, D. & Goldberger, A. L. (1997) Circulation 96, 842-848. [DOI] [PubMed] [Google Scholar]
- 32.Huikuri H. V., Mäkikallio, T. H., Peng, C.-K., Goldberger, A. L., Hintze, U., Møller, M. & the Diamond Study Group (2000) Circulation 101, 47-53. [DOI] [PubMed] [Google Scholar]
- 33.Mäkikallio T. H., Koistinen, M. J., Jordaens, L., Tulppo, M. P., Wood, N., Golosarsky, B., Peng, C.-K., Goldberger, A. L. & Huikuri, H. V. (1999) Am. J. Cardiol. 83, 880-884. [DOI] [PubMed] [Google Scholar]
- 34.Mäkikallio T. H., Høiber, S., Køber, L., Torp-Pedersen, C., Peng, C.-K., Goldberger, A. L., Huikuri, H. V. & the TRACE Investigators (1999) Am. J. Cardiol. 83, 836-839. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov P. Ch., Bunde, A., Amaral, L. A. N., Havlin, S., Fritsch-Yelle, J., Baevsky, R. M., Stanley, H. E. & Goldberger, A. L. (1999) Europhys. Lett. 48, 594-600. [DOI] [PubMed] [Google Scholar]
- 36.Bunde A., Havlin, S., Kantelhardt, J. W., Penzel, T., Peter, J.-H. & Voigt, K. (2000) Phys. Rev. Lett. 85, 3736. [DOI] [PubMed] [Google Scholar]
- 37.Iyengar N., Peng, C.-K., Morin, R., Goldberger, A. L. & Lipsitz, L. A. (1996) Am. J. Physiol. 271, R1078-R1084. [DOI] [PubMed] [Google Scholar]
- 38.Stanley H. E., Amaral, L. A. N., Goldberger, A. L., Havlin, S., Ivanov, P. Ch. & Peng, C.-K. (1999) Physica A 270, 309-324. [DOI] [PubMed] [Google Scholar]
- 39.Ivanov P. Ch., Amaral, L. A. N., Goldberger, A. L., Havlin, S., Rosenblum, M. G., Stanley, H. E. & Struzik, Z. R. (2001) Chaos 11, 641-652. [DOI] [PubMed] [Google Scholar]
- 40.Barabási A.-L., Szépfalusy, P. & Vicsek, T. (1991) Physica A 178, 17-28. [Google Scholar]
- 41.Ivanov P. Ch., Rosenblum, M. G., Peng, C.-K., Mietus, J., Havlin, S., Stanley, H. E. & Goldberger, A. L. (1996) Nature (London) 383, 323-327. [DOI] [PubMed] [Google Scholar]
- 42.Sugihara G., Allan, W., Sobel, D. & Allan, K. D. (1996) Proc. Natl. Acad. Sci. USA 93, 2608-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barahona M. & Poon, C.-S. (1996) Nature (London) 381, 215-217. [Google Scholar]
- 44.Ashkenazy Y., Ivanov, P. Ch., Havlin, S., Peng, C.-K., Goldberger, A. L. & Stanley, H. E. (2001) Phys. Rev. Lett. 86, 1900-1903. [DOI] [PubMed] [Google Scholar]
- 45.Amaral L. A. N., Ivanov, P. Ch., Aoyagi, N., Hidaka, I., Tomono, S., Goldberger, A. L., Stanley, H. E. & Yamamoto, Y. (2001) Phys. Rev. Lett. 86, 6026-6029. [DOI] [PubMed] [Google Scholar]
- 46.Marsh D. J., Osborn, J. L. & Cowley, A. W. (1990) Am. J. Physiol. 258, F1394-F1400. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi M. & Musha, T. (1982) IEEE Trans. Biomed. Eng. 29, 456-457. [DOI] [PubMed] [Google Scholar]
- 48.Lin D. C. & Hughson, R. L. (2001) Phys. Rev. Lett. 86, 1650-1653. [DOI] [PubMed] [Google Scholar]
- 49.Muzy J. F., Bacry, E. & Arneodo, A. (1991) Phys. Rev. Lett. 67, 3515-3518. [DOI] [PubMed] [Google Scholar]
- 50.Meneveau C. & Sreenivasan, K. R. (1987) Phys. Rev. Lett. 59, 1424-1427. [DOI] [PubMed] [Google Scholar]
- 51.Hausdorff J. M., Peng, C.-K., Ladin, Z., Wei, J. Y. & Goldberger, A. L. (1995) J. Appl. Physiol. 78, 349-358. [DOI] [PubMed] [Google Scholar]
- 52.Hausdorff J. M., Zemany, L., Peng, C.-K. & Goldberger, A. L. (1999) J. Appl. Physiol. 86, 1040-1047. [DOI] [PubMed] [Google Scholar]
- 53.Hausdorff J. M., Lertratanakul, A., Cudkowicz, M. E., Peterson, A. L., Kaliton, D. & Goldberger, A. L. (2000) J. Appl. Physiol. 88, 2045-2053. [DOI] [PubMed] [Google Scholar]
- 54.West B. J. & Griffin, L. (1998) Fractals 6, 101-108. [Google Scholar]
- 55.Hausdorff J. M., Purdon, P. L., Peng, C.-K., Ladin, Z., Wei, J. Y. & Goldberger, A. L. (1996) J. Appl. Physiol. 80, 1448-1457. [DOI] [PubMed] [Google Scholar]
- 56.Chau T. (2001) Gait Posture 13, 49-66. [DOI] [PubMed] [Google Scholar]
- 57.Milton J. & Black, D. (1995) Chaos 5, 8-13. [DOI] [PubMed] [Google Scholar]
- 58.Goldberger A. L., Amaral, L. A. N., Glass, L., Hausdorff, J. M., Ivanov, P. Ch., Mark, R. G., Mietus, J. E., Moody, G. B., Peng, C.-K. & Stanley, H. E. (2000) Circulation 101, e215-e220. [DOI] [PubMed] [Google Scholar]
- 59.Moody G. B., Mark, R. G. & Goldberger, A. L. (2001) IEEE Eng. Med. Biol. 20, 70-75. [DOI] [PubMed] [Google Scholar]