Abstract
Susceptibility testing of laboratory strains and clinical isolates of Borrelia burgdorferi indicates that resistance to erythromycin is present in them. Evaluation of the MICs, minimal bactericidal concentrations, and kinetics of bacterial killing of erythromycin suggests that this resistance is increased by preexposure to the antibiotic, is dependent on inoculum size, and may be the result of selection of subpopulations of bacterial cells with increased resistance.
Studies of the antibiotic susceptibility of Borrelia burgdorferi have not been performed extensively because the bacteria cannot be cultured in the majority of cases of Lyme disease (1, 6, 16). Interpretation of these assays is in any case hindered by the complex media used to grow B. burgdorferi, the need for precise control of partial O2 and CO2 pressure during culture, the slow growth of this bacterium, and the lack of standardization of MIC determinations (1, 7-9, 19). The fact that in vitro culture appears to modify biological properties of B. burgdorferi that may be relevant to its antibiotic susceptibility, including plasmid content, growth rate, and infectivity, adds further difficulties (6, 14). These problems may contribute to discrepancies between the susceptibility of B. burgdorferi and the efficacy of certain antibiotics in treating Lyme disease (7-9, 16, 19). This is clearly exemplified by the lack of agreement in the literature concerning the in vitro susceptibility of B. burgdorferi to macrolide antibiotics such as erythromycin (7-9) and the efficacy of macrolides in the treatment of Lyme disease (16, 19).
In general, the results of antibiotic susceptibility studies with B. burgdorferi have indicated that different strains of this bacterium appear to be quite homogenous in their patterns of susceptibility and resistance to many antibiotics, and together with genetic analysis, these studies have suggested that the problem of emergence of disseminated antibiotic resistance is minimal in B. burgdorferi (8, 9). The studies reported here indicate that erythromycin resistance is present in B. burgdorferi and that clinical isolates and laboratory strains differ in their susceptibilities to this antibiotic.
B. burgdorferi strains.
B. burgdorferi strains B31 (ATCC 35210), 297 (17), and N40 were obtained from the American Type Culture Collection, J. Benach, and L. Bockenstadt, respectively. Random clinical isolates obtained from patients with Lyme disease diagnosed at the Westchester Medical Center were obtained from blood cultures and skin biopsies (Table 1) (10).
TABLE 1.
MBC and MIC of erythromycin for several B. burgdorferi isolates
| Strain | No. of passages in culture | Site of isolation | Genotypea | Erythromycin
|
|
|---|---|---|---|---|---|
| MBC (μg/ml) | MIC (μg/ml) | ||||
| 206 | 2 | Blood | I | 0.06 | NDb |
| 224 | 5 | Blood | II | >500 | 10 |
| 254 | 2 | Blood | I | >100 | ND |
| 256 | 2 | Blood | I | >100 | ND |
| 264 | 3 | Blood | II | >500 | 10 |
| 282 | 1 | Blood | I | >100 | ND |
| 296 | 1 | Blood | II | >100 | ND |
| 441 | 1 | Skin biopsy | II | >100 | ND |
| 449 | 1 | Skin biopsy | III | >100 | ND |
| 452 | 1 | Skin biopsy | I | 50 | ND |
| 460 | 3 | Skin biopsy | III | >120 | 5 |
| 485 | 3 | Skin biopsy | III | 0.06 | 0.05 |
| N40 | 4 | Tick | III | >500 | 10 |
| B31 | Many | Tick | I | 0.06 | 0.005 |
| 297 | Many | Spinal fluid | II | 0.03 | 0.003 |
Determined as described in reference 10.
ND, not done.
Determination of MICs and MBCs.
MICs were determined by inoculation of 5 × 105 B. burgdorferi cells into tubes containing 300 μl of Barbour-Stoenner-Kelly II (BSK-II) medium and 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 15, 100, or 500 μg of erythromycin per ml. Cultures were incubated at 34°C for 7 to 10 days (3), and the end point was determined by turbidity, change in color of the medium, and microscopy (3). Minimal bactericidal concentrations (MBCs) were determined by inoculating 107 B. burgdorferi cells into 60-mm-diameter plating BSK agar plates containing 0.0075, 0.015, 0.03, 0.06, 0.12, 0.24, 10, 30, 60, 120, 240, or 500 μg of erythromycin per ml (3). The plates were incubated at 32°C in a humidified 5% CO2 atmosphere for 10 to 15 days, and the colonies were counted. The end point for the MBC determinations was either the lack of colonies or the presence of not more than 5 to 10 colonies based on the lack of correlation between susceptibility to erythromycin and success of treatment (3, 7-9, 12, 13, 16-19) and the fact that we were investigating the use of resistance to erythromycin as a genetic marker in Borrelia (1, 6). To determine the effect of preexposure to antibiotic on resistance to erythromycin, B. burgdorferi N40 culture was exposed to 0.01 μg of erythromycin per ml in liquid medium for 3 days before plating.
Kinetics of killing of B. burgdorferi by erythromycin.
Tubes containing 300 μl of BSK-II with erythromycin at concentrations of 0.06 and 30 μg/ml were inoculated with B. burgdorferi N40 and B31 to the starting concentration of 107 cells and incubated at 34°C (3). B. burgdorferi cells were stained with acridine orange and counted at several time intervals by fluorescent microscopy.
Luria-Delbrück fluctuation test.
The Luria-Delbrück fluctuation test was performed with B. burgdorferi N40 strain as described previously (11), with the appropriate amounts of inoculum and medium and under the appropriate growth conditions for the single large-volume culture and the independet small-volume cultures (11). Two concentrations of erythromycin (10 and 120 μg/ml) were used to select for erythromycin-resistant cells on plating BSK plates in two independent experiments.
MICs and MBCs for B. burgdorferi strains B31, 297, and N40.
We determined erythromycin susceptibility of B. burgdorferi strains B31, 297, and N40 by dilution methods with liquid broth and solid media (Table 1). The MICs of erythromycin were 0.005 and 0.003 μg/ml for strains B31 and 297, respectively, and 10 μg/ml for strain N40 (Table 1). We also observed that while the MBCs of erythromycin for B. burgdorferi B31 and 297 were 0.06 and 0.03 μg/ml, respectively, B. burgdorferi N40 cells were able to survive erythromycin concentrations of 500 μg/ml. For example, after 12 days of culture, 103 colonies were present after inoculation of 108 B. burgdorferi N40 cells on a PBSK plate containing 500 μg of erythromycin per ml, while no colonies (of 108 cells inoculated) of B. burgdorferi B31 and 297 were present on plates containing 0.06 and 0.03 μg of erythromycin per ml. Thus, these experiments confirm the results of liquid medium experiments, demonstrating that the B31 and 297 strains are susceptible to erythromycin, but N40 is not.
Attempts to grow these erythromycin-resistant N40 colonies in liquid media with and without antibiotic failed. Although B. burgdorferi N40 cells grown on solid media with antibiotics were not culturable in liquid media, microscopic observation of cells resuspended from such colonies indicated that these cells were actively motile and intact. N40 colonies growing on erythromycin-free solid media were able to grow in liquid media without erythromycin.
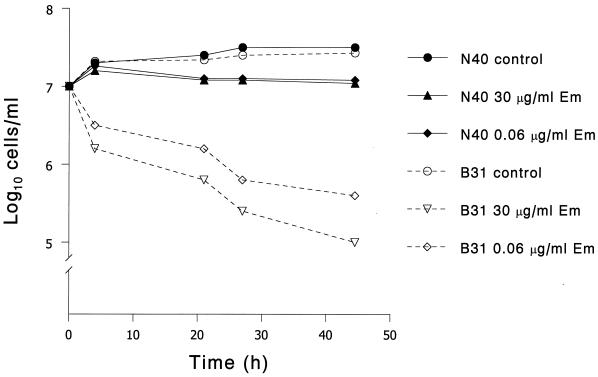
Kinetics of killing of B. burgdorferi N40 and B31 by erythromycin.
To further investigate the interaction of erythromycin with B. burgdorferi, we examined the kinetics of erythromycin killing. After 44 h of culture, there was a 1-log reduction in B. burgdorferi B31 cells in the presence of 0.06 μg of erythromycin per ml and a 2-log reduction in the presence of 30 μg of erythromycin per ml (Fig. 1). There was no significant reduction in B. burgdorferi N40 cells after exposure to the same concentrations of erythromycin (Fig. 1). These results confirm the differences in susceptibility of strains B31 and N40 to erythromycin and indicate that at the concentrations used in these experiments, erythromycin is bactericidal for strain B31, but not for strain N40.
FIG. 1.
Time-kill curves with B. burgdorferi strains B31 and N40 at different concentrations of erythromycin (em).
Effect of preexposure to erythromycin on erythromycin resistance in B. burgdorferi N40 and inoculum effect.
To determine if exposure to erythromycin increased the resistance of B. burgdorferi N40 to this antibiotic, cells were exposed to 0.01 μg of erythromycin per ml before plating. Preexposure of N40 to erythromycin increased resistance to this antibiotic only slightly, so that after inoculation of 2 × 107 B. burgdorferi N40 cells on a plate containing 10 μg of erythromycin per ml, the number of surviving bacteria increased from 104 to 105. We also observed that erythromycin resistance expressed by N40 increased as the numbers of B. burgdorferi cells inoculated on erythromycin-containing plates increased. For example, when 103 N40 cells were inoculated onto erythromycin plates, resistant bacteria were observed in plates containing 10 μg of erythromycin per ml, but not in plates containing 30 μg/ml. If 107 bacteria were inoculated, resistant bacteria grew in plates containing 500 μg of erythromycin per ml. This inoculum effect was also observed after pretreatment of B. burgdorferi cells with 0.01 μg of erythromycin per ml.
MBCs and MICs of erythromycin for B. burgdorferi clinical isolates.
These results and those of other investigators (6) indicate an important heterogeneity in the susceptibility of B. burgdorferi strains to erythromycin. Table 1 shows that the MBCs for several B. burgdorferi clinical isolates are above 100 μg/ml, confirming this heterogeneity. We also determined the MIC in liquid media for isolates 224, 460, 264, and 485 (Table 1). Although the number of strains examined was small, their resistance to erythromycin did not appear to be related to the source or genotype (10), inasmuch as there were susceptible and resistant strains in both skin and blood (Table 1).
Lack of B. burgdorferi N40 cells with an increased rate of mutation to erythromycin resistance.
To investigate whether resistance to erythromycin is the result of B. burgdorferi N40 cells with an increased rate of mutation, we performed Luria-Delbrück fluctuation tests selecting B. burgdorferi cells on erythromycin plates (11). The statistical indistinguishability in the numbers of erythromycin-resistant cells of B. burgdorferi grown in a single large-volume culture (variance, 23.7) and B. burgdorferi cells grown in multiple small-volume independent cultures (variance, 34.2) indicated that there were no individual cells with an increased rate of mutation in the population of B. burgdorferi N40. However, these results do not rule out the presence of a preexisting subpopulation of more resistant cells in the population of B. burgdorferi N40 exposed to the antibiotic.
Studies of the in vitro susceptibility of B. burgdorferi to erythromycin and other macrolide antibiotics have generally indicated that B. burgdorferi strains are usually highly susceptible to this antibiotic (3, 7-9). These findings contrast with clinical reports indicating the limited efficacy of these antibiotics in treatment of B. burgdorferi infections in animals and human beings (7-9, 12, 13, 18, 19). This apparent lack of correlation between in vitro susceptibility studies performed with erythromycin and other macrolides and in vivo studies in animals and human beings may be the result of lack of standardization of susceptibility assays, differences in B. burgdorferi populations, differences in the pharmacological properties of the macrolide antibiotic preparations in animals and human beings, and failure to detect B. burgdorferi erythromycin-resistant strains because only a limited number of strains have been tested for susceptibility (16-19).
Our measurements of MIC, MBC, and the kinetics of killing of strains B31, 297, and N40 by erythromycin indicate that there are variations in the susceptibility of these B. burgdorferi strains to the antibiotic and that it cannot be assumed that all B. burgdorferi strains display as exquisite susceptibility to this antibiotic as do strains B31 and 297 (3, 9). That the erythromycin resistance observed in B. burgdorferi N40 may be a general phenomenon in B. burgdorferi is suggested by our finding of high MBCs and MICs among several clinical isolates of B. burgdorferi (Table 1), by previous reports suggesting that erythromycin resistance is present in B. burgdorferi strains (6), and by information indicating that B. burgdorferi erythromycin resistance variants can be generated in the laboratory by exposure to erythromycin (6). The presence of undetected erythromycin-resistant strains among clinical isolates could explain the lack of correlation observed between B. burgdorferi in vitro susceptibility to erythromycin (3, 9) and the lack of consistent efficacy of erythromycin in the treatment of Lyme disease (16-19). Levels of erythromycin resistance displayed by clinical isolates similar to that shown by N40 would interfere with the therapy of Lyme disease because they are above levels reached in the bloodstream by administration of oral and intravenous erythromycin (1 to 4 and 10 μg/ml, respectively) (2). We do not believe that our findings are the result of the use of different methodology, since the inocula of B. burgdorferi used in our MIC determinations and time-killing experiments are similar to the inocula used by other investigators (3, 7-9). Moreover, it is clear that our methods can differentiate between erythromycin-susceptible and -resistant strains of B. burgdorferi, because we detected erythromycin-resistant cells in the MBC experiments even when a lower inoculum was used, and there was a direct correlation between our MIC and MBC experiments and the kinetic killing experiments. The lack of growth in liquid media of the erythromycin-resistant colonies of B. burgdorferi growing on solid media and their altered colony morphology (M. L. Sartakova and F. C. Cabello, unpublished observations) suggests that this subpopulation of bacteria may be undergoing a global metabolic change responsible for the observed antibiotic resistance (4). More extensive and standardized susceptibility studies of clinical isolates of B. burgdorferi are clearly needed to answer this point.
The fact that most low-passage infectious strains of B. burgdorferi in our study showed erythromycin resistance could indicate that these strains express mechanisms responsible for an increased rate of mutation to this antibiotic, because gene transfer in this bacterium is limited (1, 5). The results of Luria-Delbrück fluctuation testing (11) of the infectious N40 strain with concentrations of 10 and 120 μg/ml to select for erythromycin-resistant mutants showed that no cells in the culture displayed an increased rate of mutation in response to erythromycin, since there was no fluctuation in erythromycin-resistant colonies in these experiments (11). However, these results do not rule out the presence of a small number of preexisting B. burgdorferi subpopulations resistant to erythromycin in the population prior to the experiments (11). Preexposure of B. burgdorferi N40 to low levels of erythromycin increased the number of cells resistant to this antibiotic; additional studies will be needed to discern whether this increase corresponds to induction or to an increased selection of preexisting antibiotic-resistant subpopulation in a heterogeneous population. A relevant difference between infectious and noninfectious strains of B. burgdorferi that could explain their different susceptibilities to erythromycin (Table 1) is the lack of the plasmids lp25 and lp28-1 in the latter (14). Genomic analysis of the DNA sequence of these plasmids did not detect any obvious genes that could endow infectious B. burgdorferi strains with erythromycin resistance (data not shown). Erythromycin-resistant mutants in B. burgdorferi could be secondary to mutations in the 23S rRNA, because these mutations are dominant in bacteria such as B. burgdorferi that contain a decreased number of rRNA operons (15). However, preliminary PCR amplification studies with B. burgdorferi N40 to detect genes conferring erythromycin resistance and DNA sequencing of this strain to detect mutations in the rRNA genes have produced no evidence for any such hypothesis (data not shown).
In summary, some B. burgdorferi strains are able to express resistance to erythromycin. Further studies will be needed for improved characterization of the microbiological and mechanistic characteristics of this resistance and its clinical relevance. Moreover, the presence of this resistance in clinical isolates also suggests that erythromycin will not be a useful genetic marker to manipulate many clinical isolates of B. burgdorferi.
Acknowledgments
This work was supported by Public Health Service grant R01 AI48856 to F. C. Cabello.
We thank Paulina Pedraza for help with some experiments, H. V. Harrison and H. P. Godfrey for their contributions to the preparation of the manuscript, and F. Gherardini, R. Goldschmidt, and J. Sutcliffe for discussions.
REFERENCES
- 1.Cabello, F. C., M. L. Sartakova, and E. Y. Dobrikova. 2000. Genetic manipulation of spirochetes—light at the end of the tunnel. Trends Microbiol. 9:245-248. [DOI] [PubMed] [Google Scholar]
- 2.Chambers, H. F. 2000. Protein synthesis inhibitors and miscellaneous antibacterial agents, p. 1239-1271. In J. G. Hardman, L. E. Limbird, and A. G. Gilman (ed.), Goodman & Gilman's the pharmacological basis of therapeutics, 10th ed. McGraw-Hill, New York, N.Y.
- 3.Dever, L. L., J. H. Jorgensen, and A. G. Barbour. 1993. Comparative in vitro activities of clarithromycin, azithromycin, and erythromycin against Borrelia burgdorferi. Antimicrob. Agents Chemother. 37:1704-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drenkard, E., and F. M. Ausubel. 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416:740-743. [DOI] [PubMed] [Google Scholar]
- 5.Dykhuizen, D. E., and G. Baranton. 2001.The implications of a low rate of horizontal transfer in Borrelia. Trends Microbiol. 9:344-350. [DOI] [PubMed] [Google Scholar]
- 6.Hardham, J. M., and E. L. Rosey. 2000. Antibiotic selective markers and spirochete genetics. J. Mol. Microbiol. Biotechnol. 2:425-432. [PubMed] [Google Scholar]
- 7.Johnson, R. C., C. B. Kodner, P. J. Jurkovich, and J. J. Collins. 1990. Comparative in vitro and in vivo susceptibilities of the Lyme disease spirochete, Borrelia burgdorferi, to cefuroxime and other antimicrobial agents. Antimicrob. Agents Chemother. 34:2133-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson, R. C., C. Kodner, and M. Russell. 1987. In vitro and in vivo susceptibility of the Lyme disease spirochete, Borrelia burgdorferi, to four antimicrobial agents. Antimicrob. Agents Chemother. 31:164-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson, R. C., C. Kodner, M. Russell, and D. Girard. 1990. In-vitro and in-vivo susceptibility of Borrelia burgdorferi to azithromycin. J. Antimicrob. Chemother. 25(Suppl. A):33-38. [DOI] [PubMed] [Google Scholar]
- 10.Liveris, D., S. Varde, R. Iyer, S. Koenig, S. Bittker, D. Cooper, D. McKenna, J. Nowakowski, R. B. Nadelman, G. P. Wormser, and I. Schwartz. 1999. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J. Clin. Microbiol. 37:565-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luria, S. E., and M. Delbrück. 1943. Mutations of bacteria form virus sensitivity to virus resistance. Genetics 28:491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mursic, V. P., B. Wilske, G. Schierz, M. Holmburger, and E. Süβ. 1987. In vitro and in vivo susceptibility of Borrelia burgdorferi. Eur. J. Clin. Microbiol. 6:424-426. [DOI] [PubMed] [Google Scholar]
- 13.Philipson, A. 1991. Antibiotic treatment in Lyme borreliosis. Scand. J. Infect. Dis. Suppl. 77:145-150. [PubMed] [Google Scholar]
- 14.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz, J. J., A. Gazumyan, and I. Schwartz. 1992. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J. Bacteriol. 174:3757-3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 17.Steere, A. C., R. L. Grodzicki, A. N. Kornblatt, J. E. Craft, A. G. Barbour, W. Burgdorfer, G. P. Schmid, E. Johnson, and S. E. Malawista. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733-740. [DOI] [PubMed] [Google Scholar]
- 18.Weber, K. 1996. Treatment failure in erythema migrans—a review. Infection 24:73-75. [DOI] [PubMed] [Google Scholar]
- 19.Wormser, G. P., R. B. Nadelman, R. J. Dattwyler, D. T. Dennis, E. D. Shapiro, A. C. Steere, T. J. Rush, D. W. Rahn, P. K. Coyle, D. H. Persing, D. Fish, and B. J. Luft. 2000. Practice guidelines for the treatment of Lyme disease. Clin. Infect. Dis. 31(Suppl. 1):S1-S14. [DOI] [PubMed] [Google Scholar]