Abstract
Myroides odoratus and Myroides odoratimimus (formerly designated in a single species as Flavobacterium odoratum) are gram-negative aerobes and sources of nosocomial infections in humans. They have variable susceptibility to β-lactams and a decreased susceptibility to carbapenems. Using genomic DNAs of M. odoratus CIP 103105 and M. odoratimimus CIP 103073 reference strains, shotgun cloning of β-lactamase genes was performed, followed by protein expression in Escherichia coli. The deduced amino acid sequences of these β-lactamase genes revealed that TUS-1 and MUS-1 from M. odoratus CIP 103105 and M. odoratimimus CIP 103073, respectively, shared 73% amino acid identity. Mature proteins TUS-1 and MUS-1, with pI values of 7.8 and 5.2, respectively, had relative molecular masses of ca. 26 kDa. These β-lactamases are members of the subclass B1 of metallo-β-lactamases and are distantly related to other metalloenzymes, being most closely related to IND-1 from Chryseobacterium indologenes (42% amino acid identity). However, phylogenic analysis showed that TUS-1 and MUS-1 belong to the same phylogenic lineage of subclass B1 enzymes that groups the subclass B1 β-lactamases of Flavobacterium species. Kinetic parameters of purified β-lactamases TUS-1 and MUS-1 detailed their hydrolysis spectra, which encompass most β-lactams except aztreonam. β-Lactamases TUS-1 and MUS-1 were classified in functional subgroup 3a of metalloenzymes. This work further characterizes chromosome-encoded metalloenzymes from Flavobacteriaceae species that explain at least part of their intrinsic resistance to β-lactams.
The genus Flavobacterium was created in 1923 (6). Since then, the taxonomy of this genus has undergone substantial changes, especially for strains belonging to the former Flavobacterium odoratum species. In 1984, Holmes et al. had suggested that F. odoratum should be classified in a separate genus on the basis of its unique phenotypic features (17). Strains of this species, differing from most Flavobacterium species in being nonsaccharolytic, fail to produce indole. Using 16S rRNA sequencing, Bernardet et al. showed that on a molecular basis, Flavobacterium odoratum occupies an independent taxonomic position in the Flavobacterium genus (7). Finally, the genus Myroides was created for organisms removed from the genus Flavobacterium on the basis of hybridization experiments and phenotypic characteristics (34). Two species have been delineated: Myroides odoratus (to accommodate Flavobacterium odoratum) and Myroides odoratimimus (37).
Organisms of Myroides spp. are aerobic, yellow-pigmented, gram-negative rods that grow at both room temperature and 37°C. They are habitat-specific organisms, like other members of the Flavobacteriaceae family, and are commonly found in wet environments (19).
Organisms of Myroides spp. behave like low-grade opportunistic pathogens. Myroides was identified as a source of surgery wound (13, 18, 34) and urinary tract (18, 39) infections, septicemia (14, 18, 34), pneumonia (34), meningitidis (10), fasciitis (38), and ventriculitis (24). Nosocomial outbreaks have also been reported (25, 39).
Antibiotic resistance patterns of Myroides strains exhibit variable susceptibility to β-lactams (18), with a constant decreased susceptibility to cephalosporins and imipenem. In 1985, kinetic parameters of a metallo-β-lactamase of an F. odoratum strain were reported (34).
The aim of this study was to determine the β-lactamase gene content of strains of the genus previously designated F. odoratum and now separated into M. odoratus and M. odoratimimus. Kinetic parameters of two distinct metalloenzymes have been determined with purified preparations.
MATERIALS AND METHODS
Bacterial strains.
M. odoratus CIP 103105 and M. odoratimimus CIP 103073 reference strains were from the Institut Pasteur (Paris, France) strain collection. Escherichia coli DH10B, in vitro-obtained rifampin-resistant E. coli JM109, and E. coli BL21(DE3) strains were used for cloning experiments, conjugation experiments, and β-lactamase overexpression, respectively (5).
Antimicrobial agents and MIC determinations.
The antimicrobial agents used in this study were obtained in the form of standard laboratory powders and were used immediately after their solubilization. The agents and their sources have been described elsewhere (28). MICs were determined by an agar dilution technique on Mueller-Hinton agar (Sanofi-Diagnostics Pasteur, Paris, France), with an inoculum of 104 CFU per spot (26). All drugs were incorporated into Mueller-Hinton agar, at serial twofold concentrations, before determination of antimicrobial susceptibilities. The plates were incubated at 35°C for 18 h.
Plasmid DNA content and conjugation.
Plasmid DNA extractions of M. odoratus CIP 103105 and M. odoratimimus CIP 103073 were performed according to two different methods as previously described (5). Direct transfer of resistant genes into rifampin-resistant E. coli JM109 was attempted by liquid and solid mating-out assays and by electroporation of the putative plasmid DNA suspensions into E. coli DH10B (5). Transconjugants and electroporants were selected on Trypticase soy (TS) agar plates containing rifampin (200 μg/ml) and amoxicillin (30 μg/ml) and amoxicillin only, respectively.
Cloning and analysis of recombinant plasmids.
Whole-cell DNAs of M. odoratus CIP 103105 and M. odoratimimus CIP 103073 were extracted as described previously (3). All enzymes used in cloning experiments were from Amersham Pharmacia Biotech (Orsay, France). Whole-cell DNAs of M. odoratimimus CIP 103073 and M. odoratus CIP 103105 were partially digested by Sau3AI. Sau3AI DNA fragments from M. odoratus CIP 103105 or M. odoratimimus CIP 103073 strains were ligated into pBK-CMV phagemid (Stratagene, Amsterdam, The Netherlands) which had previously been digested with BamHI and were dephosphorylated with shrimp alkaline phosphatase (Roche Diagnostics, Meylan, France). Recombinant phagemids were transformed into E. coli strain DH10B by electroporation (Gene Pulser II; Bio-Rad, Ivry-sur-Seine, France). Transformants were selected on TS agar containing ampicillin (30 μg/ml) and kanamycin (30 μg/ml). Selected clones were tested for their ability to hydrolyze imipenem as described below. Recombinant plasmids were purified with the Qiagen plasmid Midi kit (Qiagen, Courtaboeuf, France), and cloned DNA inserts of recombinant plasmids were sequenced on both strands, using an Applied Biosystems sequencer (ABI 377). The nucleotide and deduced protein sequences were analyzed with software available over the Internet from the National Center for Biotechnology Information website (www.ncbi.nlm.nih.gov). A dendrogram of MUS-1 and TUS-1 β-lactamases was derived from the multiple alignment by a parsimony method, using the phylogeny package PAUP (Phylogenetic Analysis Using Parsimony) version 3.0 (35).
Southern and I-CeuI techniques.
Southern hybridization experiments (33) were performed using 0.8% electrophoresis gel containing whole-cell DNAs of M. odoratus CIP 103105 and M. odoratimimus CIP 103073 transferred onto a nylon membrane that was then hybridized with PCR-obtained internal fragments for blaTUS-1 (TuA, 5′-CTACTTTGGTCTATCCTCAATCGG-3′; TuB, 5′-ATTCGGCTATTCTATGCCCG-3′) and for blaMUS-1 (MuA, 5′-ATTAGTACACG CTCAATCTAG-3′; MuB, 5′-AGTCATAAGAGCTATACCGG-3′), respectively. Visualization of hybridization was performed using the ECL nonradioactive hybridization kit as described by the manufacturer (Amersham Pharmacia Biotech). Additionally, chromosomal locations of the β-lactamase genes were investigated using the I-CeuI technique (22). Whole-cell DNA of the Myroides reference strains was digested with endonuclease I-CeuI (New England Biolabs, Ozyme, Saint-Quentin-en-Yvelines, France), which digests a 26-bp sequence in rrn genes for the 23S large subunit rRNA. After digestion, separation of the resulting fragments was performed on a CHEF-DRII apparatus used for pulsed-field gel electrophoresis (PFGE), as described previously (22). The sizes of the I-CeuI-generated fragments were determined by comparison with those of Lambda-ladder molecular weight marker (Bio-Rad). A Southern transfer of the PFGE gel was hybridized with probes for blaTUS-1 or blaMUS-1 and a probe for 16rRNA genes made of PCR-generated fragments, using universal primers 8 to 24 (5′-AGAGTTTGATCHTGGYTYAGA-3′) and 1512 to 1491 (5′-ACGGYTACCTTGTTACGACTT-3′).
Preparation of β-lactamase extracts.
β-Lactamase production was enhanced by subcloning β-lactamase genes into plasmid PET-9a. The blaTUS-1 and blaMUS-1 genes were amplified from pBK-TUS-1 and pBK-MUS-1 recombinant plasmids, which were used as templates, with primers designed to amplify the entire sequences of blaTUS-1 and blaMUS-1 genes that contained NdeI and BamHI restriction sites. Amplification products were cloned into TOPO-Blunt plasmids (InVitrogen, Gröningen, The Netherlands) according to manufacturer instructions. Recombinant plasmids were recovered with a Qiagen Midi kit and digested with NdeI and BamHI. After purification of restricted fragments, β-lactamase genes were subcloned into plasmid PET-9 that had been previously digested with the same restriction enzymes, giving rise to recombinant plasmids pET-TUS-1 and pET-MUS-1, which were subsequently transformed into E. coli BL21. E. coli BL21(pET-TUS-1) and E. coli BL21 (pET-MUS-1) strains were cultured overnight at 37°C in 2 liters of TS broth with amoxicillin (30 μg/ml) and kanamycin (30 μg/ml). Bacterial suspensions were pelleted, resuspended in 40 ml of 100 mM phosphate buffer (pH 7), disrupted by sonication (three times at 50 W for 30 s each time, using a Vibra Cell 75022 Phospholyser; Bioblock, Illkirch, France), and centrifuged at 20,000 × g for 1 h at 4°C.
β-Lactamase purification.
β-Lactamase extracts from cultures of E. coli BL21 (pET-TUS-1) and E. coli BL21(pET-MUS-1) were filtered through a 0.45-μm-pore-size filter (Millipore, Saint-Quentin-en-Yvelines, France), dialyzed overnight at 4°C against 20 mM ethanolamine buffer (pH 7) and 20 mM diethylamine buffer (pH 9.3), respectively, and loaded twice onto a preequilibrated Q-Sepharose column (Amersham Pharmacia Biotech). The enzymes were eluted by a linear NaCl gradient (0 to 1 M) in the same buffers. Eluted fractions with high β-lactamase activity (nitrocefin test; Oxoid, Paris, France) were pooled and dialyzed against 50 mM phosphate buffer (pH 5.8) or 50 mM phosphate buffer (pH 7) supplemented with 150 mM NaCl for purification of β-lactamases TUS-1 and MUS-1, respectively. The fractions containing β-lactamase TUS-1 were loaded onto a preequilibrated S-Sepharose column (Amersham Pharmacia Biotech) and were eluted by linear NaCl gradient (0 to 1 M), whereas the fractions containing MUS-1 β-lactamase were loaded onto a preequilibrated 1.6- by 47-cm gel filtration column packed with Superdex 75 (Amersham Pharmacia Biotech) and recovered from the flowthrough. Purified extracts were finally dialyzed overnight at 4°C against 50 mM phosphate buffer (pH 7), prior to a 10-fold concentration with a Vivaspin 10,000 column (Sartorius, Stonehouse, England).
NH2-terminal sequencing.
To determine the cleavage site of the mature proteins of TUS-1 and MUS-1 β-lactamases, the purified enzymes were submitted to an Edman sequence analysis (16) (Laboratory for Protein Micro-Sequencing, Institut Pasteur, Paris, France). Purified enzymes and marker proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis (33). Proteins were then electrotransferred onto a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Guyancourt, France) by using the Mini Protean II transfer cell (8 by 7.3 cm) (Bio-Rad) in 50 mM Tris-borate buffer (pH 8.7) at room temperature (3.5 V/cm, overnight). The membrane was then rinsed in distilled water and stained with a solution made of 0.1% Coomassie brilliant blue R-250 in methanol and water (50:40 [vol/vol]). The protein band was then excised with a razor blade and allowed to air dry. The amino-terminal sequences of the mature β-lactamases were determined with an automated Edman sequencer on a 473A model gas phase sequencer (Applied Biosystems).
IEF analysis.
The purified enzymes and β-lactamase extracts from culture of Myroides sp. reference strains were subjected to analytical isoelectric focusing (IEF) on an ampholine polyacrylamide gel with a pH of 3.5 to 9.5 (Ampholine PAG plate; Amersham Pharmacia Biotech) for 90 min at 1,500 V, 50 mA, and 30 W. The focused β-lactamases were detected by overlaying the gel with a 1 mM nitrocefin solution.
Kinetic measurements.
Purified β-lactamases were used for kinetic measurements (kcat and Km), which were made at 30°C in 50 mM sodium phosphate (pH 7.0), supplemented with 50 μM ZnCl2, as described previously (32). The rates of hydrolysis were determined with a Pharmacia ULTROSPEC 2000 spectrophotometer and were analyzed using SWIFT II software (Amersham Pharmacia Biotech). Using Eadie-Hofstee linearization of the Michaelis-Menten equation as previously described (12), Km and kcat values were determined by analyzing the β-lactam hydrolysis under initial rate conditions. Various concentrations of EDTA were preincubated with the enzyme for 10 min at 30°C before testing the rate of imipenem (100 μM) hydrolysis. Fifty percent inhibitory concentrations (IC50) were determined for EDTA and clavulanic acid, and results were expressed in micromolar units.
The specific activities of β-lactamase extracts obtained after sonication and those of the purified β-lactamases from E. coli BL21(pET-TUS-1) and E. coli BL21(pET-MUS-1) strains were compared, using 100 μM imipenem as substrate, as previously described (3). The protein contents were measured by the Bio-Rad DC protein assay. Purity and relative molecular mass of enzymes were estimated using SDS-PAGE analysis (33).
Nucleotide sequence accession number.
The nucleotide sequences and deduced β-lactamase amino acid sequences reported in this work have been assigned to the GenBank and EMBL databases under no. AF441286 and no. AF441287.
RESULTS
Cloning and DNA sequence analysis of β-lactamase genes from M. odoratus and M. odoratimimus.
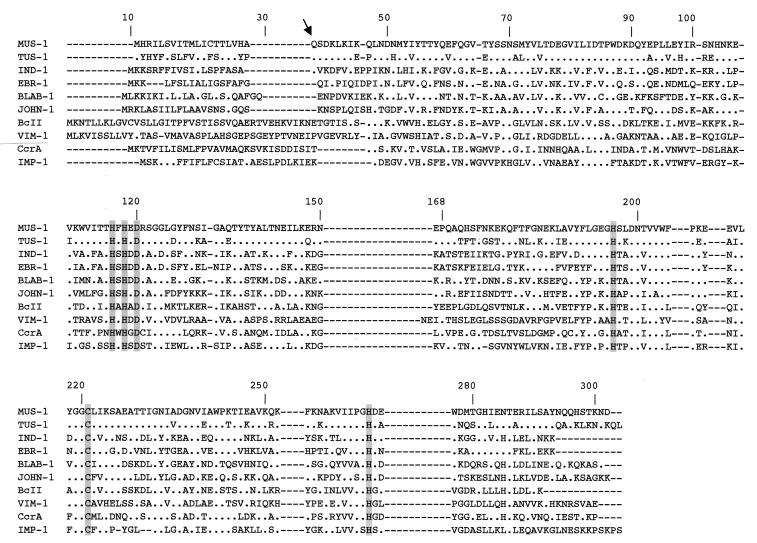
DNA sequence analysis of the 1.2- and 1.7-kb inserts of pBK-TUS-1 and pBK-MUS-1, respectively, revealed single open reading frames of 744 bp and 738 bp, with overall G+C contents of 36.2% and 38.6%, respectively, that encoded 246- and 248-amino-acid preproteins (Fig. 1). The putative cleavage sites were found between positions 19 and 20 (alanine and glutamine residues) for β-lactamase TUS-1 and at the same position (between the proline and glutamine residues) for β-lactamase MUS-1. These cleavage site positions were confirmed by Edman sequence analysis, which determined the N-terminal ends of purified β-lactamases TUS-1 and MUS-1 to be motif QSDK. β-Lactamases TUS-1 and MUS-1 had pI values of 7.8 and 5.2, respectively. Similarly, IEF analysis with culture extracts of M. odoratus CIP 103105 and M. odoratimimus CIP 103073 revealed single pI values of 7.8 and 5.2, respectively.
FIG. 1.
Comparison of amino acid sequences of TUS-1 and MUS-1 with those of other metallo-β-lactamases of subclass B1. The origins of the metallo-β-lactamases were as follows: BLAB-1, Chryseobacterium meningosepticum CIP 6058 (32); IND-1, Chryseobacterium indologenes (4); VIM-1, Pseudomonas aeruginosa VT-143/97 (21); BcII, Bacillus cereus 569/H (20); CcrA, Bacteroides fragilis (30); IMP-1, Serratia marcescens TN9106 (27); EBR-1, Empedobacter brevis (GenBank accession no. AF416700); and JOHN-1, Flavobacterium johnsoniae (GenBank accession no. AY028464). The BBL numbering scheme identification numbers are indicated above the sequences (15). The arrow indicates cleavage of the peptide leader site for TUS-1 and MUS-1. Amino acid residues that may be involved in Zn2+ binding appear in grey. Dashes were introduced to optimize the alignment, whereas dots indicate amino acid residues identical to those of β-lactamase MUS-1.
Genetic location of β-lactamase genes.
Plasmid extraction of M. odoratus CIP 103105 and M. odoratimimus CIP 103073 and transformation and conjugation experiments failed. Using PCR-prepared probes internal for blaTUS-1 and for blaMUS-1 and whole-cell DNAs of M. odoratus CIP 103105 and M. odoratimimus CIP 103073 as templates, hybridization signals were obtained at the chromosomal position of migration (data not shown), indicating a likely chromosomal location of these genes.
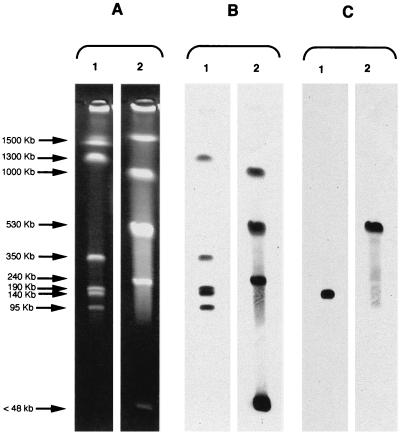
Using the I-CeuI technique, six and five DNA fragments were generated with restricted DNAs of M. odoratus CIP 103105 and M. odoratimimus CIP 103073, respectively (Fig. 2A). Except for one ca. 1,500-kb DNA fragment, all of the fragments hybridized with an rRNA probe (Fig. 2B). The blaTUS-1 and blaMUS-1 probes hybridized with a ca. 140-kb fragment of M. odoratus and a ca. 530-kb fragment of M. odoratimimus, respectively (Fig. 2C), further indicating a chromosomal location of these β-lactamase genes.
FIG. 2.
Localization of blaTUS-1 and blaMUS-1 in I-CeuI-generated chromosomal fragments of M. odoratus CIP 103105 and M. odoratimimus CIP 1033073 separated by PFGE. (A) Chromosome restriction patterns. (B) Hybridization of restricted patterns with a probe specific to 16S rRNA genes. (C) Hybridization of restricted fragments with probes specific to the blaTUS-1 and blaMUS-1 genes. Lanes: 1, M. odoratus CIP 103105; 2, M. odoratimimus CIP 103703.
Protein sequence analysis of TUS-1 and MUS-1.
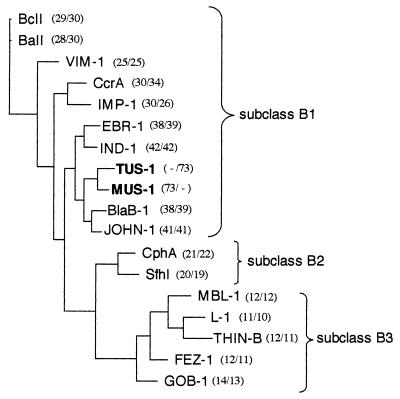
A comparison of amino acid sequences of TUS-1 and MUS-1 with those of other class B β-lactamases revealed 42% amino acid identity with IND-1 from Chryseobacterium indologenes (4), the most closely related enzyme. A dendrogram was constructed to relate TUS-1 and MUS-1 β-lactamases to representative Ambler class B enzymes (Fig. 3). MUS-1 and TUS-1 enzymes clustered with metalloenzymes produced by Chryseobacterium meningosepticum (3), Chryseobacterium indologenes (4), Flavobacterium johnsoniae (T. Naas, S. Bellais, P. Nordmann, submitted for publication), and Empedobacter brevis (S. Bellais, D. Girlich, A. Karim, P. Nordmann, submitted for publication). This cluster parallels most closely the taxonomic positions of Chryseobacterium, Flavobacterium, and Empedobacter species compared to Myroides species, thus indicating that they may have diverged from a common ancestor.
FIG. 3.
Dendrogram obtained by parsimony analysis for representative natural and acquired metallo-β-lactamases. The origins of the metallo-β-lactamases were as follows: BlaB-1 and GOB-1, Chryseobacterium meningosepticum (3); IND-1, Chryseobacterium indologenes (5); EBR-1, Empedobacter brevis (GenBank accession no. AF416700); JOHN-1, Flavobacterium johnsoniae (GenBank accession no. AY028464); CphA, Aeromonas hydrophila (23); SfhI, Serratia fonticola (GenBank accession no. AF197943); L-1, Stenotrophomonas maltophilia (40); BcII, Bacillus cereus (20); BaII, Bacillus anthracis (GenBank accession no. AF367984); VIM-1, Pseudomonas aeruginosa (21); CcrA, Bacteroides fragilis (30); IMP-1, Serratia marcescens (27); THIN-B, Janthinobacterium lividum (31); MBL-1, Caulobacter crescentus (GenBank accession no. AJ315850); and FEZ-1, Legionella gormanii (8). The alignment used for tree calculation was performed with ClustalW followed by minor adjustments. Branch lengths are drawn to scale and are proportional to the number of amino acid changes. The distance along the vertical axis has no significance. Percent amino acid identities to TUS-1 and MUS-1 are indicated in parentheses (left and right numbers, respectively).
Susceptibility testing.
MICs of β-lactams for the M. odoratus CIP 103105 strain showed that the bacteria had reduced susceptibility to cefoxitin, cefepime, cefpirome, imipenem, and meropenem and was resistant to all other β-lactams tested (Table 1). Surprisingly, it was susceptible to amoxicillin. Susceptibility of M. odoratimimus CIP 103073 to β-lactams mirrored that of the M. odoratus CIP 103105 strain but at a lower level (Table 1). MIC values of β-lactams for the E. coli DH10B strain, which harbors recombinant plasmids pBK-TUS-1 and pBK-MUS-1, were very similar. The bacteria were resistant to amoxicillin and ticarcillin and had a reduced susceptibility to piperacillin, whereas they were fully susceptible to expanded-spectrum cephalosporins, monobactams, carbapenems, and cephamycins (Table 1). Clavulanic acid and tazobactam did not restore activity of β-lactams. MICs of β-lactams for E. coli harboring recombinant plasmids did not mirror those for parental strains.
TABLE 1.
MICs of β-lactams for M. odoratus CIP 103105, M. odoratimimus CIP 103073, E. coli DH10B(pBK-TUS-1), E. coli DH10B(pBK-MUS-1), and for reference strain E. coli DH10Ba
| Substrateb | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| M. odoratus CIP 103105 | E. coli DH10B (pBK-TUS-1) | M. odoratimimus CIP 103073 | E. coli DH10B (pBK-MUS-1) | E. coli DH10B | |
| Amoxicillin | 8 | 128 | 32 | 128 | 2 |
| Amoxicillin-CLA | 8 | 128 | 32 | 128 | 2 |
| Ticarcillin | 256 | 256 | 256 | 256 | 2 |
| Piperacillin | 64 | 8 | 128 | 8 | 2 |
| Piperacillin-TZB | 64 | 8 | 128 | 8 | 2 |
| Cephalothin | 256 | 8 | 128 | 8 | 2 |
| Cefoxitin | 16 | 4 | 32 | 4 | 2 |
| Moxalactam | 32 | 0.25 | 32 | 0.12 | 0.12 |
| Cefotaxime | 32 | 0.25 | 512 | 0.06 | 0.06 |
| Cefuroxime | 128 | 32 | 256 | 8 | 2 |
| Ceftazidime | 32 | 0.25 | 128 | 0.06 | 0.06 |
| Cefepime | 16 | 0.06 | 32 | 0.06 | 0.06 |
| Cefpirome | 4 | 0.06 | 32 | 0.06 | 0.06 |
| Aztreonam | 64 | 0.06 | >512 | 0.06 | 0.06 |
| Imipenem | 4 | 0.5 | 8 | 0.25 | 0.06 |
| Meropenem | 4 | 0.25 | 4 | 0.25 | 0.06 |
M. odoratus CIP 103105 and E. coli DH10B(pBK-TUS-1) produced β-lactamase TUS-1, whereas M. odoratimimus CIP 103073 and E. coli DH10B(pBK-MUS-1) produced β-lactamase MUS-1.
CLA, clavulanic acid at 2 μg/ml; TZB, tazobactam at 4 μg/ml.
Purification of β-lactamases TUS-1 and MUS-1.
The amplification products obtained with specific primers using pBK-TUS-1 and pBK-MUS-1 as templates were subcloned into pET-9a plasmid, giving rise to recombinant plasmids pPET-TUS-1 and pPET-MUS-1, respectively. The metalloenzymes produced by the E. coli BL21(pPET-TUS-1) and E. coli BL21 (pPET-MUS-1) were then overproduced. Since TUS-1 is a cationic protein and MUS-1 is anionic, the purification technique was different for each enzyme.
After purification, the specific activities, determined with 100 μM imipenem as the substrate, were 140 μmol · min−1 · mg of protein−1 for TUS-1 and 94 μmol · min−1 · mg of protein−1 for MUS-1. Comparison of specific activities before and after purification showed purification factors of 75 and 315 for TUS-1 and MUS-1, respectively. The purity of both enzymes was estimated to be 95% by SDS-PAGE analysis (data not shown). Mature proteins had similar relative molecular masses, determined experimentally to be ca. 26 kDa (data not shown).
Kinetic measurements of TUS-1 and MUS-1.
The kinetic parameters of the purified TUS-1 and MUS-1 β-lactamases were very similar (Table 2). The enzymes had similar Km values for all substrates. A comparison of kcat values revealed no difference between MUS-1 and TUS-1 for all substrates, except for cephaloridin, for which the Km value was higher with MUS-1. The catalytic efficiencies were higher for penicillins than for cephalosporins in both cases. Cephamycins and expanded-spectrum cephalosporins were poorly hydrolyzed by TUS-1 and MUS-1, whereas aztreonam was not hydrolyzed at all. These β-lactamases showed strong activities against carbapenems, and the metal chelator EDTA was a good inhibitor of β-lactamase activities (IC50 of 120 and 80 μM for TUS-1 and MUS-1, respectively).
TABLE 2.
Kinetic parameters for the purified β-lactamases TUS-1 and MUS-1a
| Substrate | TUS-1
|
MUS-1
|
||||
|---|---|---|---|---|---|---|
| kcat (s−1)c | Km (μM)b | kcat/Km (mM−1 · s−1)c | kcat (s−1)c | Km (μM)b | kcat/Km (mM−1 · s−1)c | |
| Benzylpenicillin | 320 | 78 | 4,000 | 170 | 40 | 4,200 |
| Amoxicillin | 490 | 480 | 1,000 | 400 | 560 | 700 |
| Ticarcillin | 115 | 163 | 700 | 344 | 150 | 2,200 |
| Piperacillin | 990 | 75 | 13,500 | 330 | 160 | 2,020 |
| Cephaloridine | 12 | 600 | 20 | 100 | 580 | 170 |
| Cephalothin | 40 | 125 | 300 | 90 | 97 | 900 |
| Cefuroxime | 30 | 24 | 1,200 | 15 | 15 | 870 |
| Cefoxitin | 17 | 820 | 20 | 7 | 1,300 | 5 |
| Moxalactam | >90 | >2,000 | ND | >70 | >2,000 | ND |
| Cefotaxime | 80 | 105 | 470 | 125 | 180 | 700 |
| Ceftazidime | 15 | 1470 | 10 | 3 | 1,713 | 2 |
| Cefepime | >1 | >2,000 | ND | >1 | >2,000 | ND |
| Cefpirome | >6 | >2,000 | ND | >1 | >2,000 | ND |
| Aztreonam | ND | — | ND | ND | — | ND |
| Imipenem | 225 | 250 | 900 | 240 | 470 | 500 |
| Meropenem | 170 | 1,060 | 160 | 250 | 1,400 | 180 |
Values are expressed as the means of three independent measures (standard deviations were within 15%).
—, not hydrolyzed (i.e., the initial rate of hydrolysis was lower than 0.01 μM−1·s−1).
ND, not determinable due to too-high Km value.
DISCUSSION
The biochemical detection of β-lactamases in clinical strains of Myroides spp. (formerly F. odoratum) had been performed in 1985 by Sato et al. (34). Nevertheless, the molecular characteristics of β-lactamase had not been determined. We biochemically and genetically characterized β-lactamases expressed by M. odoratus CIP 103105 and M. odoratimimus CIP 103073, which were taken as reference strains of the Myroides genus.
The β-lactamases produced by M. odoratus CIP 103105 and by M. odoratimimus CIP 103073 shared 73% amino acid identity. This high degree of identity agrees with the phylogenic relationship between M. odoratus and M. odoratimimus (34). Similarly, the relative heterogeneity of metalloenzymes produced by a given Flavobacterium species has been previously reported, such as that found for IND β-lactamases from C. indologenes (5). These latter enzymes display identities ranging from 77% to 99% (5). Thus, amino acid sequence discrepancy between TUS-1 and MUS-1 could be due either to a regular variability that occurs among β-lactamases belonging to closely related species or to phylogenic divergence that delineates M. odoratus and M. odoratimimus.
Sequence analysis revealed that TUS-1 and MUS-1 belong to the subclass B1 of Ambler (2) and Galleni et al. (15). At the present time, each bacterial species of the Flavobacteriaceae family that has been subjected to β-lactamase characterization produces a subclass B1 metallo-β-lactamase (3, 5), with the exception of C. meningosepticum, which harbors an additional metalloenzyme of the GOB series belonging to subclass B3 (3).
The six amino acid residues that interact with the Zn2+ cofactor or with the water molecule located in the active site (His116, His118, Asp120, His196, Cys221, and His264, according to BBL numbering of metallo-β-lactamases [15]) are conserved in β-lactamases TUS-1 and MUS-1 (Fig. 3). Furthermore, as indicated by docking studies using X-ray crystallography analysis of β-lactamase IMP-1 complexed with potent inhibitors (11, 36), the residues required for ligand binding (i.e., Leu39, Glu59, Glu60, Gly65, Val61, Trp64, Val66, Val67, Pro68, Lys69, Phe87, Lys224, Tyr227, Gly232, and Asn233) were also identified in amino acid sequences of TUS-1 and MUS-1. Conservation of these residues contrasted with the low identity between MUS-1/TUS-1 and IMP-1 (26 to 30%). This result underlines the probable importance of these residues in conserved activity of these enzymes.
According to biochemical criteria established by Rasmussen and Bush to classify metallo-β-lactamases (29), TUS-1 and MUS-1 belong to functional subgroup 3a, since their catalytic efficiencies for penicillins were at least 60% of the catalytic efficiencies for imipenem. However, these data disagree with those of Rasmussen and Bush (29) and Bush (9), who reported that a metallo-β-lactamase produced by a Flavobacterium odoratum strain belongs to subgroup 3b, which contains true carbapenem-hydrolyzing β-lactamases, i.e., enzymes exhibiting very strong preference for hydrolysis of carbapenems. This discrepancy is likely due to the fact that the Rasmussen and Bush statement was based on preliminary biochemical analysis of β-lactamases of Myroides spp. (34).
Finally, it is difficult to estimate the exact role of the identified β-lactamases in the intrinsic β-lactam resistance of Myroides spp., since it is well known that metalloenzymes expressed in E. coli give much lower levels of β-lactam resistance than those seen in the original producers. Other β-lactam resistance mechanisms are likely to be involved, such as those that can contribute to resistance to aztreonam, a β-lactam molecule that is hydrolyzed by some metalloenzymes but not others, with TUS-1 and MUS-1 included in the latter category. It remains unknown why flavobacteria are a source of such a variety of metalloenzymes. This may be related to combined biosynthesis of carbapenem derivatives and carbapenem-hydrolyzing β-lactamases, as found in other environmental species such as a Streptomyces sp. (1).
Acknowledgments
This work was financed by a grant from the Ministère de l'Education Nationale et de la Recherche (grant UPRES-EA), Université Paris XI, Paris, France.
We thank C. Bizet for the gift of M. odoratus and M. odoratimimus reference strains.
REFERENCES
- 1.Alexander, D. C., and S. E. Jensen. 1998. Investigation of the Streptomyces clavuligerus cephamycin C gene cluster and its regulation by the CcaR protein. J. Bacteriol. 180:4068-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambler, R. P. 1980. The structure of beta-lactamase. Philos. Trans. R. Soc. Lond. B Biol. Sci. 289:321-331. [DOI] [PubMed] [Google Scholar]
- 3.Bellais, S., D. Aubert, T. Naas, and P. Nordmann. 2000. Molecular and biochemical heterogeneity of class B carbapenem-hydrolyzing β-lactamases in Chryseobacterium meningosepticum. Antimicrob. Agents Chemother. 44:1878-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellais, S., S. Léotard, L. Poirel, T. Naas, and P. Nordmann. 1999. Molecular characterization of a carbapenem-hydrolyzing β-lactamase from Chryseobacterium (Flavobacterium) indologenes. FEMS Microbiol. Lett. 171:127-132. [DOI] [PubMed] [Google Scholar]
- 5.Bellais, S., L. Poirel, S. Léotard, T. Naas, and P. Nordmann. 2000. Genetic diversity of carbapenem-hydrolyzing metallo-β-lactamases from Chryseobacterium (Flavobacterium) indologenes. Antimicrob. Agents Chemother. 44:3028-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergey, D. H., F. C. Harrisson, R. S. Breed, B. W. Hammer, and F. M. Huntoon. 1923. In Bergey's manual of determinative bacteriology, 1st ed, p. 97-117. The Williams and Wilkins Co., Baltimore, Md.
- 7.Bernardet, J. F., P. Segers, M. Vancanneyt, F. Berthe, K. Kersters, and P. Vandamme. 1996. Cutting a Gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis, nom. nov. (basonym, Cytophaga aquatilis Strohl and Tait 1978). Int. J. Syst. Bacteriol. 46:128-148. [Google Scholar]
- 8.Boshi, L., P. S. Mercuri, M. L. Riccio, G. Amicosante, M. Galleni, J.-M. Frère, and G. M. Rossolini. 2000. The Legionella (Fluoribacter) gormanii metallo-β-lactamase: a new member of the highly divergent lineage of molecular-subclass B3 β-lactamases. Antimicrob. Agents Chemother. 44:1538-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush, K. 1998. Metallo-β-lactamases: a class apart. Clin. Infect. Dis. 27(Suppl. 1):S48-S53. [DOI] [PubMed] [Google Scholar]
- 10.Casalta, J. P., Y. Peloux, D. Raoult, P. Brunet, and H. Galais. 1989. Pneumonia and meningitis caused by a new nonfermentative unknown gram-negative bacterium. J. Clin. Microbiol. 27:1446-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Concha, N. O., C. A. Janson, P. Rowling, S. Pearson, C. A. Cheever, B. P. Clarke, C. Lewis, M. Galleni, J.-M. Frère, D. J. Payne, J. H. Bateson, and S. S. Abdel-Meguid. 2000. Crystal structure of the IMP-1 metallo-β-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: binding determinants of a potent, broad-spectrum inhibitor. Biochemistry 39:4288-4299. [DOI] [PubMed] [Google Scholar]
- 12.Cornish-Bowden, A. 1995. Fundamentals of enzyme kinetics, p. 30-37. Portland Press, Seattle, Wash.
- 13.Davis, J. M., M. M. Peel, and J. A. Gillians. 1979. Colonization of an amputation site by Flavobacterium odoratum after gentamicin therapy. Med. J. Aust. 29:703-704. [DOI] [PubMed] [Google Scholar]
- 14.Ferrer, C., E. Jakob, G. Pastorino, and L. I. Juncos. 1995. Right-sided bacterial endocarditis due to Flavobacterium odoratum in a patient on chronic hemodialysis. Am. J. Nephrol. 15:82-84. [DOI] [PubMed] [Google Scholar]
- 15.Galleni, M., J. Lamotte-Brasseur, G. M. Rossolini, J. Spenser, O. Dideberg, J.-M. Frère, and the Metallo-β-Lactamase Working Group. 2001. Standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 45:660-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hewick, R. M., M. W. Hunkapiller, D. Le Hoo, and W. J. Dreyer. 1981. A gas-liquid solid phase peptide and protein sequenator. J. Biol. Chem. 256:7990-7997. [PubMed] [Google Scholar]
- 17.Holmes, B., R. J. Owen, and T. A. McMeekin. 1984. Genus Flavobacterium, p. 353-361. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. The Williams and Wilkins Co., Baltimore, Md.
- 18.Holmes, B., J. J. S. Snell, and S. P. Lapage. 1979. Flavobacterium odoratum: a species resistant to a wide range of antimicrobial agents. J. Clin. Pathol. 32:73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsueh, P. R., J. J. Wu, T. R. Hsiue, and W. C. Hieh. 1995. Bacteremic necrotizing fasciitis due to Flavobacterium odoratum. Clin. Infect. Dis. 21:1337-1338. [DOI] [PubMed] [Google Scholar]
- 20.Hussain, M., A. Carlino, M. J. Madonna, and J. O. Lampen. 1985. Cloning and sequencing of the metallothioprotein β-lactamase II gene of Bacillus cereus 569/H in Escherichia coli. J. Bacteriol. 164:223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauretti, L., M. L. Riccio, A. Mazzariol, G. Cornaglia, G. Amicosante, R. Fontana, and G. M. Rossolini. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, S. L., A. Hessel, and K. E. Sanderson. 1993. Genomic mapping with I-CeuI, an integron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc. Natl. Acad. Sci. USA 90:6874-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massida, O., G. M. Rossolini, and G. Satta. 1991. The Aeromonas hydrophila CphA gene: molecular heterogeneity among class B metallo-β-lactamases. J. Bacteriol. 173:4611-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFarlane, D. E., P. Baumthureen, and I. Crandon. 1985. Flavobacterium odoratum ventriculitis treated with intraventricular cefotaxime. J. Infect. 11:233-238. [DOI] [PubMed] [Google Scholar]
- 25.McGowan, J. E., and C. DelRio. 1991. Other gram-negative bacilli, p. 1790-1791. In G. L. Mandell, R. G. Douglas, and J. E. Benett (ed.), Principles and practice of infectious diseases, 3rd ed. Churchill Livingstone, New York, N.Y.
- 26.National Commitee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 27.Osano, E., Y. Arakawa, R. Wacharotayankun, M. Ohta, T. Horii, H. Ito, F. Yoshimura, and N. Kato. 1994. Molecular characterization of an enterobacterial metallo β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob. Agents Chemother. 38:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poirel, L., T. Naas, M. Guilbert, E. B. Chaibi, R. Labia, and P. Nordmann. 1999. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum β-lactamase encoded by an Escherichia coli integron gene. Antimicrob. Agents Chemother. 43:573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen, B. A., and K. Bush. 1997. Carbapenem-hydrolyzing β-lactamases. Antimicrob. Agents Chemother. 41:223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen, B. A., Y. Gluzman, and F. P. Tally. 1990. Cloning and sequencing of the class B β-lactamase gene (CcrA) from Bacteroides fragilis TAL3636. Antimicrob. Agents Chemother. 34:1590-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossolini, G. M., M. A. Condemi, F. Pantanella, J. D. Docquier, G. Amicosante, and M. C. Thaller. 2001. Metallo-β-lactamase producers in environmental microbiota: new molecular class B enzyme in Janthinobacterium lividum. Antimicrob. Agents Chemother. 45:837-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossolini, G. M., N. Franceschini, M. L. Riccio, P. S. Mercuri, M. Perilli, M. Galleni, J.-M. Frère, and G. Amicosante. 1998. Characterization and sequence of the Chryseobacterium (Flavobacterium) meningosepticum carbapenemase: a new molecular class B β-lactamase showing a broad substrate profile. J. Biochem. 332:145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Sato, K., T. Fujii, R. Okamoto, M. Inoue, and S. Mitsuhashi. 1985. Biochemical properties of β-lactamase produced by Flavobacterium odoratum. Antimicrob. Agents Chemother. 27:612-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swofford, D. L. 1989. PAUP (version 3.0): phylogenetic analysis using parsimony. Illinois Natural History Survey, Champaign, Ill.
- 36.Tonney, J. H., G. G. Hammond, P. M. D. Fitzgerald, N. Sharma, J. M. Balkovec, G. P. Rouen, S. H. Olson, M. L. Hammond, M. L. Greenlee, and Y. D. Gao. 2001. Succinic acids as potent inhibitors of plasmid-borne IMP-1 metallo-β-lactamase. J. Biol. Chem. 276:31913-31918. [DOI] [PubMed] [Google Scholar]
- 37.Vancanneyt, M., P. Segers, U. Torck, B. Hoste, J. F. Bernardet, P. Vandamme, and K. Kersters. 1996. Reclassification of Flavobacterium odoratum (Stutzer 1929) strains to a new genus, Myroides odoratus comb. nov. and Myroides odoratimimus sp. nov. Int. J. Syst. Bacteriol. 46:926-932. [Google Scholar]
- 38.Von Graevenitz, A. 1995. Nonfermentative gram-negative bacteria, p. 520-532. In B. Murray, E. Baron, M. Pfaller, F. Tenover, and H. Yolken (ed.), Manual of clinical microbiology, 6th ed., ASM Press, Washington, D.C.
- 39.Yagci, A., M. Cerikçioglu, E. Kaufmann, H. Malnick, G. Söyletir, F. Babacan, and T. L. Pitt. 2000. Molecular typing of Myroides odoratimimus (Flavobacterium odoratum) urinary tract infections in a Turkish hospital. Eur. J. Clin. Microbiol. Infect. Dis. 19:731-732. [DOI] [PubMed] [Google Scholar]
- 40.Walsh, T. R., L. Hall, S. J. Assinda, W. W. Nichols, S. J. Cartwright, A. P. MacGowan, and P. M. Bennett. 1994. Sequence and analysis of the L-1 metallo-β-lactamase from Xanthomonas maltophilia. Biochim. Biophys. Acta 1218:199-201. [DOI] [PubMed] [Google Scholar]