In the early 1990s, an unexpected phenotype—namely, premature ovarian failure (POF)—was noted among heterozygous carriers of the fragile X mutation (Cronister et al. 1991; Schwartz et al. 1994). Interestingly, only premutation carriers have been found to have an increased risk for POF (Allingham-Hawkins et al. 1999), whereas full-mutation carriers and their noncarrier sisters appear to have the same risk seen in the general population (∼1%). In the scientific community, acceptance or recognition of this phenotype, with its unusual pattern among carriers, was slow to come—as it should have been—although carriers of the fragile X mutation did not need to be convinced of the phenotype's existence. The primary reason for the slow acceptance was the lack of understanding of the molecular mechanism. The FMR1 premutation allele is unmethlyated and therefore is thought to be transcribed and translated in a manner similar to that of those alleles with a normal number of CGG repeats in the 5′ untranslated region of the gene. However, recent data have suggested that these processes may be altered—at least in male premutation carriers (Tassone et al. 2000).
Only ∼16% of premutation heterozygotes have POF. An exciting observation that potentially explains the reduced penetrance was recently reported by Hundscheid et al. (2000a). They found that paternally inherited premutations (PIP) were more likely to give rise to POF than were maternally inherited premutations (MIP). This observation motivated other investigators studying the fragile X mutation to examine their extended pedigrees for this same imprinting effect. Murray et al. (2000) and Vianna-Morgante and Costa (2000) report their findings in this issue of the Journal. Although both groups note the rigorous design and implementation of the study of Hundscheid et al., neither group was able to confirm the parent-of-origin effect or to account for the differing results. All three groups used the same definition of POF, and all personally interviewed the subjects. Both Hundscheid et al. (2000a) and Murray et al. (2000 [in this issue]) examined the hormonal profiles of those women who still had menstrual cycles, for a more precise definition of ovarian function, and the same two groups used survival analysis to extract full information from subjects of age <40 years and from those who had had nonspontaneous cessation of menses.
All three groups are well known for their study of the fragile X syndrome and have used their extensive sets of pedigrees, ascertained through individuals with the fragile X syndrome, for studies of POF among female premutation carriers. In the reply by Hundscheid et al. (2000b [in this issue]) to the letters by Murray et al. and Vianna-Morgante and Costa, several differences in the demographics of the female premutation carriers in the three study populations were identified. These demographic differences and others are presented in table 1. Most evident are the differences in the average age of the female subjects and the structure of the pedigrees from which they were drawn. The females studied by Hundscheid et al. (2000a) are older (compare age at examination and the ratio of the number of females of age ⩾40 years to the number of those of age <40 years) and most likely are ascertained from larger pedigrees with more generations (compare the average number of female premutation carriers who were ascertained per pedigree and, as an indicator of the number of generations studied, the PIP:MIP ratio). The younger age of the female premutation carriers limits the power to accurately estimate the age at menopause in general. The difference in age at examination among females with MIP and those with PIP may also be important. In the study of Hundscheid et al. (2000a), females with PIP were older than those with MIP, as indicated by the ratio of the number of subjects of age ⩾40 years compared with the number of those of age <40 years (3.41 vs. 1.80). This is not true to the same extent for the other data sets. Such differences in the amount of available information for each type of premutation carrier should be overcome by the use of survival analysis as performed by Hundscheid et al. (2000a); however, unanticipated biases may still exist.
Table 1.
Comparison of Study Populations
|
Population in Study by |
|||
| Hundscheid et al. (2000a) | Murray et al. (2000)a | Vianna-Morgante and Costa (2000)a | |
| No. premutation carriers interviewed (age) | 148 (>18 years) | 116 (not specified) | 113 (⩾25 years) |
| Median age at examination | 50 years | Not specified | 38 years |
| No. pedigrees examined (average no. females/pedigree) | 55 (2.69) | 62 (1.87) | Not specified |
| No. PIP/no. MIP/no. unknown [PIP:MIP] | 106/42/not specified [2.52] | 51/40/25 [1.27] | 32/27/54 [1.19] |
| No. females ⩾40 years at examination (PIP/MIP) [PIP:MIP] | 82/27 [3.03] | 12/18 [.67] | 15/10 [1.50] |
| Ratio of no. females ⩾40 years at examination:no. females <40 years at examination (PIP/MIP) | 3.41/1.80 | .31/.82 | .88/.59 |
| Mean age at menopause, for premutation carriersb | 45 years | 48 years | Not done |
| % POF among females ⩾40 years (PIP/MIP) | 28%/4% | 17%/28% | 33%/20% |
In this issue of the Journal.
Estimated using survival analysis.
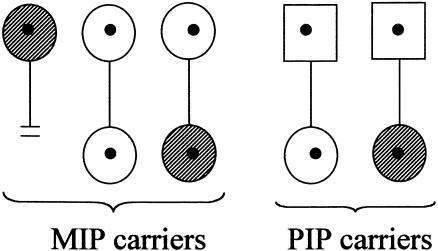
One potential bias that cannot be overcome by survival analysis is the effect of reduced fitness among female premutation carriers with POF. Presenting an extreme scenario for illustration purposes (see fig. 1), if female carriers with POF have reduced fertility at later reproductive ages (late 20s to 30s) and if there is a positive association of the risk of POF among mothers and daughters, then there will be selection against mother-daughter pairs with POF but not among father-daughter pairs. If this is true, then the ages of the study cohorts become important, since the age at reproduction has increased over the past several decades. The results of pedigree analyses show that premutation carriers with POF are not completely infertile; however, no studies have been done to assess the reduction in fitness among female premutation carriers with and without POF. To date, no familial effect of POF has been identified among families with fragile X syndrome, although the data to observe such an effect are limited. Thus, although this example of a potential bias may be exaggerated, it illustrates two important points: (1) the study of POF among premutation carriers is in its infancy, and (2) possible biases resulting from retrospective ascertainment of subjects and potential effects of reduced fitness need to be assessed.
Figure 1.
Example of a potential bias of ascertainment resulting from reduced fertility of female premutation carriers with POF. Symbols containing dots indicate premutation status; diagonally striped symbols indicate presence of POF.
As emphasized by all groups of investigators, there is a clear need to collect additional data using a rigorous definition of the phenotype, personal interviews backed by medical records, an accurate description of the study population (for comparison purposes), and appropriate statistical analyses. These data are needed not only to confirm or refute the potential imprinting effect but also to identify the factors involved in the reduced penetrance of POF among female premutation carriers.
References
- Allingham-Hawkins DJ, Babul R, Chitayat D, et al (1999) Fragile X premutation is a significant risk factor for premature ovarian failure. Am J Med Genet 83:322–325 [PMC free article] [PubMed] [Google Scholar]
- Cronister A, Schreiner R, Wittenberger M, Amiri K, Harris K, Hagerman RJ (1991) Heterozygous fragile X female: historical, physical, cognitive, and cytogenetic features. Am J Med Genet 38:269–274 [DOI] [PubMed] [Google Scholar]
- Hundscheid RDL, Sistermans EA, Thomas CMG, et al (2000a) Imprinting effect in premature ovarian failure confined to paternally inherited fragile X premutations. Am J Hum Genet 66:413–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundscheid RDL, Thomas CMG, Braat DDM, Oostra BA, Smits APT (2000b) Reply to the letters from Murray et al and Vianna-Morgante and Costa. Am J Hum Genet 67:256–258 (in this issue)10848497 [Google Scholar]
- Murray A, Ennis S, Morton N (2000) No evidence for parent of origin influencing premature ovarian failure in fragile X premutation carriers. Am J Hum Genet 67:253–254 (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Dean J, Howard-Peebles PN, et al (1994) Obstetrical and gynecological complications in fragile X carriers: a multicenter study. Am J Med Genet 51:400–402 [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ (2000) Elevated levels of FMR1 messenger RNA in carrier males: a new mechanism of involvement in the fragile X syndrome. Am J Hum Genet 66:6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna-Morgante AM, Costa SS (2000) Premature ovarian failure is associated with maternally and paternally inherited premutation in Brazilian families with fragile X. Am J Hum Genet 67:254–255 (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]