Abstract
We report the antiretroviral activity of stavudine-5′-(p-bromophenyl methoxyalaninyl phosphate) (stampidine [STAMP]), a novel aryl phosphate derivative of stavudine, against primary clinical human immunodeficiency virus type 1 (HIV-1) isolates. STAMP inhibited each one of nine clinical HIV-1 isolates of non-B envelope subtype and 20 genotypically and phenotypically nucleoside analog reverse transcriptase inhibitor-resistant HIV-1 isolates at subnanomolar to low-nanomolar concentrations.
Stavudine (STV) is a pyrimidine nucleoside analogue used in the treatment of human immunodeficiency virus type 1 (HIV-1) infection. The rate-limiting step for the generation of the bioactive STV metabolite STV-triphosphate is the conversion of STV to its monophosphate derivative (1-3, 8-10). In an attempt to overcome the dependence of STV on intracellular nucleoside kinase activation, we prepared STV-5′-(p-bromophenyl methoxyalaninyl phosphate) (stampidine [STAMP]), a novel aryl phosphate derivative of STV, and structurally similar derivatives of STV, zidovudine (ZDV), and lamivudine (19). The presence of a single para-bromine group in the phenyl moiety of STAMP contributes to its unique ability to undergo rapid hydrolysis, yielding the key active metabolite alaninyl-STV-monophosphate (4, 14, 17, 18). In preliminary studies, we found that STAMP is substantially more potent than STV in inhibiting the replication of the laboratory HIV-1 strain HTLVIIIB in thymidine kinase-deficient T cells (19). Balzarini et al. prepared similar compounds with the hypothesis that such lipophilic compounds would more easily enter HIV-infected cells, be metabolized in a thymidine kinase-independent fashion, and inhibit HIV replication in thymidine kinase-deficient cells such as macrophages (1-3). The structure-activity relationships that determine the propensity of this class of compounds to undergo hydrolysis and inhibit HIV replication have recently been published (17).
We previously investigated the in vivo pharmacokinetics, metabolism, toxicity, and antiretroviral activity of STAMP in rodent species (4). In mice and rats, STAMP was very well tolerated, without any detectable acute or subacute toxicity at single intraperitoneal or oral bolus dose levels as high as 500 mg/kg of body weight (15). Notably, daily administration of STAMP intraperitoneally or orally for up to 8 consecutive weeks was not associated with any detectable toxicity in mice or rats at cumulative dose levels as high as 6.4 g/kg (15). STAMP exhibited potent in vivo anti-HIV activity in human peripheral blood lymphocyte-SCID mice at nontoxic dose levels (16). In accordance with its safety profile in rodent species, a 4-week STAMP treatment course with twice-daily administration to dogs and cats of hard gelatin capsules containing STAMP at 25 to 100 mg/kg was very well tolerated at cumulative dose levels as high as 8.4 g/kg (F. M. Uckun, C. Chen, P. Samuel, S. Pendergrass, T. K. Venkatachalam, B. Waurzyniak, and S. Qazi, submitted for publication). A 4-week treatment course with STAMP administered in gelatin capsules twice daily showed a dose-dependent antiretroviral effect in chronically feline immunodeficiency virus (FIV)-infected cats, as evidenced by a ≥1-log decrease of the FIV load of circulating peripheral blood mononuclear cells (PBMC) within 2 weeks after initiation of STAMP therapy (Uckun et al., submitted).
The purpose of the present study was to evaluate the antiretroviral activity of STAMP against primary clinical HIV-1 isolates. Phenotypic drug susceptibility studies of HIV-1 isolates and strains were performed by measuring the production of the p24 gag protein in PBMC from seronegative healthy volunteers in the presence of increasing concentrations of the anti-HIV agent using the quantitative Coulter HIV-1 p24 antigen enzyme immunoassay and HIV-1 p24 antigen kinetic standard (Beckman Coulter), as previously described (6, 13). We used StatView in the calculation of the 50% inhibitory concentrations (IC50s) from each set of triplicate wells using the linearized form of an exponential equation (ln y = ln b0 + b1x; where y is percent inhibition and x is drug concentration). The inhibition constants were log10 transformed to homogenize the variances within each group. Paired t tests were performed in order to test for differences between means of IC50s for STAMP and STV or ZDV across each viral strain. P values below 0.05 were deemed significant (JMP software; SAS).
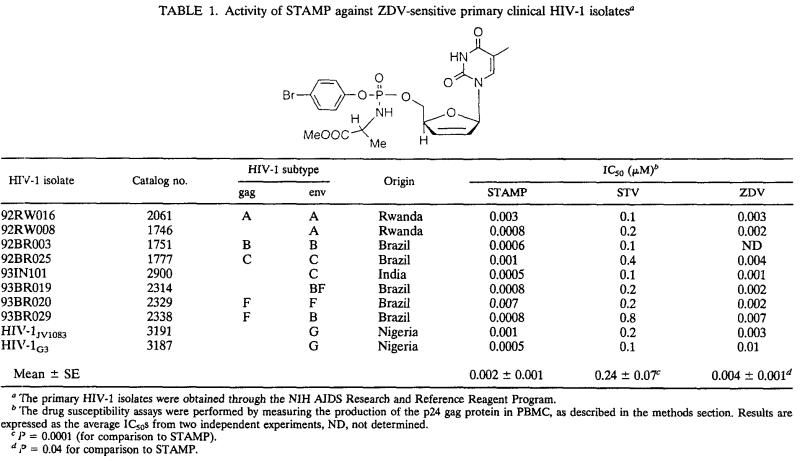
Currently available anti-HIV agents have been traditionally developed against subtype B HIV-1 strains, which are the predominant strains in the United States and Europe although worldwide the majority of HIV-infected individuals are infected with non-subtype B strains and the vast majority of new infections are caused by non-subtype B HIV-1 strains (7, 11). Therefore, there is an urgent need to identify anti-HIV agents with potent activity against non-subtype B HIV-1. STAMP (mean IC50 ± standard error [SE] = 1.7 ± 0.7 nM) was 100-fold more potent than STV (IC50 = 240 ± 7 nM; P < 0.0001 [paired t test on log10-transformed values]) and 2-fold more potent than ZDV (IC50 = 3.8 ± 0.1 nM; P = 0.039 [paired t test on log10-transformed values]) against clinical HIV-1 isolates (n = 9) of non-B envelope subtype (viz., subtypes A [n = 2], C [n = 2], F [n = 3], and G [n = 2]) originating from South America, Asia, and sub-Saharan Africa (Table 1).
TABLE 1.
Activity of STAMP against ZDV-sensitive primary clinical HIV-1 isolatesa
The primary HIV-1 isolates were obtained through the NIH AIDS Research and Reference Reagent Program.
The drug susceptibility assays were performed by measuring the production of the p24 gag protein in PBMC, as described in the methods section. Results are expressed as the average IC50s from two independent experiments, ND, not determined.
P = 0.0001 (for comparison to STAMP).
P = 0.04 for comparison to STAMP.
Contemporary antiretroviral treatment regimens employing combinations of drugs from at least two of the three classes of antiretroviral therapy—namely, nucleoside analog reverse transcriptase (RT) inhibitors (NRTI), nonnucleoside analog RT inhibitors, and protease inhibitors—exhibit a potent and sustained antiviral effect and confer consistent long-term viral suppression in patients with HIV infection (12). However, the individual components of these combination regimens can select for drug-resistant viruses, and the emergence of antiviral drug resistance limits the clinical benefit of these drugs (11). Patients failing on contemporary antiretroviral therapy programs constitute a reservoir of multidrug-resistant HIV that may limit treatment options in the future. The frequency of genotypic and phenotypic drug-resistant HIV is increasing among therapy-naïve HIV-infected seroconverters (5). Therefore, there also is an urgent need for new anti-HIV agents capable of inhibiting the replication of NRTI-resistant HIV. STAMP inhibited the in vitro replication of each one of 20 genotypically and phenotypically NRTI-resistant HIV-1 isolates carrying two to five thymidine analogue mutations associated with NRTI resistance at nanomolar concentrations, with an IC50 (mean ± SE) of 8.7 ± 2.7 nM, whereas the IC50 (mean ± SE) of ZDV against the same isolates was 1.6 ± 0.3 μM (t value, 18.1, P < 0.0001 [paired t test on log10-transformed values]) (Table 2). Notably, the phenotypically highly ZDV-resistant G190-6 and G704-2 isolates (ZDV IC50 >10 μM) carrying five thymidine analogue mutations were inhibited by STAMP, with average IC50s of 2.8 and 3.2 nM, respectively. These findings provide evidence that STAMP is a highly potent inhibitor of primary clinical HIV-1 isolates with a genotypic and/or phenotypic NRTI-resistant profile. The documented in vitro potency of STAMP against primary clinical HIV-1 isolates with genotypic and/or phenotypic NRTI resistance as well as against the non-B envelope subtype, together with a favorable toxicity profile in rodent (15, 16) and nonrodent (Uckun et al., submitted) animal species, and the in vivo antiretroviral activity of STAMP in HIV-infected human peripheral blood lymphocyte-SCID mice (16) as well as in FIV-infected cats (Uckun et al., submitted) warrant the further development of this promising new NRTI compound.
TABLE 2.
Activity of STAMP against ZDV-resistant primary clinical HIV-1 isolates
| HIV-1b isolate | RT gene mutations | IC50 (μM)a
|
|
|---|---|---|---|
| ZDV | STAMP | ||
| RT-MDR | M41L, L74V, T215Y, V106N | >10 | <0.001 |
| G910-6 | M41L, D67N, K70R, T215Y, K219Q | >3.2 | 0.0028 |
| T156-3 | M41L, E44D, D67N, T69D, L210W, T215Y | >3.2 | 0.0033 |
| G890-1 | K20R, M41L, D67N, T69N, K70R, L210W, T215Y | >3.2 | 0.006 |
| G780-1 | M41L, D67N, K70R, T215F, K219Q | >3.2 | 0.035 |
| C467-4 | K20R, D67N, K70R, Y188L, T215F, K219Q | 3.2 | 0.042 |
| G691.2 | ND | 2.6 | 0.005 |
| G704-2 | M41L, D67N, K70R, L210W, T215Y | 1.8 | 0.0032 |
| P798-1 | M41L, T215Y | 1.8 | 0.0075 |
| Q252-2 | M41L, L210W, T215Y | 1.7 | 0.0019 |
| Y270-7 | M41W, Y188L, T215N | 1.5 | 0.009 |
| S762-4 | M41L, T215Y | 1.2 | 0.008 |
| X165-8 | D67N, T69N, K70R, K103N, Y181C, T215F, K219E | 0.9 | 0.013 |
| X165-9 | K20R, D67N, K103N, T215Y | 0.9 | 0.002 |
| S159-2 | M41L, K103N, T215Y | 0.7 | 0.00078 |
| X267-5 | M41L, F116S, T215Y | 0.6 | 0.03 |
| X267-1 | M41L, L74V, L210W, T215Y | 0.6 | 0.00015 |
| X267-2 | M41L, T215Y | 0.4 | 0.00018 |
| R416-10 | M41L, T215Y | 0.3 | 0.0002 |
| 92BR019 | D67N, L214F, T215D, K219Q | 0.2 | 0.002 |
| C140 | M41L, M184V, T215Y | 0.1 | 0.0025 |
| Mean ± SEc | 1.6 ± 0.3 | 0.0087 ± 0.0027 | |
The results presented were obtained from a representative antiviral assay. The SE between individual antiviral assays was <10% of the average IC50.
All primary HIV-1 isolates except for 92BR019 were recovered from peripheral blood leukocytes-of HIV-infected individuals who had been treated with NRTI using previously described culture techniques (11, 13). RT-MDR is an NRTI-resistant and nonnucleoside analog RT inhibitor-resistant laboratory strain of HIV-1, which was included as a control. HIV-1 RT-MDR-1/MT-2 (catalog no. 252) and 92BR019 (catalog no. 1778; envelope subtype B) were obtained through the NIH AIDS Research and Reference Reagent Program. The drug susceptibility assays were performed using PBMC, as described in the methods section.
P < 0.0001 (for comparison between ZDV and STAMP; paired t test).
REFERENCES
- 1.Balzarini, J., P. Herdewijin, and E. De Clercq. 1989. Differential patterns of intracellular metabolism of 2′,3′-didehydro-2′,3′-dideoxythymidine and 3′-azido-2′,3′-dideoxythymidine, two potent anti-human immunodeficiency virus compounds. J. Biol. Chem. 264:6127-6133. [PubMed] [Google Scholar]
- 2.Balzarini, J., H. Egberink, K. Hartmann, D. Cahard, T. Vahlenkamp, H. Thormar, E. De Clercq, and C. McGuigan. 1996. Antiretrovirus specificity and intracellular metabolism of 2′,3′-didehydro-2′,3′-dideoxythymidine (stavudine) and its 5′-monophosphate triester prodrug So324. Mol. Pharmacol. 50:1207-1213. [PubMed] [Google Scholar]
- 3.Balzarini, J., A. Karlsson, S. Aquaro, C. F. Perno, D. Cahard, L. Naesens, and E. De Clercq. 1996. Mechanism of anti-HIV action of masked alaninyl d4T-MP derivatives. Proc. Natl. Acad. Sci. USA 93:7295-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, C., T. K. Venkatachalam, Z. Zhu, and F. M. Uckun. 2001. In vivo pharmacokinetics and metabolism of stampidine, a novel aryl phosphate derivative of d4T with potent anti-HIV activity. Drug Metab. Dispos. 29:1035-1041. [PubMed] [Google Scholar]
- 5.Duwe, S., M. Brunn, D. Altman, O. Hamouda, B. Schmidt, H. Walter, G. Pauli, and C. Kucherer. 2001. Frequency of genotypic and phenotypic drug-resistant HIV-1 among therapy naïve patients of the German Seroconverter Study. J. Acquir. Immune Defic. Syndr. 26:266-273. [DOI] [PubMed] [Google Scholar]
- 6.Erice, A., H. H. Balfour, Jr., D. E. Myers, V. L. Leske, K. J. Sannerud, V. Kuebelbeck, J. D. Irvin, and F. M. Uckun. 1993. Inhibition of zidovudine (AZT)-sensitive strains of human immunodeficiency virus type 1 by pokeweed antiviral protein targeted to CD4+ cells. Antimicrob. Agents Chemother. 37:835-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu, D. J., T. J. Dondero, M. A. Rayfield, J. R. George, G. Schochetman, H. W. Jaffe, C. C. Luo, M. L. Kalish, B. G. Weniger, C. P. Pau, C. A. Schable, and J. W. Curran. 1996. The emerging genetic diversity of HIV. The importance of global surveillance for diagnostics, research, and prevention. JAMA 275:210-216. [PubMed] [Google Scholar]
- 8.McGuigan, C., D. Cahard, H. M. Sheeka, E. De Clercq, and J. Balzarini. 1996. Phosphoramidate derivatives of d4T with improved anti-HIV efficacy retain full activity in thymidine kinase deficient cells. Bioorg. Med. Chem. Lett. 6:1183-1186. [Google Scholar]
- 9.McGuigan, C., D. Cahard, H. M. Sheeka, E. De Clercq, and J. Balzarini. 1996. Arylphosphoramidate derivatives of d4T have improved anti-HIV efficacy in tissue culture and may act by the generation of a novel intracellular metabolite. J. Med. Chem. 39:1748-1753. [DOI] [PubMed] [Google Scholar]
- 10.McGuigan, C., H. W. Tsang., D. Cahard., S. Turner., Velazquez., A. Salgado., L. Bidois., L. Naesens, E. De Clercq, and J. Balzarini. 1997. Phosphoramidate derivatives of d4T as inhibitors of HIV: the effect of amino acid variation. Antivir. Res. 35:195-204. [DOI] [PubMed] [Google Scholar]
- 11.Richman, D. D., et al. 1993. In vitro evaluation of experimental agents for anti-HIV activity, p. 1-21. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, W. Strober (ed.), Current protocols in immunology, supplement 8. John Wiley & Sons, Inc., Brooklyn, N.Y. [DOI] [PubMed]
- 12.Rey, D., M. P. Schmitt, M. Partisani, G. Hess-Kempf, V. Krantz, E. de Mautort, H. C. Bernard, M. Priester, C. Cheneau, and J. M. Lang. 2001. Efavirenz as a substitute for protease inhibitors in HIV-1 infected patients with undetectable plasma viral load on HAART: a median follow up of 64 weeks. J. Acquir, Immune Defic. Syndr. 27:459-462. [DOI] [PubMed] [Google Scholar]
- 13.Uckun, F. M., L. M. Chelstrom, L. Tuel-Ahlgren, I. Dibirdik, J. D. Irvin, M. Chandan-Langlie, and D. E. Myers. 1998. TXU (anti-CD7)-pokeweed antiviral protein as a potent inhibitor of human immunodeficiency virus. Antimicrob. Agents and Chemother. 42:383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uckun, F. M., and R. Vig. February 2000. Aryl phosphate derivatives of d4T having anti-HIV activity. U.S. patent 6,030,957.
- 15.Uckun, F. M., C. L. Chen, E. Lisowski, G. C. Mitcheltree, T. K. Venkatachalam, D. Erbeck, H. Chen, and B. Waurzyniak. Toxicity and pharmacokinetics of stampidine in rats. Arzneimittelforschung/Drug Res., in press. [DOI] [PubMed]
- 16.Uckun, F. M., S. Qazi, S. Pendergrass, E. Lisowski, B. Waurzyniak, C. L. Chen, and T. K. Vankatachalam. 2002. In vivo toxicity, pharmacokinetics, and anti-human immunodeficiency virus activity of stavudine-5′-(p-bromophenyl methoxyalaninyl phosphate) (stampidine) in mice. Antimicrob. Agents Chemother. 46:3428-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ucku F. M., S. Pendergrass, S. Qazi, P. Samuel, C. L. Chen, and T. K. Venkatachalam. Effects of aryl substituents on the anti-HIV activity of the arylphosphoramidate derivatives of stavudine. Antivir. Chem. Chemother., in press. [DOI] [PubMed]
- 18.Venkatachalam, T. K., H. L. Tai, R. Vig, C. L. Chen, S. T. Jan, and F. M. Uckun. 1998. Enhanced effects of a mono-bromo substitution at the para position of the phenyl moiety on the metabolism and anti-HIV activity of D4T-phenyl methoxylalaninyl phosphate derivatives. Bioorg. Med. Chem. Lett. 8:3121-3126. [DOI] [PubMed] [Google Scholar]
- 19.Vig, R., T. K. Venkatachalam, and F. M. Uckun. 1998. D4T-5′-[p-bromophenyl methoxyalaninyl phosphate] as a potent and non-toxic anti-human immunodeficiency virus agent. Antivir. Chem. Chemother. 9:445-448. [PubMed] [Google Scholar]