Abstract
Ertapenem (INVANZ) is a new once-a-day parenteral β-lactam antimicrobial shown to be effective as a single agent for treatment of various community-acquired and mixed infections. The single- and multiple-dose pharmacokinetics of ertapenem at doses up to 3 g were examined in healthy young men and women volunteers. Plasma and urine samples collected were analyzed using reversed-phase high-performance liquid chromatography with UV detection. Ertapenem is highly bound to plasma protein. The protein binding changes from ∼95% bound at concentrations of <50 μg/ml to ∼92% bound at concentrations of 150 μg/ml (concentration at the end of a 30-min infusion following the 1-g dose). The nonlinear protein binding of ertapenem resulted in a slightly less than dose proportional increase in the area under the curve from 0 h to infinity (AUC0-∞) of total ertapenem. The single-dose AUC0-∞ of unbound ertapenem was nearly dose proportional over the dose range of 0.5 to 2 g. The mean concentration of ertapenem in plasma ranged from ∼145 to 175 μg/ml at the end of a 30-min infusion, from ∼30 to 34 μg/ml at 6 h, and from ∼9 to 11 μg/ml at 12 h. The mean plasma t1/2 ranged from 3.8 to 4.4 h. About 45% of the plasma clearance (CLP) was via renal clearance. The remainder of the CLP was primarily via the formation of the β-lactam ring-opened metabolite that was excreted in urine. There were no clinically significant differences between the pharmacokinetics of ertapenem in men and women. Ertapenem does not accumulate after multiple once-daily dosing.
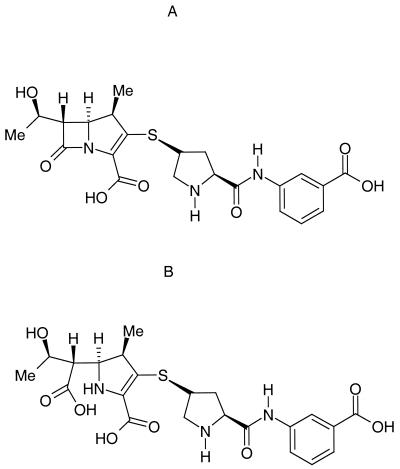
Ertapenem (INVANZ; MK-0826; Merck & Co., Inc.) is a once-a-day parenteral β-lactam antimicrobial agent with excellent in vitro activity against gram-positive and gram-negative aerobic and anaerobic bacteria generally associated with community-acquired and mixed infections (1; C. J. Gill, J. J. Jackson, J. G. Sundelof, H. Rosen, and H. Kropp, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F125, p. 121, 1996). Moreover, ertapenem has been shown to be effective for treating several community-acquired and mixed infections, including intra-abdominal infections, skin and skin-structure infections, community-acquired pneumonia, acute pelvic infections, and urinary tract infections (3, 5; J. S. Solomkin, K. A. Choe, N. V. Christou, et al., Abstr. 21st Surg. Infect. Soc., abstr. 3, 2001; K. Tomera, E. Burdmann, et al., Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-1053, 2001; S. Roy, I. Higareda, E. Angel-Muller, et al., Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-888, 2001; N. Vetter, E. Cambronero-Hernandez, J. Rohlf, et al., Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-855, 2001). This structurally unique carbapenem (Fig. 1) exhibits a long plasma half-life (t1/2) (about 4 h) due largely to its high plasma protein binding and stability against human renal dehydropeptidase.
FIG. 1.
Structures of ertapenem (A) and its β-lactam open-ring form (B)
The objectives of this study were to (i) assess the dose proportionality of intravenous (i.v.) doses of ertapenem across the dose range of 0.5 to 3 g, (ii) evaluate the plasma protein binding of ertapenem in vivo, (iii) compare the pharmacokinetic parameters of ertapenem across studies following the proposed therapeutic dose of 1 g i.v. (infused over 30 min), (iv) compare the pharmacokinetic parameters of ertapenem in men and women, and (v) compare the pharmacokinetic parameters of ertapenem following single versus multiple i.v. doses.
MATERIALS AND METHODS
Study design.
This report includes data from five clinical studies. The design of these studies are as follows. Study 1 was a two-part, double-blind, placebo-controlled study; part I was a two-panel, four-period single rising dose study with doses of 0.04, 0.25, 1, and 2 g in one panel and 0.1, 0.5, 1.5, and 3 g in the second panel. Pharmacokinetic analysis was performed for doses of 0.25 g and over; the 0.04-mg dose was included as a conservative staring dose. The second panel also included a group of healthy women who received the 1-g dose. Part II was a sequential five-panel multiple i.v. dose study with doses of 0.25, 0.5, 1, 1.5, and 3 g once daily for 8 days. Study 2 was an open-label, randomized, four-period crossover study with single i.v. doses of 0.5, 1, 2, and 3 g. Study 3 was an open-label study to investigate the disposition of [14C]ertapenem following a 1-g (∼100-μCi) i.v. dose. The data of unchanged ertapenem are included here. Study 4 was a two-part, randomized, placebo-controlled, single 1-g i.v. or intramuscular dose three-period (part A) and multiple 1-g intramuscular dose (part B) study. The data from the 1-g i.v. dose are included here. Study 5 was an open-label, randomized, two-period crossover ertapenem and probenecid interaction study. The data from the 1-g i.v. ertapenem-alone treatment arm of the study are included here. In each of the studies, all the volunteers, between 18 and 49 years old, were considered healthy based upon clinical evaluations. The volunteers did not take any over-the-counter or prescription medications that may interfere with pharmacokinetic assessment.
Bioanalytical methods.
Quantitation of total ertapenem in plasma and urine involved on-line extraction using column-switching, reversed-phase high-performance liquid chromatography, and UV absorbance detection (4). Determination of unbound ertapenem in plasma involved separation of unbound drug from plasma protein by ultrafiltration and subsequent assay of the plasma ultrafiltrate sample for ertapenem using reversed-phase high-performance liquid chromatography with UV absorbance detection [D. G. Musson, K. L. Birk, A. Majumdar, and J. D. Rogers, abstract from the 1997 AAPS Annu. Meet., Pharm. Res. 14(Suppl.):S376, 1997]. In studies 1 and 2, plasma samples were stabilized with 0.1 M 2-(N-morpholino)ethanesulfonic acid sodium salt (MES) buffer (pH 3.5)-ethylene glycol (1:1, vol/vol) and stored at −70°C at the clinical site. Subsequently, plasma samples were collected without the addition of stabilizer, as adequate stability was demonstrated. The urine samples were collected, stabilized with 0.1 M MES buffer, pH 6.5, and stored at −70°C at the clinical site. The samples were generally mixed, stabilized, and centrifuged before analysis. The lower limit of quantitation for the assay was 0.125 μg/ml for total drug in plasma, 0.25 μg/ml for unbound drug in plasma; and 1.25 or 2.5 μg/ml in urine. The concentrations for the standard curves ranged from 0.125 to 50 μg/ml for the total-drug assay and 0.25 to 100 μg/ml for the unbound-drug assay. The control concentrations were 0.25, 10, and 40 μg/ml for the total-drug assay and 0.25, 12, and 80 μg/ml for the unbound-drug assay. The intraday coefficient of variation for standards was <10%, and the interday coefficient of variation for control concentrations was <20%.
Pharmacokinetic methods.
The area under the plasma concentration-time curve(AUC) from 0 h to the last quantifiable concentration was estimated using the linear trapezoidal rule up to the end of infusion and the log trapezoidal rule thereafter. The apparent terminal elimination rate constant (β) was estimated by regression of the terminal log-linear concentration time points using commercial software RS-1, EXCEL, or Sigmaplot. The apparent t1/2 was estimated at ln 2/β. Total AUC from 0 h to infinity (AUC0-∞) was calculated from the sum of AUC from 0 h to the last quantifiable concentration and the extrapolated area obtained by dividing the concentration at the last quantifiable point by the terminal elimination rate constant. The apparent plasma clearance, CLP, was obtained by dividing the actual dose administered by the corresponding AUC0-∞. The apparent renal clearance (CLR) was estimated from urinary recovery of drug and corresponding increments in AUC. The apparent volume of distribution at steady state (Vss) was calculated based on the following formula: Vss = (AUMC/AUC − τ/2) · (dose/AUC), where AUMC is the area under the first moment curve from time zero to infinity, AUC is from 0 h to infinity, and τ is the duration of infusion. This method assumes linear kinetics. The percentage of dose excreted as unchanged drug in urine (fe) was obtained by dividing the amount of unchanged drug excreted in urine by the corresponding dose.
Statistical methods.
To assess dose proportionality (studies1 and 2), dose-adjusted AUCs at doses of 0.25, 0.5, 1.5, 2, and 3 g were compared with those at the 1- or 1.5-g dose depending on the panel. All individual AUC data were dose adjusted, and then a log transformation was applied to the AUC data to perform the analysis of variance model appropriate for the design of the study. Dose proportionality was also explored using a regression approach (2) in the single-dose, dose proportionality study (study 2). To compare AUCs in men and women, geometric mean AUCs for men and women, geometric mean ratios (GMR) of those data (women/men) and the corresponding 90% confidence interval (CI) were assessed using analysis of variance including sex and period effects. To compare AUC on day 8 and day 1, GMR (day 8/day 1) and associated 90% CIs were calculated using analysis of variance.
RESULTS
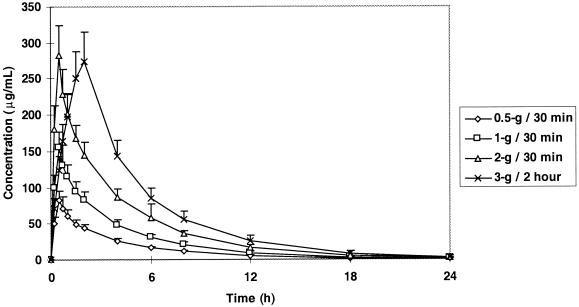
The single-dose pharmacokinetics of ertapenem in healthy young adults were evaluated in studies 1 and 2. Figure 2 shows the mean plasma concentration-time profiles of total ertapenem following each dose in study 2. The shape of the plasma-concentration profiles was similar across the range of doses studied in the two single-dose studies, with the exception of the more prolonged peak corresponding to the 2-h infusion of the 3-g dose as opposed to the 30-min infusion of all lower doses.
FIG. 2.
Mean (n = 16) plasma concentration profiles of total ertapenem following single i.v. doses in healthy young volunteers
Dose proportionality.
In both single-dose studies (studies1 and 2), the mean AUC increased nearly dose proportionally. Summary statistics of total ertapenem plasma AUC0-∞ from study 1, the alternating-panel rising dose study, are shown in Table 1. In both panels of volunteers, the GMR of ertapenem AUC at the low and high dose compared with the middle dose was nearly 1.0, indicative of approximate dose proportionality.
TABLE 1.
Geometric mean and GMR of ertapenem AUC0-∞ for total drug in two panels of healthy men
| Panel | Dose (g) | n | Geometric mean AUC0-∞ (μg · h/ml) | Dose-adjusteda geometric mean AUC0-∞ | Dose-adjusteda AUC0-∞ GMR | 90% CI for dose-adjusteda AUC0-∞ GMR |
|---|---|---|---|---|---|---|
| A | 0.25 | 5 | 165.55 | 662.20 | 1.14 | 1.07, 1.21 |
| 1 | 6 | 581.16 | 581.16 | |||
| 2 | 6 | 1,007.90 | 503.95 | 0.87 | 0.82, 0.92 | |
| B | 0.5 | 6 | 269.08 | 807.24 | 1.13 | 1.07, 1.19 |
| 1.5 | 6 | 715.21 | 715.21 | |||
| 3 | 6 | 1,216.24 | 608.12 | 0.85 | 0.81, 0.90 |
Dose-adjusted to 1 g for panel A; dose-adjusted to 1.5 g for panel B.
The slight nonlinearity observed in the AUC of total drug concentration is consistent with the nonlinear protein binding characteristics of ertapenem seen in vitro and in animals (8). Hence, the protein-binding characteristics of ertapenem and the pharmacokinetics of both total and unbound ertapenem were evaluated in the dose proportionality study (study 2). Table 2 shows the summary statistics of ertapenem plasma AUC0-∞ for both total and unbound drug from this study. Based on both total and unbound drug, the GMR of AUC at each dose level compared to the 1-g dose was again nearly but not precisely 1.0, indicative of approximate dose proportionality. The GMR of total-drug AUC for the 2- and 3-g doses relative to the 1-g dose was 0.88 and 0.82, respectively, indicative of a slight negative deviation from dose proportionality (90% CI at the 3-g dose = 0.78, 0.85). For unbound drug, the GMR of AUC for the 2- and 3-g doses relative to the 1-g dose was 1.15 and 1.24, respectively, indicating a slightly greater than dose proportional increase (90% CI for the 3-g dose = 1.19, 1.30).
TABLE 2.
Geometric mean AUC0-∞ and AUC0-∞ GMR for total and unbound ertapenem for men and women combined (n = 16)
| Pharmacokinetic parameter | Dose (g) | Geometric meana,b | AUC GMRc (90% CI) | P |
|---|---|---|---|---|
| AUC0-∞ for total drugb (μg · h/ml) | 0.5 | 607.14 | 1.07 (1.02, 1.12) | 0.0157 |
| 1 | 568.40 | |||
| 2 | 502.37 | 0.88 (0.85, 0.92) | 0.0001 | |
| 3 | 463.76 | 0.82 (0.78, 0.85) | 0.001 | |
| AUC0-∞ for unbound druga (μg · h/ml) | 0.5 | 31.27 | 0.95 (0.91, 1.00) | 0.0901 |
| 1 | 32.84 | |||
| 2 | 37.70 | 1.15 (1.10, 1.20) | 0.0001 | |
| 3 | 40.78 | 1.24 (1.19, 1.30) | 0.0001 |
AUC root mean square error on the log scale is 0.080 for unbound drug. Dose adjusted to 1 g.
AUC root mean square error on the log scale is 0.074 for total drug. Dose adjusted to 1 g.
GMR relative to 1-g dose.
The dose proportionality of total and unbound AUC was also evaluated using a power model described by Gough et al. (2). The results exhibited a slight deviation from linearity. The estimate for the slope was 0.848 (90% CI = 0.82 to 0.88) for total drug and 1.152 (90% CI = 1.12 to 1.18) for unbound drug. Hence, the results of this model were consistent with those obtained by pairwise analysis of the dose-adjusted AUC ratios; i.e., the AUC is nearly dose proportional.
Pharmacokinetic parameters.
Table 3 shows the mean values of the various pharmacokinetic parameters of total ertapenem from the dose proportionality study. The CLP of total ertapenem increased slightly with dose, consistent with the slightly less than dose-proportional increase in AUC over this dose range. At each dose, CLR was slightly less than half of CLP, and the remainder is, by definition, nonrenal clearance (CLNR). Both CLR and CLNR increased slightly with dose. The fe was approximately 45% at each dose. Mean t1/2 was relatively constant at 3.6 to 3.8 h across the doses.
TABLE 3.
Mean (n = 16) pharmacokinetic parameters for total ertapenem in healthy volunteers receiving various single i.v. doses
| Pharmacokinetic parameter | Mean value (SD) at indicated dose
|
|||
|---|---|---|---|---|
| 0.5 g | 1 g | 2 g | 3 g | |
| AUC0-∞ (μg · h/ ml) | 305.6 (36.8) | 572.1 (68.6) | 1,011.4 (118.0) | 1,407.2 (230.1) |
| CLP (ml/min) | 27.6 (3.2) | 29.5 (3.4) | 33.4 (4.1) | 36.3 (5.2) |
| CLR (ml/min) | 12.7 (3.4)a | 12.9 (4.3)b | 14.9 (4.6) | 16.1 (4.9)b |
| CLNR (ml/min) | 15.0 (4.0)a | 16.1 (5.4)b | 18.5 (5.5) | 20.3 (5.9)b |
| VSS (liter) | 7.8 (1.3) | 8.2 (1.5) | 9.2 (1.4) | 9.4 (1.7) |
| Cmaxe (μg/ml) | 83.0 (12.1) | 154.9 (22.0) | 282.9 (41.4) | 274.3 (41.3) |
| C12 (μg/ml) | 5.2 (1.1) | 9.3 (2.8) | 16.4 (3.6) | 25.3 (8.3) |
| C24 (μg/ml) | 0.6 (0.3) | 1.2 (0.6) | 2.0 (1.0) | 3.0 (1.9) |
| fe (% dose)c | 45.6 (12.1)a | 44.4 (14.8)b | 44.3 (13.3) | 44.1 (12.5)b |
| t1/2β (h)d | 3.8 | 3.8 | 3.8 | 3.6 |
Based on 15 subjects; 1 subject whose urine collection during one of the intervals appeared incomplete was not included.
Based on 14 subjects; 2 subjects whose urine collections in one of the intervals appeared incomplete or missing were not included.
Percent excreted during 0 to 24 h.
Harmonic mean.
Cmax = concentration at end of infusion. The length of infusion was 0.5 h except for the 3-g dose, which was administered over 2 h.
The mean pharmacokinetic parameters for unbound ertapenem are summarized in Table 4. The slight decrease in CLP, CLR, and CLNR with increasing dose is consistent with the slightly greater than dose-proportional increase in AUC of unbound drug with increasing dose. The apparent deviation from precise linearity may be due to drug concentrations approaching the saturation point at the higher dose levels for one or more of the elimination pathways.
TABLE 4.
Mean (n = 16) pharmacokinetic parameters for unbound ertapenem in healthy volunteers receiving various single i.v. doses
| Pharmacokinetic parameter | Mean value (SD) at indicated dose
|
|||
|---|---|---|---|---|
| 0.5 g | 1 g | 2 g | 3 g | |
| AUC0-∞ (μg · h/ ml) | 15.8 (2.2) | 33.2 (5.5) | 76.6 (13.2) | 124.7 (24.6) |
| CLP (ml/min) | 538.7 (81.7) | 513.6 (80.8) | 450.5 (98.4) | 417.1 (89.3) |
| CLR (ml/min) | 243.3 (59.1)a | 223.3 (67.8)b | 194.4 (50.8) | 177.6 (53.1)b |
| CLNR (ml/min) | 294.1 (95.3)a | 289.8 (117.8)b | 256.2 (109.5) | 232.9 (93.6)b |
| VSS (liter) | 138.1 (41.9) | 123.1 (37.2) | 94.1 (33.1) | 78.5 (25.7) |
| Cmaxc (μg/ml) | 5.3 (1.2) | 12.9 (3.2) | 43.3 (14.8) | 39.4 (10.6) |
Based on 15 subjects; 1 subject whose urine collection during one of the intervals appeared incomplete was not included.
Based on 14 subjects; 2 subjects whose urine collections in one of the intervals appeared incomplete or missing were not included.
Cmax = concentration at end of infusion. The length of infusion was 0.5 h except for the 3-g dose, which was administered over 2 h.
Protein binding.
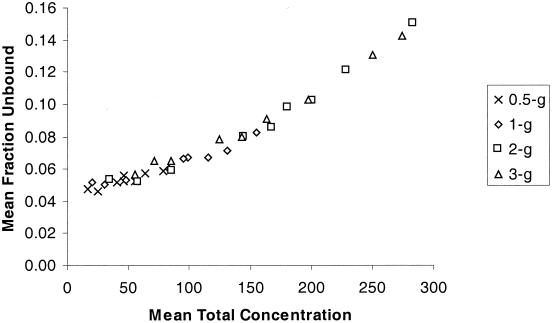
Based on in vitro data (8), it was anticipated that concentration-dependent protein binding would be most evident in plasma samples obtained following the 2- and 3-g doses. Figure 3 indicates that ertapenem exhibits concentration-dependent plasma protein binding in humans, which was most evident at concentrations near the end of the infusion associated with the 2- and 3-g doses. The percentage of unbound ertapenem is about 5% at plasma concentrations of total drug below 50 μg/ml, about 8% at a concentration of 150 μg/ml (approximate concentration at the end of infusion following a 1-g dose), and about 15% at a concentration of about 280 μg/ml (the approximate concentration at the end of the 30-min infusion following the 2-g dose and the end of the 2-h infusion following the 3-g dose). Thus, in healthy volunteers administered a 1-g dose in whom peak concentrations average approximately 150 μg/ml, the nonlinearity of percent unbound drug was modest, whereas the nonlinearity in percent unbound drug is more pronounced for the concentrations achieved following the 2- and 3-g doses. The increase in unbound drug for a substantial part of the plasma profile following the 2- and 3-g doses probably contributed to the slightly higher plasma clearance in terms of total drug at those doses.
FIG. 3.
Mean fraction unbound versus mean concentration of total ertapenem in plasma following the 0.5-, 1-, 2-, and 3-g single doses to healthy young volunteers.
Single-dose pharmacokinetics of ertapenem across studies.
The results of five studies which investigated the pharmacokinetics of total ertapenem in healthy young adult subjects following a 1-g i.v. dose are shown in Table 5. The mean pharmacokinetic parameters of total ertapenem were similar across the five studies. The mean CLP of total ertapenem ranged from approximately 27 to 30 ml/min across the five studies. The mean CLR was slightly less than half of the CLP in each of these studies. The mean plasma concentration ranged from about 145 to 175 μg/ml at the end of infusion, from about 9 to 11 μg/ml at 12 h postdose (C12), and from 1.2 to 1.9 μg/ml at 24 h (C24). The mean percentage of dose excreted as unchanged drug in urine ranged from 39 to 47%. The harmonic mean t1/2 in plasma ranged from 3.8 to 4.4 h.
TABLE 5.
Mean pharmacokinetic parameters of total ertapenem in healthy young volunteers following a 1-g single i.v. dose infused over 30 min across studies
| Pharmacokinetic parameter | Mean value for indicated study no.
|
||||
|---|---|---|---|---|---|
| 1 (n = 12a) | 2 (n = 16) | 3 (n = 7) | 4 (n = 19) | 5 (n = 14) | |
| AUC0-∞ (μg · h/ml) | 588.7 | 572.1 | 627.3 | 608.2 | 619.0 |
| CLP (ml/min) | 29.3 | 29.5 | 26.9 | 28.4 | 27.2 |
| CLR (ml/min) | 11.3 | 12.9b | 10.5 | 12.7 | 12.8 |
| CLNR (ml/min) | 18.0 | 16.1b | 16.4 | 15.7 | 14.4 |
| Cmax (μg/ml) | 145.6 | 154.9 | 165.6 | 164.6 | 175.3 |
| C12 (μg/ml) | 10.5 | 9.3 | 11.3 | 10.2 | 10.4 |
| C24 (μg/ml) | 1.6 | 1.2 | 1.9 | 1.4 | 1.5 |
| fe (% dose)c | 40.6 | 44.4b | 38.7 | 42.3 | 46.7 |
| t1/2β (h)d | 4.1 | 3.8 | 4.4 | 3.8 | 4.0 |
Based on 11 subjects (six males and five females); 1 subject whose drug concentrations in plasma at a few time points appeared anomalous was not included.
Based on 14 subjects; 2 subjects who appeared to have incomplete or missing urine collection in one of the intervals were not included.
Based on 0- to 48-h collection of all 12 subjects in study 1, 0- to 24-h collection for 14 subjects in study 2, 0 to infinity for all 7 subjects in study 3, 0- to 24-h collection for 18 subjects in study 4, and 0- to 24-h collection for all 14 subjects in study 5.
Harmonic mean for t1/2.
Single-dose pharmacokinetics of ertapenem in young men and women.
Formal comparison of single-dose pharmacokinetics of ertapenem in young men and women was performed in the single-dose, dose proportionality study (study 2). The plasma concentration profiles of total ertapenem in young adult men and women following the 1-g i.v. dose were similar.
Table 6 shows the mean pharmacokinetic parameters in men and women following 0.5-, 1-, 2-, and 3-g single doses in study 2. Statistical analysis of the AUC data in men and women following the 1- and 2-g doses in this study revealed no significant difference between the AUC of total ertapenem in men and women. The geometric mean (95% CI) AUC0-∞ ratio (women/men) of total ertapenem was 0.99 (0.86, 1.15) following the 1-g dose and 1.06 (0.92, 1.21) following the 2-g dose.
TABLE 6.
Mean pharmacokinetic parameters for total ertapenem in men and women volunteers following various single i.v. doses (study 2)
| Pharmacokinetic parameter | Mean value (SD) for indicated dose
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Men (n = 8)
|
Women (n = 8)
|
|||||||
| 0.5 ga | 1 g | 2 g | 3 gb | 0.5 g | 1 g | 2 g | 3 gb | |
| AUC0-∞ (μg · h/ml) | 298 (40.3) | 575.0 (83.7) | 986.3 (144.1) | 1,376.1 (302.3) | 312.3 (34.2) | 569.1 (55.3) | 1,036.5 (87.5) | 1,438.4 (140.9) |
| CLP (ml/min) | 28.3 (3.7) | 29.5 (4.1) | 34.4 (4.9) | 37.6 (6.5) | 27.0 (2.8) | 29.5 (2.8) | 32.4 (3.0) | 35.1 (3.5) |
| CLR (ml/min) | 10.9 (3.1) | 10.1 (3.4) | 12.2 (4.1) | 13.6 (5.8) | 14.3 (3.0) | 15.7 (3.1) | 17.6 (3.3) | 18.7 (2.2) |
| CLNR (ml/min) | 17.6 (3.2) | 18.8 (6.0) | 22.3 (5.1) | 23.7 (6.2) | 12.7 (3.2) | 13.3 (3.1) | 14.8 (2.6) | 16.9 (3.1) |
| Cmax (μg/ml) | 78.2 (13.2) | 144.2 (20.6) | 268.2 (48.1) | 253.0 (47.7) | 87.9 (9.4) | 165.5 (18.7) | 297.7 (29.2) | 295.7 (18.6) |
| C12 (μg/ml) | 5.5 (1.1) | 10.3 (2.1) | 17.5 (4.0) | 26.9 (9.5) | 4.8 (1.2) | 8.4 (3.2) | 15.4 (3.0) | 23.8 (7.3) |
| C24 (μg/ml) | 0.8 (0.2) | 1.5 (0.5) | 2.5 (1.1) | 3.8 (2.1) | 0.5 (0.2) | 1.0 (0.6) | 1.6 (0.6) | 2.2 (1.2) |
| fe(0-24) (% dose) | 37.5 (8.4) | 35.2 (13.2) | 34.8 (10.1) | 35.8 (12.1) | 52.7 (10.5) | 53.7 (9.9) | 53.8 (8.4) | 52.3 (5.7) |
| t1/2 (h) | 4.1 (0.3) | 4.2 (0.3) | 4.0 (0.5) | 3.9 (0.5) | 3.5 (0.3) | 3.5 (0.5) | 3.5 (0.3) | 3.3 (0.4) |
| VSS (liter) | 8.6 (1.3) | 8.9 (1.6) | 10.1 (1.5) | 10.4 (1.8) | 7.0 (0.8) | 7.5 (0.9) | 9.2 (1.4) | 9.4 (1.7) |
CLR, CLNR, and fe for the 0.5-g dose are based on 7 subjects (one subject's urine collection during one of the intervals appeared incomplete).
CLR, CLNR, and fe for the 3-g dose are based on seven out of eight subjects in each group (one subject's urine collection in one of the intervals appeared incomplete or missing).
The AUC of unbound ertapenem appeared similar for women and men at a 1-g i.v. dose (34.8 μg · h/ml in women versus 31.0 μg · h/ml in men; P = 0.163). It appeared slightly higher for women at a 2-g i.v. dose (84.1 μg · h/ml in women versus 67.6 μg · h/ml in men; P = 0.035).
Pharmacokinetics of ertapenem were also compared in young adult men and women following the 1-g dose in the initial rising dose study (study 1) and in the control periods of the probenecid interaction study (study 5). AUC0-∞ and CLP values were very similar between men and women in each study, indicative of comparable overall drug exposure. In other pharmacokinetic parameters, there were small, but consistent differences between men and women. Women exhibited a slightly higher maximum concentration of drug in plasma (Cmax), a slightly shorter apparent t1/2, a slightly lower Vss, a slightly faster CLR, and a somewhat higher fe than men. The body weight-adjusted Vss for men was similar to that for women (0.11 and 0.12 liter/kg, respectively, following the 1-g dose). The higher Cmax, shorter t1/2, and lower Vss are consistent with the lower body weight of women
Multiple-dose pharmacokinetics of ertapenem in healthy young volunteers.
The pharmacokinetics of multiple i.v. doses of ertapenem were studied in young men as part II of the initial rising dose study (study 1). Mean plasma concentration profiles of total ertapenem were very similar on day 1 and day 8 following multiple dosing at the 0.25- to 2-g dose levels.
To assess accumulation after multiple dosing, Table 7 shows the ratio of day 8 AUC0-24 over day 1 AUC0-24 for each dose. The ratio was close to or slightly lower than 1 for each dose, indicating that ertapenem does not accumulate following once-daily (0.25 to 3 g) dosing over 8 days. The ratio at the 3-g dose appears slightly less than one. There is no clear explanation for the slightly lower plasma concentrations on day 8 following the 3-g dose.
TABLE 7.
Ratios of day 8 AUC0-24 to day 1 AUC0-24 for total ertapenem
| Panel | Dose (g) | N | Geometric mean AUC0-24 on day:
|
AUC GMR day 8/day 1 | 90% CI of AUC GMR | |
|---|---|---|---|---|---|---|
| 1 | 8 | |||||
| A | 0.25 | 6 | 148.36 | 151.43 | 1.02 | 0.97, 1.07 |
| B | 0.5 | 6 | 274.76 | 293.41 | 1.07 | 0.92, 1.24 |
| C | 1 | 6 | 480.70 | 501.44 | 1.04 | 0.98, 1.11 |
| D | 2 | 6 | 1,041.67 | 1,019.30 | 0.98 | 0.89, 1.08 |
| E | 3 | 6 | 1,547.55 | 1,266.06 | 0.82 | 0.76, 0.88 |
DISCUSSION
The five studies reviewed herein examined the pharmacokinetic characteristics of i.v. ertapenem in healthy young men and women volunteers following single and multiple doses ranging from 0.25 to 3 g.
Ertapenem is highly plasma protein bound in the therapeutic concentration range (95%). This high plasma protein binding of ertapenem contributes to its long plasma t1/2. The protein binding of ertapenem changes somewhat with concentration, like that of ceftriaxone (6, 7). However, the effect of this nonlinear protein binding on the pharmacokinetics of ertapenem is small, as evidenced by the near-dose-proportional increase in the AUC of ertapenem over the dose of 0.25 to 2 g or 0.5 to 3 g. Thus, near-linear pharmacokinetics can be reasonably assumed for applying AUC data to clinical situations.
The CLR of ertapenem was slightly less than half of its CLP. The CLR of unbound drug is above the normal range of the glomerular filtration rate that would be anticipated in these subjects, indicating that the urinary excretion of ertapenem was via renal tubular secretion in addition to glomerular filtration. The urinary excretion of ertapenem was about the same (∼45%) across 0.5- to 3-g doses and was thus independent of dose.
The AUCs and CLR of total ertapenem were similar between men and women. In women, the mean t1/2 and Vss were slightly lower, and the end of infusion concentration and CLR were slightly higher and faster, respectively. A slightly lower Vss, a slightly higher Cmax, and a slightly shorter t1/2 in women are consistent with the generally lower body weight of women. However, these differences were judged not likely to be clinically significant given the similarity in overall drug exposure (AUC) and CLP. No dose adjustment based on sex was incorporated into studies of efficacy and safety in patients.
Ertapenem does not accumulate after multiple dosing (0.25 to 3 g once daily for 8 days), consistent with the approximately 4 h t1/2 of ertapenem.
REFERENCES
- 1.Gill, C. J., J. J. Jackson, L. S. Gerckens, B. A. Pelak, R. K. Thompson, J. G. Sundelof, H. Kropp, and H. Rosen. 1998. In vivo activity and pharmacokinetic evaluation of a novel long-acting carbapenem antibiotic, MK-0826 (L-749345). Antimicrob. Agents Chemother. 42:1996-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gough, K., M. Hutchinson, O. Keene, B. Byron, S. Ellis, L. Lacey, and J. McKellar. 1995. Assessment of dose proportionality: report from the statisticians in the pharmaceutical industry/pharmacokinetic joint working party. Drug Inform. J. 29:1039-1048. [Google Scholar]
- 3.Graham, D. R., C. Lucasti, O. Malafaia, R. L. Nichols, P. Holtom, N. Qunitero Perez, A. McAdams, G. L. Woods, T. P. Ceesay, R. Gesser, and the Ertapenem CSSSI Study Group. 2002. Ertapenem once daily versus piperacillin-tazobactam 4 times per day for treatment of complicated skin and skin structure infections in adults: results of a prospective, randomized, double-blind multicenter study. Clin. Infect. Dis. 34:1460-1468. [DOI] [PubMed] [Google Scholar]
- 4.Musson, D. G., K. L. Birk, A. M. Cairns, A. K. Majumdar, and J. D. Rogers. 1998. High performance liquid chromatographic methods for the determination of a new carbapenem antibiotic, L-749345, in human plasma and urine. J. Chromatogr. B 720:99-106. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz-Ruiz. G., J. Caballero-Lopez, I. R. Friedland, G. L. Woods. A. Carides, and the Ertapenem Community Acquired Pneumonia in Adults. 2002. A study evaluating the efficacy, safety, and tolerability of ertapenem versus ceftriaxone in the treatment of community-acquired pneumonia in adults.Clin. Infect. Dis. 34:1076-1083. [DOI] [PubMed] [Google Scholar]
- 6.Popick, A. C., W. G. Crouthamel, and L. Bekersky. 1987. Plasma protein binding of ceftriaxone. Xenobiotica 17:1139-1145. [DOI] [PubMed] [Google Scholar]
- 7.Stoeckel, K., P. J. McNamara, R. Brandt, H. Plozza-Nottebrock, and W. Ziegler. 1981. Effect of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin. Pharmacol. Ther. 29:650-657. [DOI] [PubMed] [Google Scholar]
- 8.Wong, B. K., P. J. Bruhin, and J. H. Lin. 1999. Dose-dependent plasma clearance of MK-0826, a carbapenem antibiotic, arising from concentration dependent plasma protein binding in rats and monkeys. J. Pharm. Sci. 88:277-280. [DOI] [PubMed] [Google Scholar]