Abstract
North American Indian childhood cirrhosis (NAIC, or CIRH1A) is an isolated nonsyndromic form of familial cholestasis reported in Ojibway-Cree children and young adults in northwestern Quebec. The pattern of transmission is consistent with an autosomal recessive mode of inheritance. To map the NAIC locus, we performed a genomewide scan on three DNA pools of samples from 13 patients, 16 unaffected siblings, and 22 parents from five families. Analysis of 333 highly polymorphic markers revealed 3 markers with apparent excess allele sharing among affected individuals. Additional mapping identified a chromosome 16q segment shared by all affected individuals. When the program FASTLINK/LINKAGE was used and a completely penetrant autosomal recessive mode of inheritance was assumed, a maximum LOD score of 4.44 was observed for a recombination fraction of 0, with marker D16S3067. A five-marker haplotype (D16S3067, D16S752, D16S2624, D16S3025, and D16S3106) spanning 4.9 cM was shared by all patients. These results provide significant evidence of linkage for a candidate gene on chromosome 16q22.
North American Indian childhood cirrhosis (NAIC; also known as “CIRH1A” [MIM 604901]) is an isolated nonsyndromic form of cholestasis found in Ojibway-Cree children from First Nations communities in the Abitibi region of northwestern Quebec, Canada (Weber et al. 1981). The disease typically presents, in a child who is otherwise well, with transient neonatal jaundice that progresses to biliary cirrhosis requiring hepatic transplantation in childhood or young adulthood. The biochemical and histopathologic features of the disease suggest involvement of the bile ducts, rather than of the bile canaliculi. They include elevated gamma glutamyl transferase and alkaline phosphatase levels and, typically, marked fibrosis around portal bile ducts. Results of imaging studies are not typical of known cholangiopathies, such as sclerosing cholangitis. Clinical and physiological investigations to discover the underlying cause of NAIC have been unsuccessful. Under the assumption of autosomal recessive transmission and complete penetrance, the carrier frequency in at-risk populations was estimated to be ∼9% (E. Drouin, unpublished data). Hypothesizing that a major founder mutation underlies most or all cases of NAIC, we used a DNA-pooling strategy to search for an excess of shared homozygosity due to identity by descent (Sheffield et al. 1998) among patients, compared with their unaffected first-degree relatives.
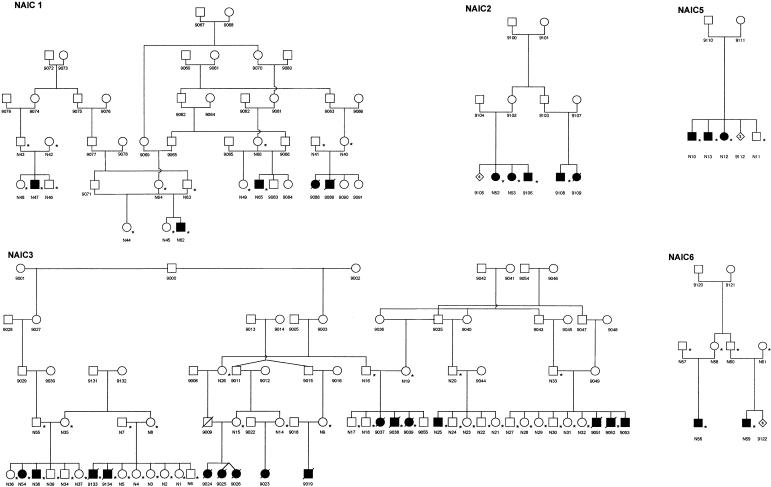
Samples were obtained with ethics approval from the Institutional Review Boards of Hôpital Sainte-Justine and Montreal General Hospital. Of note, all known patients have been followed by A.R.-W. and/or E.D. Consenting individuals were visited by A.R.-W., E.D., and/or G.A.M., who explained the study and obtained written consent, pedigree information, and blood samples. The detailed clinical features of the patients in the study will be described separately (E. Drouin, unpublished data). Leukocyte DNA samples were obtained from 13 affected individuals, as well as from their parents and unaffected siblings, when available. These 51 individuals from five families are shown, with the identification prefix “N,” in figure 1. Three DNA pools were used in the genomewide scan. The three DNA pools contained equal quantities of DNA from the following individuals: all available obligate heterozygote parents (N=22), all 13 affected individuals with identification prefix “N,” and unaffected siblings (48, 46, 44, 45, 49, 39, 34, 5, 4, 17, 18, 24, 23, 27, 28, and 11). We limited the number of subjects in the pool of unaffected siblings to two individuals from each nuclear family, to avoid overrepresentation of specific alleles. A genomewide scan was performed with a set of 380 simple-sequence-length polymorphisms (SSLPs). This marker panel is a modified version of the Cooperative Human Linkage Center Human Screening set version 6.0 (Dubovsky et al. 1995) and includes selected Généthon markers (Dib et al. 1996). The three DNA pools were used as templates for DNA amplification. The DNA pool from affected individuals was genotyped in duplicate. Each marker was independently amplified using fluorescently labeled primers (Life Technologies). The PCR products were multiplexed and electrophoretically separated on an ABI 377 DNA sequencer (PE Biosystems) using 5% acrylamide gels. The gels were analyzed with GeneScan version 2.0.2 peak-calling software (PE Biosystems) and were inspected visually to identify markers with a predominant allele in the pools of affected individuals, compared with the DNA pools of the parents and of unaffected siblings. Genealogical data from an additional 79 individuals were used to construct the pedigrees (fig. 1). All patients were of northern-Ojibway or Cree origin and had both a history of neonatal jaundice and clinical and biochemical evidence of a cholangiopathic process. In all patients from whom liver samples were available, the presence of biliary cirrhosis was confirmed. After the initial mapping with pooled DNA, samples from small archived liver samples from an additional seven patients (designated “9038,” “9039,” “9052,” “9106,” “9108,” “9133,” and “9134”) became available and were used for further analysis.
Figure 1.
Pedigrees of five families with NAIC. Blackened symbols represent individuals with clinically and biochemically documented NAIC. An asterisk (*) indicates an individual whose DNA sample was genotyped. The identification prefix “N” denotes the initial 51 family members who provided DNA samples for the study; DNA samples from 7 additional NAIC patients (designated “9038,” “9039,” “9052,” “9106,” “9108,” “9133,” and “9134”) became available later and were included in the linkage and haplotype analyses. The number in a diamond-shaped symbol is the number of additional family members for whom DNA samples were not available.
After the genomewide scan, selected markers chosen from the Center for Medical Genetics, Marshfield Medical Research Foundation genetic map for chromosomes 4, 8, and 16 were genotyped using individual DNA from the five NAIC pedigrees. Fluorescently labeled oligonucleotides (Research Genetics) were used for GATA138C05, D16S3398, D16S3067, D16S752, D16S2624, D16S3025, and D16S518. ABI 377 gel files were analyzed using an automated allele-calling software package developed by Mark Daly atthe Whitehead Institute/Massachusetts Institute of Technology Center for Genome Research. For D16S3140, D16S3089, D16S514, D16S3106, D16S512, D16S3083, and D16S3125, the marker alleles were amplified by PCR with one of the primers end-labeled with [32P]-γATP, under standard conditions, and were analyzed as described elsewhere (Richter et al. 1999).
Linkage between the NAIC locus and microsatellite markers was analyzed using the FASTLINK software package (version 4.1P [Cottingham et al. 1993; Schäffer et al. 1994; Schäffer 1996; Becker et al. 1998]) of the LINKAGE programs (version 5.2 [Lathrop and Lalouel 1984; Lathrop et al. 1984, 1986]). ILINK was used for two-point linkage analysis assuming an autosomal recessive trait with complete penetrance. The disease-allele frequency was estimated to be .045. LOD scores were computed from the likelihoods maximized over marker-allele frequencies for selected values of recombination fraction (θ)<.5 and at θ=.5. (Terwilliger and Ott 1994; Annunen et al. 1999). Genetic distances between microsatellite markers were obtained from the Center for Medical Genetics, Marshfield Medical Research Foundation Web site.
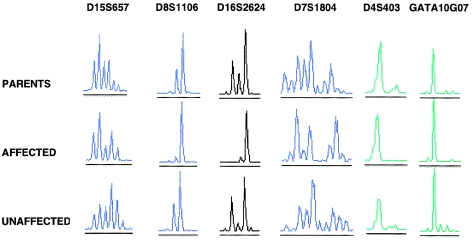
The DNA-pooling scheme allowed the genomewide scan to be performed with ∼1,500 PCR reactions (4×380 markers) loaded onto 14 ABI 377 gels. We scored the ABI 377 trace data for 333 SSLPs, after discarding 47 because of weak, absent, or incorrectly sized PCR products. Three markers (D4S403, D8S1106, and D16S2624) were potential candidates for close linkage to the NAIC locus, since the affected DNA pools appeared to contain a predominant allele, compared with the control DNA pools (fig. 2).
Figure 2.
ABI 377 electropherograms for six SSLPs, for three DNA pools (parents, unaffected siblings, and patients). D15S657 and D7S1804 are representative markers that have multiple peaks in all DNA pools, corresponding to distinguishable alleles. GATA10G07 appears to be monomorphic in all three DNA pools. Three markers (D16S2624, D8S1106, and D4S403) had a predominant peak in the DNA pool of affected children but differed by the presence of two or more peaks in the parent and unaffected-sibling pools.
Available family members were genotyped for D4S403, D8S1106, and D16S2624. All affected individuals were homozygous for the same D16S2624 allele (143 bp), whereas the marker was polymorphic in the remaining family members. A LOD score of 4.04 at θ=0 was obtained for linkage of the NAIC locus and D16S2624. A single D4S403 allele was present in 23 of 26 chromosomes from the patients. However, recombination under the assumed genetic model was observed between the NAIC locus and between NAIC and D4S403 and D8S1106. There was little or no evidence for linkage to D4S403 (and two distal markers) or to D8S1106.
Thirteen additional chromosome 16 markers closely linked to D16S2624 were genotyped. The maximum LOD score was 4.44 at D16S3067 (table 1). The closest recombinant flanking markers of the NAIC-associated haplotype were D16S3398 and D16S3083, and these define a 7.4-cM interval. Furthermore, all patients were homozygous for a five-marker haplotype, composed of D16S3067, D16S752, D16S2624, D16S3025, and D16S3106 (table 2). The NAIC-associated haplotype is flanked on the centromeric side by D16S3398 and on the telomeric side by D16S512, an interval of 4.9 cM. Two or more alleles of D16S3398 and D16S512 were observed in the sample of NAIC chromosomes, suggesting that ancestral recombination events occurred between these markers and the disease locus.
Table 1.
LOD Scores for Linkage of NAIC and Chromosome 16 Markers
| LOD Score at θ = |
||||||||
| Marker | Genetic-Map Positiona(cM) | .00 | .01 | .05 | .10 | .20 | .30 | .40 |
| D16S3140 | 74.44 | −∞ | −1.74 | .40 | .99 | .96 | .54 | .15 |
| D16S3089 | 80.00 | −∞ | −.69 | .67 | 1.05 | .94 | .54 | .17 |
| GATA138C05 | 81.15 | 1.41 | 1.48 | 1.52 | 1.37 | .91 | .46 | .13 |
| D16S514 | 81.15 | −∞ | .03 | 1.01 | 1.25 | 1.05 | .63 | .20 |
| D16S3398 | 84.75 | −∞ | 3.49 | 3.64 | 3.28 | 2.32 | 1.32 | .44 |
| D16S3067 | 85.94 | 4.44 | 4.34 | 3.85 | 3.24 | 1.97 | .90 | .13 |
| D16S752 | 87.06 | .81 | .82 | .81 | .71 | .46 | .23 | .06 |
| D16S2624 | 87.62 | 4.04 | 3.91 | 3.40 | 2.80 | 1.72 | .84 | .24 |
| D16S3025 | 88.18 | 2.58 | 2.52 | 2.31 | 2.03 | 1.47 | .88 | .31 |
| D16S3106 | 88.18 | .44 | .45 | .43 | .37 | .23 | .11 | .03 |
| D16S512 | 89.63 | 1.20 | 1.41 | 1.76 | 1.77 | 1.34 | .77 | .25 |
| D16S3083 | 92.10 | −∞ | −2.17 | −.22 | .43 | .43 | .22 | .00 |
| D16S3125 | 93.78 | −∞ | .91 | 1.98 | 2.06 | 1.54 | .85 | .24 |
| D16S518 | 95.08 | −∞ | −1.80 | −.59 | −.14 | .10 | .09 | .03 |
Map positions from the Center for Medical Genetics, Marshfield Medical Research Foundation Web site.
Table 2.
Genotypes for Chromosome 16 Markers Observed in Patients with NAIC[Note]
|
Genotype for Markera |
||||||||||||||
| Pedigree and ID | D16S3140(74.44) | D16S3089(80.00) | GATA138C05(81.15) | D16S514(81.15) | D16S3398(84.75) | D16S3067(85.94)b | D16S752(87.06)b | D16S2624(87.62)b | D16S3025(88.18)b | D16S3106(88.18)b | D16S512(89.63) | D16S3083(92.10) | D16S3125(93.78) | D16S518(95.08) |
| 1: | ||||||||||||||
| N47 | 163/151 | 184/196 | 160 | 121/125 | 201 | 142 | 105 | 143 | 104 | 196 | 201/205 | 207 | 181 | 279/275 |
| N62 | 167/151 | 184/196 | 160/143 | 121 | 201 | 142 | 105 | 143 | 104 | 196 | 201/207 | 200/212 | 203/207 | 283 |
| N65 | 167/147 | 182/196 | 160/152 | 125 | 201 | 142 | 105 | 143 | 104 | 196 | 205 | 209/213 | 181/207 | 281/279 |
| 2: | ||||||||||||||
| N52 | 169/165 | 196 | 160 | 125 | 201 | 142 | 105 | 143 | 104 | 196 | 205 | 209 | 203 | 279 |
| N53 | 169/165 | 196 | 160 | 125 | 201 | 142 | 105 | 143 | 104 | 196 | 205 | 209 | 203 | 279 |
| 9106 | 169/165 | 196 | 160 | 125 | 201 | 142 | 105 | 143 | 104 | 196 | 205 | 209 | 203 | 279 |
| 9108 | 165 | 196 | 160 | 125 | 201 | 142 | 105 | 143 | 104 | 196 | 205 | 209 | 203 | 279 |
| 3: | ||||||||||||||
| N25 | 165/151 | 190/194 | 160/155 | 121/125 | 201 | 142 | 105 | 143 | 104 | 196 | 201/207 | 207/213 | 203 | 283/273 |
| N38 | 167/151 | 184/192 | 152 | 121 | 213/201 | 142 | 105 | 143 | 104 | 196 | 205/207 | 209/213 | 203/205 | 283/273 |
| N54 | 165/151 | 184/192 | 152 | 121 | 213/201 | 142 | 105 | 143 | 104 | 196 | 205/207 | 209/213 | 203/205 | 283/273 |
| 9038 | 165/151 | 192/196 | nd | 121/125 | 201 | 142 | 105 | 143 | 104 | 196 | 201/207 | 199/207 | 203/205 | 273 |
| 9039 | 165/151 | 192/196 | 164/152 | 121/125 | 201 | 142 | 105 | 143 | 104 | 196 | 201/207 | 207/213 | 203 | 273/283 |
| 9052 | … | … | … | … | … | 142 | 105 | 143 | 104 | 196 | … | … | … | … |
| 9133 | … | … | … | … | … | 142 | 105 | 143 | 104 | nd | … | … | … | … |
| 9134 | 167/165 | 186/196 | 160/152 | 121/125 | 213/201 | 142 | 105 | 143 | 104 | 196 | 201/205 | 207/209 | 203/205 | 273 |
| 5: | ||||||||||||||
| N10 | 151 | 192/194 | 152 | 121/125 | 201 | 142 | 105 | 143 | 104 | 196 | 201 | 213 | 183/195 | 281 |
| N12 | 151 | 192/194 | 152 | 121/125 | 201 | 142 | 105 | 143 | 104 | 196 | 201 | 213 | 183/195 | 281/273 |
| N13 | 169/151 | 184/194 | 152 | 125 | 201 | 142 | 105 | 143 | 104 | 196 | 201 | 213 | 183/195 | 281 |
| 6: | ||||||||||||||
| N56 | 169/167 | 184/196 | 143 | 125 | 201 | 142 | 105 | 143 | 104 | 196 | 207 | 213 | 203 | 283 |
| N59 | 167/147 | 184/196 | 143 | 125 | 201 | 142 | 105 | 143 | 104 | 196 | 205/207 | 213 | 199/203 | 281/273 |
Note.— Each entry represents the two disease-associated haplotypes of the patient. Where the patient was homozygous for a marker locus, only the allele is given; otherwise the heterozygous genotype is given.
An ellipsis (…) denotes that the marker was not typed; nd = Genotype was not determined because it could not be reliably identified.
Marker with identical alleles among all patients in the study.
NAIC belongs to a heterogeneous group of autosomal recessively inherited liver disorders characterized by an early onset of cholestasis and progressing to cirrhosis and often to liver failure before adulthood (Gray and Saunders 1966; Toussaint and Gros 1966; Williams et al. 1972). The clustering and segregation pattern of the disease are consistent with a common ancestral mutation causing the disease in all affected individuals. A founder effect is consistent with the demographic history of Canadian Ojibway-Cree (McMillan 1995). Founder effect has been observed, in other Native American populations, for autosomal recessive traits including type I glutaric acidemia (Haworth et al. 1991; Greenberg et al. 1995), pyruvate carboxylase deficiency (Robinson et al. 1984), and limb-girdle muscular dystrophy (Weiler et al. 1996) in Manitoba; for microcephaly-micromelia (Ives and Houston 1980), hyperammonemia-hyperornithinemia-homocitrullinuria syndrome (Camacho et al. 1999), and Sandhoff disease (Lowden et al. 1978) in Saskatchewan; and, possibly, for an infantile leukoencephalopathy in northern Manitoba and Quebec (Black et al. 1988). In addition, founder effect has been suggested for dominantly inherited conditions (Birt and Davis 1975) and multigenic traits, such as type II diabetes and hyperlipidemia (Hegele et al. 1998, 1999).
The multipoint LOD score of 4.44 provides strong support for the location of the NAIC locus on distal chromosome 16q. The observation of a haplotype shared among all five families with NAIC is consistent with the hypothesis that these individuals share the same mutation, which is identical by descent. The 4.9-cM interval defined by D16S3398 and D16S512 encompasses the haplotype common to all NAIC-bearing chromosomes and is likely to contain the disease locus.
Clinically, NAIC has been suspected to be distinct from previously described nonsyndromic familial cholestases, because of its marked cholangiopathic features and severe degree of fibrosis on liver histology. Three such disorders, designated “progressive familial intrahepatic cholestases” (PFICs), are caused by mutations in genes coding for canalicular transport proteins. In PFIC-1, mutations in a P-type ATPase gene on chromosome 18q21-q22 cause both Byler disease (Clayton et al. 1969) and benign recurrent intrahepatic cholestasis (Bull et al. 1998). PFIC-2 results from mutations in the sister of the P-glycoprotein gene on chromosome 2q24, which encodes a liver-specific ATP-binding cassette transporter thought to be the human bile salt–export pump (Strautnieks et al. 1998). PFIC-3 is caused by mutations in the multidrug resistance–3 P glycoprotein gene on chromosome 7q21 (de Vree et al. 1998). The present study shows that NAIC is genetically distinct from the three known PFICs. However, other familial infantile cholestases have been reported in Greenland Inuit (Nielsen et al. 1986), Turks (Koçak et al. 1997), and Arab Israelis (Naveh et al. 1997). Available descriptions have not permitted detailed clinical and pathological comparisons with NAIC. It is now possible to test these patients for linkage to the NAIC locus. The mapping of this locus is an important step toward understanding this frequent and potentially fatal disease of First Nations children in northwestern Quebec.
Acknowledgments
We thank the patients and their families for their participation; Noella Bouchard, R.N., Department of Indian and Northern Affairs, Canada, for invaluable assistance in sample collection; Mélanie Primeau and Alexandra Girodet for expert technical help; Christine Bieri and J. Conceptión Loredo-Osti for assistance with graphics; and Robin Casey for stimulating discussion of Native Canadian health issues. This research was partially supported by grants from the Canadian Genetic Diseases Network, Federal Networks of Centres of Excellence Program (to T.J.H., G.A.M., and K.M.), and the Medical Research Council of Canada (to T.J.H., K.M., A.R., and G.A.M.). T.J.H. and G.A.M. are recipients of Clinician Scientist Awards from the Medical Research Council of Canada.
Electronic-Database Information
The accession number and URLs for data in this article are as follows:
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://www.marshmed.org/genetics/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CIRH1A [MIM 604901])
References
- Annunen S, Paassilta P, Lohiniva J, Perälä M, Pihlajamaa T, Karppinen J, Tervonen O, et al (1999) An allele of COL9A2 associated with intervertebral disc disease. Science 285:409–412 [DOI] [PubMed] [Google Scholar]
- Becker A, Geiger D, Schäffer AA (1998) Automatic selection of loop breakers for genetic linkage analysis. Hum Hered 48:49–60 [DOI] [PubMed] [Google Scholar]
- Birt AR, Davis RA (1975) Hereditary polymorphic light eruption of American Indians. Int J Dermatol 14:105–111 [DOI] [PubMed] [Google Scholar]
- Black DN, Booth F, Watters GV, Andermann E, Dumont C, Halliday WC, Hoogstraten J, et al (1988) Leukoencephalopathy among native Indian infants in northern Quebec and Manitoba. Ann Neurol 24:490–496 [DOI] [PubMed] [Google Scholar]
- Bull LN, van Eijk MJT, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, Klomp LW, et al (1998) A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet 18:219–224 [DOI] [PubMed] [Google Scholar]
- Camacho JA, Obie C, Biery B, Goodman BK, Hu C-A, Almashanu S, Steel G, et al (1999) Hyperornithinaemia-hyperammonaemia-homocitrullinuria syndrome is caused by mutations in the gene encoding the mitochondrial ornithine transporter. Nat Genet 22:151–158 [DOI] [PubMed] [Google Scholar]
- Clayton RJ, Iber FL, Ruebner BH, McKusick VA (1969) Byler’s disease: fatal familial intrahepatic cholestasis in an Amish kindred. Am J Dis Child 117:112–114 [PubMed] [Google Scholar]
- Cottingham RW Jr, Idury RM, Schäffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263 [PMC free article] [PubMed] [Google Scholar]
- de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, et al (1998) Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA 95:282–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib C, Faure S, Fizames C, Drouot N, Vignal A, Millasseau P, Marc S, et al (1996) A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152–154 [DOI] [PubMed] [Google Scholar]
- Dubovsky J, Sheffield VC, Duyk GM, Weber JL (1995) Sets of short tandem repeat polymorphisms for efficient linkage screening of the human genome. Hum Mol Genet 4:449–452 [DOI] [PubMed] [Google Scholar]
- Gray OP, Saunders RA (1966) Familial intrahepatic cholestatic jaundice in infancy. Arch Dis Child 41:320–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg CR, Reimer D, Singal R, Triggs-Raine B, Chudley AE, Dilling LA, Philipps S, et al (1995) A G-to-T transversion at the +5 position of intron 1 in the glutaryl CoA dehydrogenase gene is associated with the Island Lake variant of glutaric acidemia type I. Hum Mol Genet 4:493–495 [DOI] [PubMed] [Google Scholar]
- Haworth JC, Booth FA, Chudley AE, deGroot GW, Dilling LA, Goodman SI, Greenberg CR, et al (1991) Phenotypic variability in glutaric aciduria type I: report of fourteen cases in five Canadian Indian kindreds. J Pediatr 118:52–58 [DOI] [PubMed] [Google Scholar]
- Hegele RA, Harris SB, Connelly PW, Hanley AJ, Tsui LC, Zinman B, Scherer SW (1998) Genetic variation in paraoxonase-2 is associated with variation in plasma lipoproteins in Canadian Oji-Cree. Clin Genet 54:394–399 [DOI] [PubMed] [Google Scholar]
- Hegele RA, Sun F, Harris SB, Anderson C, Hanley AJ, Zinman B (1999) Genome-wide scanning for type 2 diabetes susceptibility in Canadian Oji-Cree, using 190 microsatellite markers. J Hum Genet 44:10–14 [DOI] [PubMed] [Google Scholar]
- Ives EJ, Houston CS (1980) Autosomal recessive microcephaly and micromelia in Cree Indians. Am J Med Genet 7:351–360 [DOI] [PubMed] [Google Scholar]
- Koçak N, Gurakan F, Yuce A, Caglar M, Kale G, Gogus S (1997) Nonsyndromic paucity of interlobular bile ducts: clinical and laboratory findings of 10 cases. J Pediatr Gastroenterol Nutr 24:44–48 [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM (1984) Easy calculation of LOD scores and genetic risks on small computers. Am J Hum Genet 36:460–465 [PMC free article] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM, White, RL (1986) Construction of human linkage maps: likelihood calculations for multilocus linkage analysis. Genet Epidemiol 3:39–52 [DOI] [PubMed] [Google Scholar]
- Lowden JA, Ives EJ, Keene DL, Burton AL, Skomorowski MA, Howard F (1978) Carrier detection in Sandhoff disease. Am J Hum Genet 30:38–45 [PMC free article] [PubMed] [Google Scholar]
- McMillan AD (1995) Native peoples and cultures of Canada: an anthropological overview, 2d ed. Douglas & McIntyre, Vancouver [Google Scholar]
- Naveh Y, Bassan L, Rosenthal E, Berkowitz D, Jaffe M, Mandel H, Berant M (1997) Progressive familial intrahepatic cholestasis among the Arab population in Israel. J Pediatr Gastroenterol Nutr 24:548–554 [DOI] [PubMed] [Google Scholar]
- Nielsen I-M, Ornvold K, Jacobsen B, Ranek L (1986) Fatal familial cholestatic syndrome in Greenland Eskimo children. Acta Paediatr Scand 75:1010–1016 [DOI] [PubMed] [Google Scholar]
- Richter A, Rioux JD, Bouchard JP, Mercier J, Mathieu J, Ge B, Poirier J, et al (1999) Location score and haplotype analyses of the locus for autosomal recessive spastic ataxia of Charlevoix-Saguenay in chromosome region 13q11. Am J Hum Genet 64:768–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BH, Oei J, Sherwood WG, Applegarth D, Wong L, Haworth J, Goodyer P, et al (1984) The molecular basis for the two different clinical presentations of classical pyruvate carboxylase deficiency. Am J Hum Genet 36:283–294 [PMC free article] [PubMed] [Google Scholar]
- Schäffer AA (1996) Faster linkage analysis computations for pedigrees with loops or unused alleles. Hum Hered 46:226–235 [DOI] [PubMed] [Google Scholar]
- Schäffer AA, Gupta SK, Shriram K, Cottingham RW Jr (1994) Avoiding recomputation in linkage analysis. Hum Hered 44:225–237 [DOI] [PubMed] [Google Scholar]
- Sheffield VC, Stone EM, Carmi R (1998) Use of isolated inbred human populations for identification of disease genes. Trends Genet 14:391–396 [DOI] [PubMed] [Google Scholar]
- Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, et al (1998) A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet 20:233–238 [DOI] [PubMed] [Google Scholar]
- Terwilliger JD, Ott J (1994) Handbook of human genetic linkage. Johns Hopkins University Press, Baltimore [Google Scholar]
- Toussaint W, Gros H (1966) Familiaerer Icterus durch intrahepatische Cholestase. Dtsch Z Verdau Stoffwechselkr 26:23–31 [PubMed] [Google Scholar]
- Weber AM, Tuchweber B, Yousef I, Brochu P, Turgeon C, Gabbiani G, Morin, et al (1981) Severe familial cholestasis in North American Indian children: a clinical model of microfilament dysfunction? Gastroenterology 81:653–662 [PubMed] [Google Scholar]
- Weiler T, Greenberg CR, Nylen E, Halliday W, Morgan K, Eggertson D, Wrogemann K (1996) Limb-girdle muscular dystrophy and Miyoshi myopathy in an aboriginal Canadian kindred map to LGMD2B and segregate with the same haplotype. Am J Hum Genet 59:872–878 [PMC free article] [PubMed] [Google Scholar]
- Williams CN, Kaye R, Baker L, Hurwitz R, Senior JR (1972) Progressive familial cholestatic cirrhosis and bile acid metabolism. J Pediatr 81:493–500 [DOI] [PubMed] [Google Scholar]