Abstract
The aggressiveness of prostate cancer (PCa) varies widely: some tumors progress to invasive, potentially life-threatening disease, whereas others stay latent for the remainder of an individual’s lifetime. The mechanisms resulting in this variability are not yet understood, but they are likely to involve both genetic and environmental influences. To investigate genetic factors, we conducted a genomewide linkage analysis of 513 brothers with PCa, using the Gleason score, which reflects tumor histology, as a quantitative measure of PCa aggressiveness. To our knowledge, this is the first time that a measure of PCa aggressiveness has been directly investigated as a quantitative trait in a genomewide scan. We employed a generalized multipoint Haseman-Elston linkage-analysis approach that regresses the mean-corrected cross product between the brothers’ Gleason scores on the estimated proportion of alleles shared by brothers identical by descent at each marker location. Our results suggest that candidate regions on chromosomes 5q, 7q, and 19q give evidence for linkage to PCa-aggressiveness genes. In particular, the strongest signals detected in these regions were at the following markers (with corresponding P values): for chromosome 5q31-33, between markers D5S1480 and D5S820 (P=.0002); for chromosome 7q32, between markers D7S3061 and D7S1804 (P=.0007); and, for chromosome 19q12, at D19S433 (P=.0004). This indicates that one or more of these candidate regions may contain genes that influence the progression of PCa from latent to invasive disease. Identification of such genes would be extremely valuable for elucidation of the mechanism underlying PCa progression and for determination of treatment in men in whom this disease has been diagnosed.
Introduction
Prostate cancer is the most common neoplasm among men in the United States: in the year 2000, ∼180,400 men in the United States will receive a diagnosis of prostate cancer (PCa [MIM 176807]), a disease accounting for ∼31,900 deaths annually (Greenlee et al. 2000). This disease will be diagnosed in ∼15% of men in the United States, and the results of autopsy studies suggest that 30% of men of age >45 years may have prostate lesions that are histologically identifiable as PCa (Dhom 1983; Kosary et al. 1995). While a good number of these lesions will remain latent for a man’s lifetime, little is currently known about what makes some PCa biologically aggressive and more likely to progress to metastatic and potentially lethal disease. One compelling possibility is that genetic factors help drive the mechanisms underlying PCa aggressiveness.
A key pathological measure of aggressiveness is the Gleason score (Gleason 1992) assigned to a prostate tumor. The Gleason score reflects the patterns of tissue architecture observed by a pathologist in two prostate biopsy or surgery samples. Each pattern is given a whole-number score between 1 and 5, so the total Gleason-score range is 2–10. For tissue with heterogeneous scores, the maximum two scores are summed to obtain the total score. Low Gleason scores (i.e., 2–4) indicate that the tumor cells are well differentiated and are organized into glandular structures. In contrast, higher scores (i.e., 8–10) signify less differentiation of the tumor cells: they appear solid and dissipate together. Poor differentiation and, hence, a high Gleason score, is a strong prospective gauge of which tumors will penetrate the prostate capsule, invade the seminal vesicles, and spread to the lymph nodes. Therefore, genes linked to Gleason score and, thus, tissue architecture could serve as molecular markers of PCa aggressiveness. In addition, such genes may increase our knowledge about the biological mechanisms underlying the progression of PCa from latent to invasive disease.
Previous work searching for genetic markers of PCa aggressiveness has included loss-of-heterozygosity (LOH) and candidate-gene approaches (Ware 1994; Nupponen and Visakorpi 1999). Genomic regions that have been shown to contain LOH and thus, possibly, tumor-suppressor genes for more-aggressive PCa include those on chromosomes 5, 7, 10, and 16 (Takahashi et al. 1994; Oakahashi 1995; Cunningham et al. 1996; Komiya et al. 1996; Elo et al. 1997; Elo et al. 1999). Other work has detected associations between PCa aggressiveness and variants in numerous candidate genes, including CYP3A4 (Rebbeck et al. 1998; Paris et al. 1999), PTEN (McMenamin et al. 1999), the androgen receptor (Giovannucci et al. 1997), and the vitamin D receptor (Ingles et al. 1997). In contrast with these studies of tumor aggressiveness, linkage analyses have focused on the detection of regions containing susceptibility genes—that is, those genes implicated in the development of PCa. Several regions that have been identified by linkage analysis may contain genes for PCa development at chromosomal locations 1q24-25 (HPC1 [MIM 601518]) (Smith et al. 1996), 1q42.2-43 (HPC2 [MIM 602759]) (Berthon et al. 1998), 1p36 (CAPB [MIM 603688]) (Gibbs et al. 1999), and Xq27-28 (HPCX [MIM 300147]) (Xu et al. 1998). We have recently undertaken a sib-pair genomewide linkage analysis that detected another PCa-susceptibility locus at chromosome 16q (Suarez et al. 2000), a region showing high levels of LOH (Carter et al. 1990a; Elo et al. 1997; Latil et al. 1997; Elo et al. 1999; Paris et al., in press).
To our knowledge, however, no genomewide scan has been done to search for PCa-aggressiveness genes. Therefore, in the present paper, we give results from a linkage analysis of markers located throughout the genome, to determine whether they are inherited along with a predisposition to more-aggressive PCa, as indicated by Gleason score.
Material and Methods
Subjects
Probands were recruited into this study from urology practices in St. Louis, Missouri, and Cleveland, Ohio, as well as from general referrals to the study. The probands’ self-reported family history of PCa was used as a guide for recruitment of their affected brothers. In the present analysis, a total of 513 men from concordant sibships (i.e., two or more brothers with PCa) in which at least two brothers had information available on their Gleason scores were recruited into the study and were genotyped. A total of 189 families had two affected brothers, 41 families had three affected brothers, two families had four affected brothers, and one family had two pairs of affected brothers who were cousins (for the equivalent of 326 sib pairs). In addition to 48 new subjects, this sample includes the 465 men who were used in our recent genomewide scan of susceptibility genes and who had complete Gleason-score information. The median age at diagnosis among these men was 65 years (range 42–91 years). Further details about the original subjects are given in our previous report (Suarez et al. 2000).
We use Gleason score as a measure of tumor aggressiveness, since, as noted above, it is generally considered to be a strong predictor of survival with PCa (Gleason 1992). Our collaborating pathologists ascribed Gleason scores to biopsy specimens and, when such specimens existed, to radical-prostatectomy specimens. For the present analyses, we used the Gleason score from the prostatectomy specimen whenever available (78% of all samples). If it was not available, we used the Gleason score from the biopsy specimen. The institutional review boards of Washington University and University Hospitals of Cleveland approved this study, and all subjects gave informed consent to take part in the project.
Genotyping
DNA was extracted from the study subject’s blood, by use of standard methods, and was sent to the Center for Medical Genetics, Marshfield Medical Research Foundation for genotyping. Samples were typed using the Marshfield Screening Set 9 (Yuan et al. 1997). With use of this screening set, 364 autosomal simple-tandem-repeat polymorphisms, which are spaced at ∼9-cM intervals across the genome (sex-equal maps) and which have an average heterozygosity of 77% (Broman et al. 1998), were genotyped. We used these markers to confirm reported relationships among the sib pairs, using RELTEST (S.A.G.E. 2000). Resulting exclusions in the original data are given elsewhere (Suarez et al. 2000). In the new samples with complete Gleason-score information, we detected one pair of monozygotic twins, which was excluded from our linkage analysis.
Statistical Analysis
For our statistical analysis, we used a multipoint generalized Haseman-Elston (HE) linkage test (Elston et al. 2000; S.A.G.E. 2000). In particular, we used linear regression in which the dependent variable—the mean-corrected cross product (i.e., of Gleason scores) between brothers—is regressed on the estimated proportion of alleles at a particular marker that are shared among brothers identical by descent (IBD), which is denoted as πj for sib pair j. The form of the model is as follows:
where xij is the Gleason score for individual i in sib pair j and where μ is the population mean of all Gleason scores. Since Gleason scores only exist for men with PCa, we estimated μ from the mean Gleason score in our sample. The expected value of the dependent variable is equal to the sibling covariance. In the presence of linkage, sib pairs that share the region—that is, those sib pairs with high πj values—are expected to have highly correlated Gleason scores, which imply high sib covariance. In contrast, sib pairs that do not share the region—that is, those sib pairs with low πj values—are expected to have less-correlated Gleason scores and low sib covariance. The regression coefficient β can be written as follows:
where θ is the recombination fraction and where σ2g equals the total genetic variance (Elston et al. 2000). From (2) we see that, in the presence of tight linkage (i.e., θ≅0), β estimates the genetic variance. Thus, values of β that are statistically significantly >0 suggest linkage. The intercept α equals the residual sibling covariance. S.A.G.E. (2000) uses a generalized least-squares approach to fit model (1), estimating a covariance matrix that accounts for the correlation among the residuals.
We also reanalyzed the data by use of the original HE approach, which regresses the squared difference of brothers’ Gleason scores on the estimated proportion of alleles shared IBD (Haseman and Elston 1972; S.A.G.E. 2000). This approach has been widely used in the past, and, although it is generally less powerful than the new HE approach, there are situations where it is more powerful than the new HE method (Palmer et al. 2000).
Because of the pathological criteria used in the assignment of Gleason scores, unit increases in scores might not represent equidistant changes in cellular differentiation. For example, the increase in Gleason score from 5 to 6 likely represents a smaller change in differentiation than does an increase from 6 to 7. This is because the two values resulting in a Gleason score of 7 are usually 3 and 4, and a value of 4 is given only if there is relatively poor tumor differentiation. Instead of the ordinal ranking of Gleason scores used here, one could instead combine scores into the following four groups, to indicate similar levels of differentiation: (a) 2–4 (well differentiated); (b) 5 and 6 (moderately differentiated); (c) 7 (less moderately differentiated); and (d) 8–10 (poorly differentiated) (Sakr and Grignon 1997; Stanford et al. 1999). Therefore, we repeated our analyses by combining Gleason scores in this manner, treating the midpoints of the categories (i.e., 3, 5.5, 7, and 9) as a continuous variable.
For sibships with more than two brothers, we assumed that all pairs were independent, since estimates of πj are pairwise independent under the null hypothesis of no linkage (Hodge 1984). Allele frequencies were calculated by random selection of one individual from each family.
Results
The median Gleason score among the men in the present study was 6 (range 2–10). The frequency distribution of Gleason scores was as follows: Gleason 2 (1%), Gleason 3 (9%), Gleason 4 (7%), Gleason 5 (26%), Gleason 6 (29%), Gleason 7 (20%), Gleason 8 (4%), Gleason 9 (3%), and Gleason 10 (.2%).
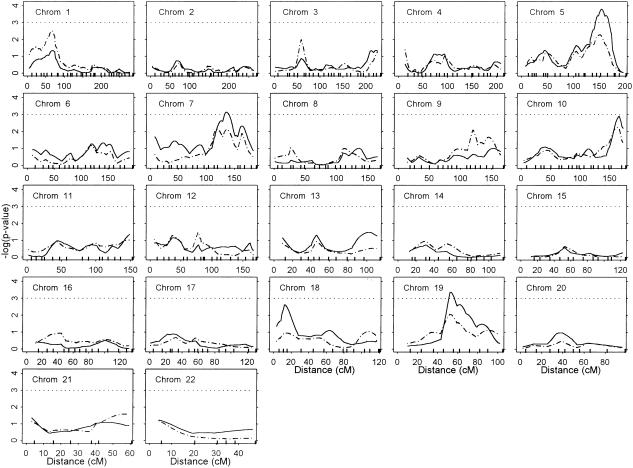
Figure 1 presents the multipoint linkage results from the new and original HE approaches across all chromosomes (except the X and Y chromosomes). The vertical axis of each chromosome’s plot gives the −log(P value) from the t test of the departure of β from zero, with 324 df (number of affected sib pairs [326] minus the number of parameters estimated [2—that is, α and β]) (S.A.G.E. 2000). The dotted horizontal lines indicate where P<.001 (−log[.001]=3). With use of the new HE approach, we found evidence (P<.001) for linkage with Gleason score in three regions on chromosomes 5q, 7q, and 19q. On chromosome 5q, we observed a relatively broad region (∼26 cM in length) within which P<.001 was maintained and a peak (P=.0002) approximately halfway between markers D5S1480 (5q31.3) and D5S820 (5q33.3). For chromosome 7q, P<.001 for an ∼8-cM region, with a peak (P=.0007) ∼2 cM centromeric to D7S1804 (7q32.3). On chromosome 19q, P<.001 was observed across ∼5 cM, with a peak (P=.0004) at D19S433 (19q12). In addition to these three regions, chromosomes 10 and 18 exhibited intriguing, albeit statistically weaker, peaks at D10S1248 (P=.0012) and 2 cM centromeric to D18S976 (P=.0024).
Figure 1.
Results from a genomewide scan of PCa-aggressiveness genes; Gleason score was used as a quantitative trait. Unbroken lines denote results from the new HE analysis; broken lines denote results from the original HE analysis. Tick marks across horizontal axes indicate marker locations (in cM) (as specified on the Center for Human Genetics, Marshfield Medical Research Foundation Web site). P values are from a t test of the HE regression coefficient, with 324 df (number of affected sib pairs [326] minus the number of parameters [2]). Dotted horizontal lines indicate where P=.001 (−log[P] = 3); thus, for peaks above these lines, P<.001.
The original HE approach indicated potential regions of linkage similar to those seen with the new HE approach, although generally with statistically weaker results (fig. 1, broken lines). In particular, for the three chromosomal regions demonstrating linkage with P<.001, the original HE approach gave the following peak P values: chromosome 5q, P=.0053; chromosome 7q, P=.0076; and chromosome 19q, P=.0088. This result is not surprising, in light of the increased power that is potentially available with use of the new HE approach; this increased power is especially apparent when the residual correlation is limited (Elston et al. 2000; Palmer et al. 2000). With use of the original HE approach, chromosomes 1p and 9q also demonstrated slight linkage to Gleason score (P<.01), with peaks between D1S1622 and D1S3721 (P=.003) and at D9S930 (P=.008).
Table 1 gives additional details for the Gleason-score linkage regions on chromosomes 5q, 7q, and 19q. In particular, for markers within and bordering these regions, intermarker spacing, regression coefficients from the new HE analysis (plus their standard errors), and corresponding P values are presented. The regression coefficients indicate that, in each region, the genetic variance accounted for by the linkage is slightly greater than one Gleason-score unit. Furthermore, low P values extend across relatively broad regions on these chromosomes, providing additional support for the presence of genes that influence the aggressiveness of PCa (Terwilliger et al. 1997).
Table 1.
Multipoint Results from the New HE Analysis, for Regions on Chromosomes 5q, 7q, and 19q That Show Linkage with Gleason Score (i.e., with Peak P<.001).
| Chromosome and Markera | Intermarker Distancesb (cM) | βc | Standard Error of β | Pd |
| 5q: | ||||
| D5S816 | 8.16 | 1.11 | .40 | .0025 |
| D5S1480 | 12.28 | 1.32 | .39 | .0004 |
| D5S820 | 12.36 | 1.37 | .41 | .0004 |
| D5S1471 | 1.06 | .40 | .0046 | |
| 7q: | ||||
| D7S3061 | 8.54 | 1.12 | .40 | .0026 |
| D7S1804 | 12.95 | 1.21 | .38 | .0008 |
| D7S1824 | .80 | .39 | .0190 | |
| 19q: | ||||
| D19S714 | 9.60 | .26 | .44 | .2781 |
| D19S433 | 6.81 | 1.37 | .41 | .0004 |
| D19S245 | 9.39 | 1.14 | .41 | .0029 |
| D19S178 | .94 | .39 | .0085 |
All markers for which P<.01 or that border the peaks are shown and are listed centromeric to telomeric from top to bottom.
Spacing between contiguous markers in genome scan. Distances shown are between the corresponding marker and the marker listed immediately below.
Regression coefficient from HE model (1).
From a t test with 324 df (number of affected sib pairs [326] minus number of parameters [2]).
When the four groups of Gleason scores reflecting tumor differentiation were used, the minimum (new) HE P values indicated slightly more support for linkage on chromosome 5q31.3-33.3 but somewhat less evidence for linkage on chromosomes 7q32.3 and 19q12. In particular, for chromosome 5q, the strongest signal now gave P=.00008, with the peak shifted 4 cM telomeric to the original peak. For chromosome 7q, P=.0048 (shifted 2 cM centromeric to the original peak). For chromosome 19q, peak P=.013 (same location). The larger P values for chromosomes 7q and 19q are not necessarily unexpected, because combining scores reduces variation crucial to the power of our linkage analysis. Even with this recoding of the Gleason scores, our results continue to suggest linkage to three regions that might contain PCa-aggressiveness genes. Use of different allele frequencies (e.g., equal across all markers) did not materially alter our results (not shown).
Discussion
The results of the present study give evidence (P<.001) that loci on chromosomes 5q31.3-33.3, 7q32.3, and 19q12 might contain genes linked to Gleason score, which is a measure of PCa aggressiveness. Recent LOH and candidate-gene work lends some additional support to the possibility that PCa-aggressiveness genes reside within or near these candidate regions. An association between LOH and tumor-node-metastasis stage has been detected on the border of the chromosome 5q candidate region (Cunningham et al. 1996). The candidate gene α-catenin is also located near this region. This gene is part of the E-cadherin pathway and has been associated with PCa aggressiveness in numerous studies (Ewing et al. 1995; Richmond et al. 1997; Umbas et al. 1997; Morita et al. 1999). LOH in PCa has also been reported in a region (chromosome 7q31.1) near the candidate locus that we have detected on chromosome 7q (Zenklusen et al. 1994; Latil et al. 1995; Cunningham et al. 1996). Recent work has also found associations between LOH and Gleason score on chromosome 7 (Takahashi et al. 1994; Oakahashi 1995). To our knowledge, there has been no LOH or candidate-gene work near the linkage region on chromosome 19.
In addition to primary linkage on chromosomes 5q, 7q, and 19q, we detected weaker (.001<P<.01) signals on chromosomes 1p, 9q, 10q, and 18p. The chromosome 1p region was also linked to PCa susceptibility in our earlier work (Suarez et al. 2000) among those with a positive history of breast cancer, and it is in close proximity to the CAPB locus (Gibbs et al. 1999). If there is a single gene driving all of these results, CAPB may also contribute to PCa aggressiveness. For chromosomes 9q, 10q, and 18p, the peak regions are relatively narrow, primarily reflecting linkage only between the peak markers and Gleason score (fig. 1).
Although none of the results reported in the present study fulfill the alpha-level criterion for “significant” linkage (Lander and Kruglyak 1995), the magnitude of the results may simply reflect sample-size issues in our sib-pair study as well as the potential for numerous genetic and environmental factors interacting in the multistep progression of PCa from latent to aggressive disease (Witte et al. 1996).
This multistep process—whereby numerous malignant events are required for a normal cell to transform into a malignant cancer cell (Carter et al. 1990b)—provides the biological rationale for why a gene might be linked to PCa aggressiveness but not to PCa development. The multiple steps appear to be driven by different regulating factors that distinguish which cancers progress to advanced, lethal disease (Isaacs et al. 1995; Hayward et al. 1998). More specifically, some factors may initiate the development of PCa, whereas others may affect progression from relatively indolent to invasive disease. In the present study, we have focused on genetic loci that may regulate one of the latter steps in PCa: progression to aggressive disease. More specifically, since Gleason score reflects the potential progression of PCa to metastatic and lethal disease, the regions detected in this report could harbor tumor-aggressiveness genes. In contrast, previous linkage analyses of PCa have searched for chromosomal regions that may contain genes that regulate an earlier step in the process: initiation of disease. For example, we have previously detected a region on chromosome 16q23 that may harbor a PCa-susceptibility gene (Suarez et al. 2000). Since the steps for initiation and progression may be unique, it is not surprising that mostly different loci were detected in our present and previous reports. While the discovery of susceptibility genes will be valuable for screening purposes, aggressiveness genes will provide important information about the most-appropriate treatment among men in whom PCa has already been diagnosed. Since most of the men (78%) we studied had radical prostatectomies, any aggressiveness genes within the loci reported here would be primarily relevant to men with localized PCa diagnosed at a younger age (i.e., those men amenable to having a prostatectomy). Nevertheless, such patients with PCa constitute a large proportion of men with the disease who need important information about the most adequate course of treatment.
A benefit of most of our subjects having undergone prostatectomies is that the resulting specimens provide the most accurate measure of Gleason score. In contrast, a Gleason score from a needle-biopsy specimen may underestimate the true score. Differential misclassification of Gleason scores between siblings—arising from their having different treatments and, thus, different specimens—will, on average, decrease the magnitude and statistical significance of the HE regression coefficients (Ott 1999). To increase the coefficient’s magnitude and statistical significance, the degree of Gleason-score misclassification would have to be related to the brothers' IBD sharing at the corresponding marker allele. We have investigated the impact of misclassification by statistically adjusting our linkage analysis for whether sibling pairs were concordant or discordant for PCa treatment (66% of the pairs were concordant). In particular, in a subsequent linkage analysis, we have used the conventional adjustment approach of inclusion of a covariate indicating treatment concordance (or discordance) between pairs of siblings. This adjustment left our results fundamentally unchanged, and we observed no linkage between PCa treatment and IBD sharing of marker alleles at the putative aggressiveness loci. Therefore, any misclassification resulting from different types of specimens used in the measurement of Gleason score do not appear to have affected our findings. Furthermore, any misclassification would lead to our results underestimating the actual linkage. Another potential source of misclassification is the fact that a number of different pathologists assigned Gleason scores to the specimens. Our past experience reviewing thousands of cases of prostate cancer indicates that the scores are reproducible 70% of the time and that they otherwise disagree by no more than one unit. More importantly, it is inconceivable that misclassification by different pathologists would be associated with the brothers' IBD sharing of marker alleles at the aggressiveness loci. Therefore, any misclassification of the brothers' Gleason scores arising from different pathologists would, at most, lead to a limited underestimation of the true linkage.
In light of the expansion of prostate-specific–antigen (PSA) screening during part of this project’s recruitment period (i.e., in the early to mid-1990s), the potential impact of this diagnostic tool on our results merits consideration. Our primary concern is that, within brother pairs, earlier detection resulting from PSA screening could result in one brother having a Gleason score that is lower than that which would have been observed prior to the widespread use of PSA tests. Therefore, any bias resulting from PSA screening will reflect whether the brothers' diagnoses were made within concordant or discordant PSA-screening eras (i.e., pre- and post-PSA eras) and whether PSA era is linked to the brothers' IBD sharing of alleles. We investigated this possibility by undertaking an adjusted linkage analysis that incorporated a covariate indicating whether they both had been given a diagnosis in the same PSA era, using 1994 as a cutoff point for when PSA was fully established as a screening tool (63% were concordant for PSA-screening era). We observed no notable differences in the linkage peaks and found that PSA-screening era is not linked to IBD sharing of alleles at these aggressiveness loci. (Using 1992 as a cutoff point for establishment of PSA screening gave similar results.) This indicates that the introduction of PSA testing does not appear to have had any impact on our linkage results.
In summary, our novel genomewide scan of Gleason score has detected loci on chromosomes 5q31.3-33.3, 7q32.3, and 19q12 that might harbor PCa-aggressiveness genes. Detection of such genes may provide important insights into the underlying mechanism driving the progression of PCa from latent to invasive disease. Moreover, knowledge about aggressiveness genes could help guide treatment plans among men with diagnosed PCa.
Acknowledgments
We thank Alethia Paradis, Sandy Stadnick, Kristi Freese, and Cindy Sebrasky, for helping coordinate this study, and Kevin Jacobs, for his technical help with S.A.G.E. This work was supported in part by grants from the Urologic Research Foundation (to J.S.W., B.K.S., J.K.B., and W.J.C.); National Institutes of Health grants CA73270 (to J.S.W.), MH31302 (to B.K.S.), GM28356 (to R.C.E.), RR03655 (to R.C.E.), HV48141 (to J.L.W.), and CA50515 (to W.J.C.); and U.S. Army grant DAMD17-98-1-8589 (to J.S.W.).
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://www.marshmed.org/genetics/ (for all markers used here)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for PCa [MIM 176807], HPC1 [MIM 601518], HPC2 [MIM 602759], CAPB [MIM 603688], and HPCX [MIM 300147])
References
- Berthon P, Valeri A, Cohen-Akenine A, Drelon E, Paiss T, Wöhr G, Latil A, et al (1998) Predisposing gene for early-onset prostate cancer, localized on chromosome 1q42.2-43. Am J Hum Genet 62:1416–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman KW, Murray JC, Sheffield VC, White RL, Weber JL (1998) Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am J Hum Genet 63:861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BS, Ewing CM, Ward WS, Treiger BF, Aalders TW, Schalken JA, Epstein JL, et al (1990a) Allelic loss of chromosomes 16q and 10q in human prostate cancer. Proc Natl Acad Sci 87:8751–8755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter HB, Piantadosi S, Isaacs JT (1990b) Clinical evidence for and implications of the multistep development of prostate cancer. J Urol 143:742–746 [DOI] [PubMed] [Google Scholar]
- Cunningham JM, Shan A, Wich MJ, McDonnell SK, Schaid DJ, Tester DJ, Qian J, et al (1996) Allelic imbalance and microsatellite instability in prostatic adenocarcinoma. Cancer Res 56:4475–4482 [PubMed] [Google Scholar]
- Dhom G (1983) Epidemiologic aspects of latent and clinically manifest carcinoma of the prostate. J Cancer Res Clin Oncol 106:210–218 [DOI] [PubMed] [Google Scholar]
- Elo JP, Harkonen P, Kyllonen AP, Lukkarinen O, Poutanen M, Vihko R, Vihko P (1997) Loss of heterozygosity at 16q24.1-q24.2 is significantly associated with metastatic and aggressive behavior of prostate cancer. Cancer Res 57:3356–3359 [PubMed] [Google Scholar]
- Elo JP, Harkonen P, Kyllonen AP, Lukkarinen O, Vihko P (1999) Three independently deleted regions at chromosome arm 16q in human prostate cancer: allelic loss at 16q24.1-q24.2 is associated with aggressive behaviour of the disease, recurrent growth, poor differentiation of the tumor and poor prognosis for the patient. Br J Cancer 79:156–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston RC, Buxbaum S, Jacobs KB, Olson JM (2000) Haseman and Elston revisited. Genet Epidemiol (in press) [DOI] [PubMed] [Google Scholar]
- Ewing CM, Ru N, Morton RA, Robinson JC, Wheelock MJ, Johnson KR, Barrett JC, et al (1995) Chromosome 5 suppresses tumorigenicity of PC3 prostate cancer cells: correlation with re-expression of α-catenin and restoration of E-caherin function. Cancer Res 55:4813–4817 [PubMed] [Google Scholar]
- Gibbs M, Stanford JL, McIndoe RA, Jarvik GP, Kolb S, Goode EL, Chakrabarti L, et al (1999) Evidence for a rare prostate cancer–susceptibility locus at chromosome 1p36. Am J Hum Genet 64:776–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Stampfer MJ, Krithivas K, Brown M, Dahl D, Brufsky A, Talcott J, et al (1997) The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci 94:3320–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason DF (1992) Histologic grading of prostate cancer: a perspective. Hum Pathol 23:273–279 [DOI] [PubMed] [Google Scholar]
- Greenlee RT, Murray T, Bolden S, Wingo PA (2000) Cancer statistics, 2000. CA Cancer J Clin 50:7–33 [DOI] [PubMed] [Google Scholar]
- Haseman JK, Elston RC (1972) The investigation of linkage between a quantitative trait and a marker locus. Behav Genet 2:3–19 [DOI] [PubMed] [Google Scholar]
- Hayward SW, Grossfeld GD, Tlsty TD, Cunha GR (1998) Genetic and epigenetic influences in prostatic carcinogenesis. Int J Oncol 13:35–47 [PubMed] [Google Scholar]
- Hodge SE (1984) The information contained in multiple independent sibling pairs. Genet Epidemiol 1:109–122 [DOI] [PubMed] [Google Scholar]
- Ingles SA, Ross RK, Yu MC, Irvine RA, La Pera G, Haile RW, Coetzee GA (1997) Association of prostate cancer risk with genetic polymorphisms in vitamin D receptor and androgen receptor. J Natl Cancer Inst 89:166–170 [DOI] [PubMed] [Google Scholar]
- Isaacs WB, Bova GS, Morton RA, Bussemakers MJ, Brooks JD, Ewing CM (1995) Molecular biology of prostate cancer progression. Cancer Surv 23:19–32 [PubMed] [Google Scholar]
- Komiya A, Suzuki H, Ueda T, Yatani R, Emi M, Ito H, Shimazaki J (1996) Allelic losses at loci on chromosome 10 are associated with metastasis and progression of human prostate cancer. Genes Chromosomes Cancer 17:245–253 [DOI] [PubMed] [Google Scholar]
- Kosary CL, Ries LAG, Miller BA, Hankey BF, Harras A, Edwards BK (1995) SEER cancer statistics review, 1973–1991: tables and graphs. NIH pub. 96-2789. National Cancer Institute, Bethesda, MD [Google Scholar]
- Lander E, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11:241–247 [DOI] [PubMed] [Google Scholar]
- Latil A, Cussenot O, Fournier G, Baron JC, Lidereau R (1995) Loss of heterozygosity at 7q31 is a frequent and early event in prostate cancer. Clin Cancer Res 1:1385–1389 [PubMed] [Google Scholar]
- Latil A, Cussenof O, Fournier G, Driouch K, Lidereau R (1997) Loss of heterozygosity at chromosome 16q in prostate adenocarcinoma: identification of three independent regions. Cancer Res 57:1058–1062 [PubMed] [Google Scholar]
- McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR (1999) Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res 59:4291–4296 [PubMed] [Google Scholar]
- Morita N, Uemura H, Tsumatani K, Cho M, Hirao Y, Okajima E, Konishi N, et al (1999) E-cadherin and α-, β- and γ-catenin expression in prostate cancers: correlation with tumour invasion. Br J Cancer 79:1879–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nupponen N, Visakorpi T (1999) Molecular biology of progression of prostate cancer. Eur Urol 35:351–354 [DOI] [PubMed] [Google Scholar]
- Ott J (1999) Analysis of human genetic linkage, 3d ed. Johns Hopkins University Press, Baltimore, p 243 [Google Scholar]
- Palmer LJ, Jacobs KB, Elston RC (2000) Haseman and Elston revisited: the effects of ascertainment and residual familial correlations on power to detect linkage. Genet Epidemiol (in press) [DOI] [PubMed] [Google Scholar]
- Paris PL, Kupelian PA, Hall JM, Williams TL, Levin H, Klein EA, Casey G, et al (1999) Association between a CYP3A4 genetic variant and clinical presentation in African American prostate cancer patients. Cancer Epidemiol Biomarkers Prev 8:901–905 [PubMed] [Google Scholar]
- Paris PL, Witte JS, Kupelian PA, Levin H, Klein EA, Catalona W, Casey G. Identification and fine mapping of a region showing a high frequency of allelic instability on chromosome 16q23.2 that corresponds to a prostate cancer susceptibility locus. Cancer Res (in press) [PubMed] [Google Scholar]
- Rebbeck TR, Jaffe JM, Walker AH, Wein AJ, Malkowicz SB (1998) Modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J Natl Cancer Inst 90:1225–1229 [DOI] [PubMed] [Google Scholar]
- Richmond PJ, Karayiannakis AJ, Nagafuchi A, Kaisary AV, Pignatelli M (1997) Aberrant E-cadherin and α-catenin expression in prostate cancer: correlation with patient survival. Cancer Res 57:3189–3193 [PubMed] [Google Scholar]
- SAGE (2000) Statistical analysis for genetic epidemiology, release 4.0. Department of Epidemiology and Biostatistics, Rammelkamp Center for Education and Research, MetroHealth Campus, Case Western Reserve University, Cleveland [Google Scholar]
- Sakr WA, Grignon DJ (1997) Prostate cancer: indicators of aggressiveness. Eur Urol 32(Suppl 3):15–23 [PubMed] [Google Scholar]
- Smith JR, Freije D, Carpten JD Grönberg H, Xu J, Isaccs SD, Brownstein MJ, et al (1996) Major susceptibility locus for prostate cancer on chromosome 1 suggested by a genome-wide search. Science 274:1371–1374 [DOI] [PubMed] [Google Scholar]
- Stanford JL, Stephenson RA, Coyle LM, Cerhan J, Correa R, Eley JW, Gilliland F, et al (1999) Prostate cancer trends 1973–1995, SEER Program, National Cancer Institute. NIH pub. 99-4543. National Institutes of Health, Bethesda, MD [Google Scholar]
- Suarez BK, Lin J, Burmester JK, Broman KW, Weber JL, Banerjee TK, Goddard KA, et al (2000) A genome screen of muliplex sibships with prostate cancer. Am J Hum Genet 66:933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Qian J, Brown JA, Alcaraz A, Bostwick DG, Lieber MM, Jenkins RB (1994) Potential markers of prostate cancer aggressiveness detected by fluorescence in situ hybridization in needle biopsies. Cancer Res 54:3574–3579 [PubMed] [Google Scholar]
- Takahashi S, Shan AL, Ritland SR, Delacey KA, Bostwick DG, Lieber MM, Thibodeau SN, et al (1995) Frequent loss of heterozygosity at 7q31.1 in primary prostate cancer is associated with tumor aggressiveness and progression. Cancer Res 55:4114–4119 [PubMed] [Google Scholar]
- Terwilliger JD, Shannon WD, Lathrop GM, Nolan JP, Goldin LR, Chase GA, Weeks DE (1997) True and false positive peaks in genomewide scans: applications of length-based sampling to linkage mapping. Am J Hum Genet 61:430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbas R, Isaacs WB, Bringuier PP, Xue Y, Debruyne FM, Schalken JA (1997) Relation between aberrant α-catenin expression and loss of E-cadherin function in prostate cancer. Int J Cancer 74:374–377 [DOI] [PubMed] [Google Scholar]
- Ware JL (1994) Prostate cancer progression: implications of histpathology. Am J Pathol 145:983–993 [PMC free article] [PubMed] [Google Scholar]
- Witte JS, Elston RC, Schork NJ (1996) Genetic dissection of complex traits. Nat Genet 12:355–356 [DOI] [PubMed] [Google Scholar]
- Xu J, Meyers D, Freije D, Isaacs S, Wiley K, Nusskern D, Ewing C, et al (1998) Evidence for a prostate cancer susceptibility locus on the X chromosome. Nat Genet 20:175-179 [DOI] [PubMed] [Google Scholar]
- Yuan B, Vaske D, Weber JL, Beck J, Sheffield VC (1997) Improved set of short-tandem-repeat polymorphisms for screening the human genome. Am J Hum Genet 60:459–460 [PMC free article] [PubMed] [Google Scholar]
- Zenklusen JC, Thompson JC, Troncoso P, Kagan J, Conti CL (1994) Loss of heterozygosity in human primary prostate carcinomas: a possible tumor suppressor gene at 7q31.1. Cancer Res 54:6370–6373 [PubMed] [Google Scholar]