Abstract
We have studied 23 children from 13 families with a clinical diagnosis of Aicardi-Goutières syndrome. Affected individuals had developed an early-onset progressive encephalopathy that was characterized by a normal head circumference at birth, basal ganglia calcification, negative viral studies, and abnormalities of cerebrospinal fluid comprising either raised white cell counts and/or raised levels of interferon-α. By means of genomewide linkage analysis, a maximum-heterogeneity LOD score of 5.28 was reached at marker D3S3563, with α=.48, where α is the proportion of families showing linkage. Our data suggest the existence of locus heterogeneity in Aicardi-Goutières syndrome and highlight potential difficulties in the differentiation of this condition from pseudo-TORCH (toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus types 1 and 2) syndrome.
In 1984, Jean Aicardi and Françoise Goutières described eight children with an early-onset progressive encephalopathy characterized by basal ganglia calcification, leukodystrophy, chronic cerebrospinal fluid (CSF) lymphocytosis, and negative serological investigations for common prenatal infections (Aicardi-Goutières syndrome [MIM 225750]; Aicardi and Goutières 1984). The observation of familial cases, affected females, and parental consanguinity prompted the authors to propose that the condition was inherited as an autosomal recessive trait. Further reports (Giroud et al. 1986; Mehta et al. 1986; Diament et al. 1986; Bönnemann and Meinecke 1992; Tolmie et al. 1995; Verrips et al. 1997; Kumar et al. 1998; McEntagart et al. 1998; Goutières et al. 1998; Østergaard et al. 1999) have supported this conclusion. These findings, taken together with the identification of families with affected siblings who are separated in age by a number of years and in birth order by intervening normal children, challenge the possibility that Aicardi-Goutières syndrome could result solely as a consequence of occult embryopathic infection. However, it was the resemblance of the clinical phenotype of Aicardi-Goutières syndrome to the sequelae of congenital infection that led to the measurement of interferon-α (IFN-α) in children with the disease (Lebon et al. 1988). A report of raised levels of CSF IFN-α in 14/15 patients with Aicardi-Goutières syndrome suggests a close association between this parameter and the other clinical and laboratory features (Goutières et al. 1998), such that an elevation of CSF IFN-α in the absence of infection is currently considered to be a marker for the condition (Goutières 2000).
Intrafamilial phenotypic variability was recognized in the initial description of Aicardi-Goutières syndrome and has since been confirmed (Aicardi and Goutières 1984; Goutières et al. 1998). Patients with apparently static or slowly progressive encephalopathy are on record (Verrips et al. 1997; McEntagart et al. 1998), and, importantly, discordance for the presence of CSF pleocytosis has been described (Østergaard et al. 1999). These reports raise fundamental questions regarding the definition of Aicardi-Goutières syndrome and its distinction from pseudo-TORCH (toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus types 1 and 2) syndrome (MIM 251290) (Baraitser et al. 1983; Ishitsu et al. 1985; Burn et al. 1986; Bolthauser et al. 1991; Reardon et al. 1994; Monastiri et al. 1997; al-Dabbous et al. 1998). Both conditions share an early-onset encephalopathy with basal ganglia calcification, leukoencephalopathy, and brain atrophy. They are said to differ because of the presence, in pseudo-TORCH syndrome, of congenital microcephaly, neonatal disturbance of liver function, thrombocytopenia, and a normal CSF examination, although this latter finding has only been reported in a minority of described cases. However, in a recent review of 27 patients with Aicardi-Goutières syndrome, two children showed a prenatal microcephaly, one exhibited a neonatal thrombocytopenia, two had hepatosplenomegaly, and three had transient elevation of liver enzymes, with one child undergoing liver biopsy (Goutières et al. 1998). These findings, together with the observations of Østergaard et al. (Østergaard et al. 1999), the absence of CSF IFN-α measurements in patients described with pseudo-TORCH syndrome, and the identification of cases showing clear overlap between that disease and Aicardi-Goutières syndrome (Stephenson et al. 1997), suggest the possibility that these clinical phenotypes may represent a single disorder.
In the present study, we report the results of linkage studies in 23 children from 13 families who were affected with Aicardi-Goutières syndrome. Our results demonstrate significant linkage to chromosome 3p21 in a proportion of the families and suggest the existence of at least one additional disease locus.
All affected singletons and at least one sibling from every family in which more than one child was affected fulfilled inclusion criteria by showing: (1) a progressive neurological disorder with onset in the first year of life; (2) a normal head circumference at birth; (3) calcification involving the basal ganglia and sometimes extending to the white matter; (4) a CSF pleocytosis (>5 cells/mm3) and/or a raised level of CSF IFN-α (>2 IU/liter); and (5) negative TORCH studies. Details for all subjects are given in table 1, and clinical descriptions of five of these families (families 2 and 8–11) have been published elsewhere (Tolmie et al. 1995; Stephenson et al. 1997; Verrips et al. 1997; Kumar et al. 1998; McEntagart et al. 1998). Families 1–3 and 8–11 were genotyped in an initial genome search, and the remaining families were analyzed with markers in regions of interest.
Table 1.
Clinical Characteristics of 23 Affected Individuals
|
Occiptiofrontal Circumferencea |
|||||||
| Family/ Patient | Age at Presentation (mo) | At Birth (Gestational age) | After Birth (Age) | Brain Calcificationb | White-Matter Hypodensitiesc | CSF WCCd/mm3 [% Lymphocytes] (Age) | IFN-α IU/liter in [CSF/serum]e (Age) |
| 1/V:1 | 4 | 30 cm (36 wk) | 37.5 cm (6 mo) | BG, DN, WM | + | 60 [50] (6 mo) | NA |
| 1/V:2 | 4 | 32.5 cm (38 wk) | 40 cm (36 mo) | BG, WM | Unknown | 52 (1 mo) | NA |
| 2/V:1 | 1 | 33.5 cm (37 wk) | −4 SD (12 mo) | BG (trace) | Minimal | <5 (1 mo) | 200 IU/liter [NA] (1 mo) |
| 2/V:2 | 1 | 34 cm (40 wk) | −2 SD (6 mo) | BG | + | <5 (6 mo) | 12 IU/liter [25 IU/liter] (6 mo) |
| 3/IV:1 | 1 | “Normal” | 42 cm (14 mo) | BG, DN, WM | +f | <5 (12 mo and 18 mo) | 50 IU/liter [50 IU/liter] (12 mo) |
| 4/II:1 | 1 | 33 cm (37 wk) | 41.5 cm (11 mo) | Not on MRI | + | NA | NA |
| 4/II:2 | 1 | 35 cm (40 wk) | 43 cm (10 mo) | BG, DN | Unknown | 27 (6 mo) | 100 IU/liter [NA] (6 mo) |
| 5/V:1 | 4 | 36 cm (40 wk) | 41 cm (12 mo) | BG | + | 38 [40] (3 mo); 27 [60] (8 mo) | 50 IU/liter [200 IU/liter] (3 mo) |
| 6/II:1 | <1 | 35 cm (40 wk) | 45 cm (93 mo) | BG, WM | Unknown | 9 [70] (60 mo) | 6 IU/liter [NK] (60 mo) |
| 6/II:2 | <1 | 34 cm (40 wk) | 41 cm (46 mo) | WM | + | 23 (2 mo); 52 [70] (4 mo); 14 [80] (24 mo) | 9 IU/liter [NK] (24 mo) |
| 7/II:1 | 2 | 35.6 cm (40 wk) | 46 cm (42 mo) | BG, WM | + | 54 [99] (2 mo) | NA |
| 7/II:2 | 1 | 35 cm (40 wk) | 40.8 cm (5 mo) | BG, DN | + | 17 [67] (13 mo) | NA |
| 8/IV:1 | 8 | “Normal” | 49 cm (60 mo) | BG | + | 14 (13 mo) | NA |
| 8/IV:2 | 12 | “Normal” | 52 cm (36 mo) | BG | + | 550 [75] (30 mo) | NA |
| 9/IV:2 | 8 | “Normal” | 40.9 cm (9 mo) | BG | Unknown | 0 (84 mo) | <2 IU/liter [<2 IU/liter] (84 mo) |
| 9/IV:3 | 6 | 50th percentile (34 wk) | 41.5 cm (9 mo) | BG | Unknown | 4 (11 mo) | 3 IU/liter [<2 IU/liter] (11 mo) |
| 10/IV:1 | 7 | 35.5 cm (40 wk) | 54 cm (108 mo) | BG | + | 16 (12 mo); 8 (108 mo) | <2 IU/liter [<2 IU/liter] (108 mo) |
| 10/IV:3 | <12 | 34.5 cm (40 wk) | 47 cm (21 mo) | BG | + | 13 (12 mo); 13 (21 mo) | 25 IU/liter [<2 IU/liter] (21 mo) |
| 11/II:1 | 1 | 33 cm (40 wk) | −6 SD (36 mo) | BG, WM | + | 25 (10 mo) | 25 IU/liter [50 IU/liter] (10 mo) |
| 11/II:2 | 2 | 34 cm (40 wk) | 45.5 cm (22 mo) | BG, DN | Not obvious | 10 (2 mo) | 37 IU/liter [12 IU/liter] (2 mo) |
| 12/II:2 | <1 | 34 cm (40 wk) | <.4th percentile (6 mo) | BG, WM | + | 60 [70] (2 mo) | NA |
| 13/II:1 | 6 | 38 cm (40 wk) | −1 SD (60 mo) | BG | Unknown | 38 (6 mo) | 25 IU/liter [NA] (6 mo) |
| 13/II:2 | 2 | 35 cm (40 wk) | −4 SD (12 mo) | BG, WM | Unknown | 44 (2 mo) | NA |
SD = standard deviations below mean.
BG = basal ganglia; DN = dentate nuclei; WM = white matter; MRI = magnetic-resonance imaging.
A plus sign (+) denotes presence.
WCC = white cell count.
NA = not assessed.
Marked.
The Cooperative Human Linkage Center Human Screening Set/Weber version 8 (Research Genetics) was used in an autosomal genomewide search. This panel contains 386 microsatellite repeat markers spaced at ∼10-cM intervals, with an average heterozygosity of .76. PCR amplification of all markers was performed according to the manufacturer's specifications, by use of a Roboseq 4200 (MWG BioTech). Amplified markers were pooled and electrophoresed on an ABI Prism 377 gene sequencer (PE Biosystems) with 4.2% polyacrylamide gels at 3,000 V and 52°C for 2.5 h. Fragment-length analysis was undertaken with the use of the ABI Prism GENESCAN and GENOTYPER 1.1.1 analysis packages. For fine mapping on chromosome 3p, five polymorphic markers were selected from the Genome Database: tel-D3S1768-D3S3527-D3S3559-D3S3563-D3S1289-cen. Information regarding marker order and relative distances was obtained from linkage maps from the Center for Medical Genetics, Marshfield Medical Research Foundation. An autosomal recessive mode of inheritance with 95% penetrance was assumed. The disease-allele frequency was estimated to be 1/500. For markers used in the initial genome screen, equal allele frequencies were calculated on the basis of the observed number of alleles with a lower frequency set at .1. For further analysis of the candidate region, marker frequencies were calculated from transmitted and nontransmitted parental alleles with a minimum frequency set at .1. Pedigree-allele inconsistencies were identified with the use of PedCheck (O’Connell and Weeks 1998). Two-point analysis was performed using the LINKAGE analysis programs (Lathrop et al. 1984). Multipoint LOD scores were computed by means of the GENEHUNTER program, version 2.0 beta (Kruglyak et al. 1996). Heterogeneity testing was performed with the use of GENEHUNTER and HOMOG, version 3.33 (Morton 1956; Ott 1991). The study was approved by the Leeds Health Authority/United Teaching Hospitals National Health Service Trust Research Ethics Committee.
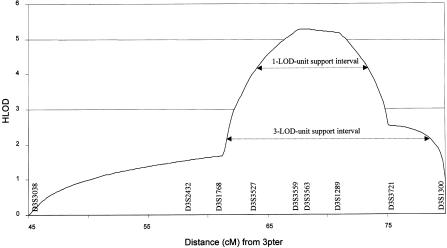
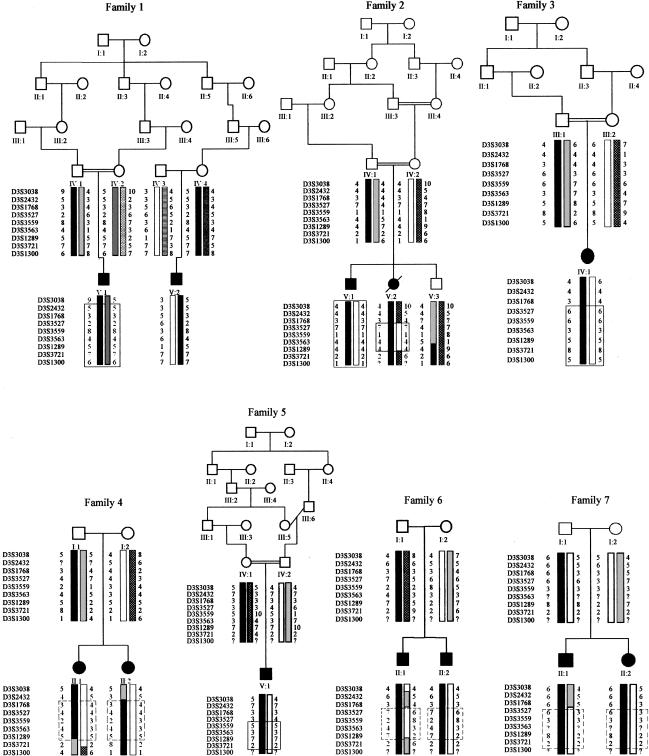
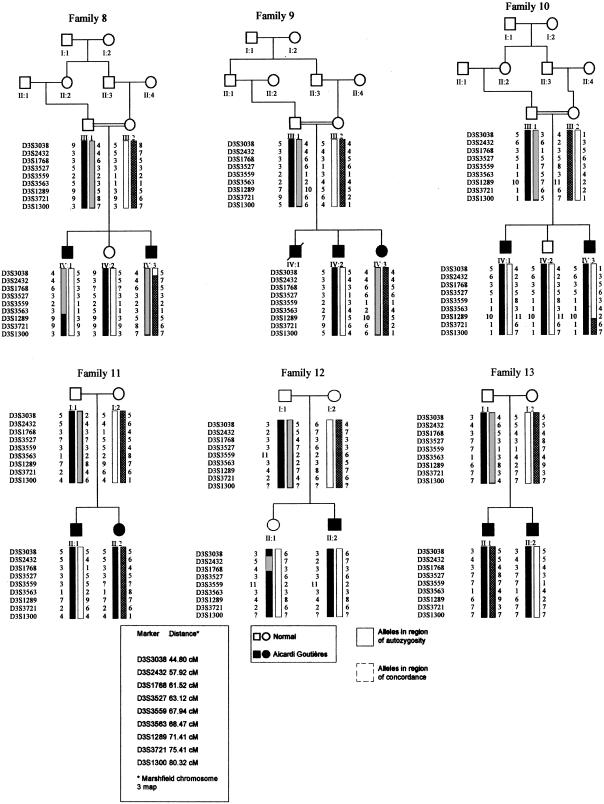
After an initial genome scan, no single region compatible with linkage in all families could be determined. However, heterogeneity analysis of the linkage data identified two regions with a multipoint heterogeneity LOD (HLOD) score >2 (data not shown). The addition of further marker information left a single locus, on chromosome 3p21, with an HLOD score greater than this value. Under the hypothesis of locus homogeneity, linkage analysis of the complete pedigree set gave a maximum two-point LOD score of 2.18 (recombination fraction [θ] = 0.1) at D3S3527 (table 2). Multipoint analysis using the hypothesis of locus homogeneity produced negative values (maximum LOD −7) between D3S3038 and D3S1300, but seven families had positive individual LOD scores in this region (table 3). In contrast, under an assumption of locus heterogeneity, a maximum multipoint HLOD score of 5.28 was obtained at D3S3563, with α=.48, where α is the proportion of families showing linkage (fig. 1). The results of both haplotype inspection (fig. 2) and heterogeneity testing of the multipoint data by use of HOMOG (tables 4 and 5) were consistent with these findings. The highest multipoint LOD score for an individual pedigree was reached in family 1 (LOD 2.98; table 3) and is based on the presence of a homozygous region in one child (patient V:1) and the transmission of a common haplotype to patient V:2 (fig. 2fig. 2). Although no corresponding region of homozygosity could be identified in patient V:2, this finding is in keeping with the results of a genealogy search that failed to identify any common ancestry between individuals IV:3 and IV:4. The other families showing results consistent with linkage at this position included a Pakistani sib pair, a Pakistani singleton, a Turkish singleton, and three white sib pairs. When the results from these seven families are combined, the minimum critical region—that is, the smallest region that is found to be identical by descent in affected children from consanguineous matings and that is also overlapped by the common haplotype in patient V:2 in family 1 and by regions of concordance in affected siblings from nonconsanguineous pedigrees—would lie between D3S3527 and D3S3721 and would cover a genetic distance of ∼12 cM. However, the 3-LOD-unit support interval defines a distance that is considerably larger than this (fig. 1).
Table 2.
Maximum Two-Point LOD Score at Each Marker, under the Hypothesis of Locus Homogeneity
| Marker | Maximum Total Two-Point LOD Score | θ |
| D3S3038 | .02 | .3 |
| D3S2432 | .13 | .2 |
| D3S1768 | .05 | .4 |
| D3S3527 | 2.18 | .1 |
| D3S3559 | 1.73 | .1 |
| D3S3563 | 1.85 | .1 |
| D3S1289 | .95 | .1 |
| D3S3721 | .33 | .3 |
| D3S1300 | .01 | .4 |
Table 3.
Multipoint LOD Score at Specified Markers, for Each Family
|
Multipoint LOD Score at Marker |
|||||
| Family Number | D3S1768 | D3S3527 | D3S3559 | D3S3563 | D3S1289 |
| 1 | 2.95 | 2.97 | 2.98 | 2.98 | 2.96 |
| 2 | .06 | 1.29 | 1.86 | 1.86 | 1.86 |
| 3 | −.05 | 1.14 | 1.19 | 1.19 | 1.19 |
| 4 | .60 | .60 | .60 | .60 | .47 |
| 5 | −.22 | −.70 | .99 | 1.00 | 1.01 |
| 6 | −1.20 | .03 | .60 | .60 | .59 |
| 7 | −.59 | .58 | .54 | .54 | .52 |
| 8 | −4.44 | −3.15 | −4.30 | −4.32 | −8.80 |
| 9 | −8.40 | −7.60 | −7.80 | −7.00 | −7.14 |
| 10 | −4.93 | −4.65 | −5.90 | −6.16 | −4.04 |
| 11 | −2.80 | −1.80 | −2.31 | −3.13 | −3.08 |
| 12 | .09 | −1.17 | −1.18 | −1.18 | −1.17 |
| 13 | −.90 | −2.90 | −3.05 | −2.59 | −3.22 |
Figure 1.
Graph of multipoint HLOD scores against distance (in cM) from 3pter. Maximum HLOD = 5.28, with α=.48. Confidence limits are given as 1-LOD-unit and 3-LOD-unit support intervals
Figure 2.
Pedigrees showing linkage (families 1–7) and pedigrees not showing linkage (families 8–13), as assigned by HOMOG analysis, with the most-likely haplotypes for region of interest on chromosome 3p. Unaffected children that were not genotyped have been excluded from the pedigrees displayed.
Table 4.
HOMOG Analysis of Multipoint Data
| Hypothesisa | df | χ2 | Likelihood Ratio |
| H2 vs. H1 | 1 | 24.232 | 1.82 × 105 |
| H1 vs. H0 | 1 | .000 | 1.00 |
| H2 vs. H0 | 2 | 24.232 | 1.82 × 105 |
H0 = no linkage; H1 = linkage, homogeneity; H2 = linkage, heterogeneity.
Table 5.
HOMOG Analysis: Conditional Probability of Linkage to AGS1, by Family
| 1-LOD-Unit Support Interval |
|||
| Family Number | Conditional Probability of Linkage | Lower | Upper |
| 1 | .99 | .99 | .99 |
| 2 | .98 | .91 | .99 |
| 3 | .94 | .73 | .98 |
| 4 | .79 | .28 | .93 |
| 5 | .91 | .52 | .98 |
| 6 | .80 | .39 | .94 |
| 7 | .78 | .37 | .93 |
| 8 | .00 | … | … |
| 9 | .00 | … | … |
| 10 | .00 | … | … |
| 11 | .00 | … | … |
| 12 | .06 | .01 | .2 |
| 13 | .00 | … | … |
We have followed the recommendation of Terwilliger and Ott, in setting the threshold value of 3.3 for a HLOD score, to declare significant linkage in the presence of locus heterogeneity (Terwilliger and Ott 1994). On this basis, our data establish the existence of a locus, AGS1, for Aicardi-Goutières syndrome on chromosome 3p and suggest that mutations in at least one other gene produce a similar phenotype. Careful comparison of the clinical and biological characteristics of families with or without putative linkage in this study failed to identify any obvious differentiating features. We consider locus heterogeneity to be the most likely explanation for difficulties in identification of genetic linkage experienced elsewhere (Fauré et al. 1999).
We note that, in two of the seven families showing results compatible with linkage to AGS1, affected individuals did not demonstrate a CSF lymphocytosis, which is a feature that was previously considered to be mandatory for the diagnosis of Aicardi-Goutières syndrome. However, the recent description of female siblings that were affected with clinically typical disease but were discordant for the presence of a chronic CSF pleocytosis suggests that this feature is not always present (Østergaard et al. 1999). Moreover, the affected children in these families (families 2 and 3) had raised levels of IFN-α in the CSF—a recognized marker for Aicardi-Goutières syndrome—and a normal head circumference at birth, which would be atypical for pseudo-TORCH syndrome. Although it is not possible to definitively categorize such cases showing overlap between the two conditions, we consider our clinical and genetic data to add weight to the previous assertion that some cases of Aicardi-Goutières syndrome and pseudo-TORCH syndrome may represent the same disorder (Stephenson et al. 1997).
A number of extraneurological features were observed in our patients (table 6). It is of note that discordance for each of these findings was present within at least one sib pair. All of these features have previously been described in Aicardi-Goutières syndrome (Goutières et al. 1998), and they highlight again the possibility for misdiagnosis of congenital infection. Interestingly, vasculitic lesions bearing similarity to the chilblains seen in Aicardi-Goutières syndrome (Tolmie et al. 1995; Stephenson et al. 1997) have been reported during treatment with IFN-α (Bachmeyer et al. 1996; Creutzig and Freund 1996; Campo-Voegeli et al. 1998; Cid et al. 1999).
Table 6.
Details of Extraneurological Features Noted in Affected Individuals[Note]
| Family/Patient | Neonatal Thrombocytopenia | HSa | Asymptomatic Elevation of Liver Enzymes | Chilblain-Like Lesions |
| 1/V:1 | − | − | − | − |
| 1/V:2 | Purpura and bruising | − | − | − |
| 2/V:1 | Platelets 32 × 109/liter (at birth) and 100 × 109/liter (at age 3 wk); bone marrow normal | + | ALT 126 and AST 177 (at age 2 mo) | + |
| 2/V:2 | − | − | − | − |
| 3/IV:1 | Platelets 59 × 109/liter (at birth) and normal (by age 6 mo) | + | ALT 207 and bilirubin 13 (at age 6 mo) | − |
| 4/II:1 | − | − | − | − |
| 4/II:2 | − | − | − | − |
| 5/V:1 | Platelets 103 × 109/liter (at age 9 wk) | − | − | − |
| 6/II:1 | − | − | − | +b |
| 6/II:2 | Platelets 111 × 109/liter (at age 2 d) | − | − | − |
| 7/II:1 | − | − | − | − |
| 7/II:2 | − | − | − | − |
| 8/IV:1 | − | − | − | − |
| 8/IV:2 | − | − | − | − |
| 9/IV:2 | − | − | − | − |
| 9/IV:3 | − | − | − | − |
| 10/IV:1 | − | − | − | − |
| 10/IV:3 | − | − | − | − |
| 11/II:1 | − | + | + | + |
| 11/II:2 | − | − | AST 172 and ALT 251 (at age 6 wk) | − |
| 12/II:2 | − | − | − | + |
| 13/II:1 | − | − | ALT 400 and AST 170 (at age 18 mo) | − |
| 13/II:2 | − | − | + | − |
Note.— A plus sign (+) denotes presence; a minus sign (−) denotes absence.
Hepatosplenomegaly.
Treated with steroids.
The development of transgenic mice with astrocyte-targeted chronic overproduction of IFN-α showing neuropathologic features that mimic those found in Aicardi-Goutières syndrome (Akwa et al. 1998; Goutières et al. 1998; Kumar et al. 1998; Barth et al. 1999; Campbell et al. 1999) suggests a direct causal relationship between abnormal intrathecal synthesis of this cytokine and the disease. We hypothesize that the elevated levels of IFN-α seen in Aicardi-Goutières syndrome may result from loss of function of a normal repressor of IFN-α production, but, although the mechanism of cytokine signal generation is well understood, little is known about the mechanisms of signal attenuation (Foster and Finter 1998; Schindler 1999). Unfortunately, although >70 genes and many more partial transcripts map between markers D3S3527 and D3S3721, none are as yet recognized as being directly involved in IFN-α metabolism.
In summary, genomewide linkage analysis of 23 children with Aicardi-Goutières syndrome has identified a locus for the disorder on chromosome 3p and has suggested the existence of locus heterogeneity. We speculate that classification of the group of genetic conditions masquerading as the sequelae of congenital infection is likely to require adjustment as the responsible genes are characterized.
Acknowledgments
We wish to express our gratitude to the families for their participation in this study. We thank Professor D. T. Bishop for advice on locus-heterogeneity analysis; Professor Pierre Lebon, for measurements of IFN-α in a number of the families; Dr. Duncan McHale, for discussion of the data; and Abigail Carradice, for help with genotyping.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://www.marshmed.org/genetics/ (for information regarding marker order and relative genetic distances)
- Genome Database, http://gdbwww.gdb.org/ (for information on polymorphic markers on chromosome 3p)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Aicardi-Goutières syndrome [MIM 225750] and pseudo-TORCH syndrome [MIM 251290])
References
- Aicardi J, Goutières F (1984) A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol 15:49–54 [DOI] [PubMed] [Google Scholar]
- Akwa Y, Hassett DE, Eloranta ML, Sandberg K, Masliah E, Powell H, Whitton JL, et al (1998) Transgenic expression of IFN-α in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol 161:5016–5026 [PubMed] [Google Scholar]
- al-Dabbous R, Sabry MA, Farah S, al-Awadi SA, Simeonov S, Farag TI (1998) The autosomal recessive congenital intrauterine infection-like syndrome of microcephaly, intracranial calcification, and CNS disease: report of another Bedouin family. Clin Dysmorphol 7:127–130 [DOI] [PubMed] [Google Scholar]
- Bachmeyer C, Farge D, Gluckman E, Miclea JM, Aractingi S (1996) Raynaud's phenomenon and digital necrosis induced by interferon-alpha. Br J Dermatol 135:481–483 [PubMed] [Google Scholar]
- Baraitser M, Brett EM, Piesowicz AT (1983) Microcephaly and intracranial calcification in two brothers. J Med Genet 20:210–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth PG, Walter A, van Gelderen I (1999) Aicardi-Goutières syndrome: a genetic microangiopathy? Acta Neuropathol 98:212–216 [DOI] [PubMed] [Google Scholar]
- Bolthauser E, Steinlin M, Boesch C, Martin E, Schubiger G (1991) Magnetic resonance imaging in infantile encephalopathy with cerebral calcification and leukodystrophy. Neuropediatrics 22:33–35 [DOI] [PubMed] [Google Scholar]
- Bönnemann CG, Meinecke P (1992) Encephalopathy of infancy with intracerebral calcification and chronic spinal fluid lymphocytosis—another case of the Aicardi-Goutières syndrome. Neuropediatrics 23:157–161 [DOI] [PubMed] [Google Scholar]
- Burn J, Wickramasinghe HT, Harding B, Baraitser M (1986) A syndrome with intracranial calcification and microcephaly in two sibs, resembling intrauterine infection. Clin Genet 30:112–116 [DOI] [PubMed] [Google Scholar]
- Campbell IL, Krucker T, Steffensen S, Akwa Y, Powell HC, Lane T, Carr DJ, et al (1999) Structural and functional neuropathology in transgenic mice with CNS expression of IFN-α. Brain Res 835:46–61 [DOI] [PubMed] [Google Scholar]
- Campo-Voegeli A, Estrach T, Marti RM, Corominas N, Tuset M, Mascaró JM (1998) Acrocyanosis induced by interferon α2a. Dermatology 196:361–363 [DOI] [PubMed] [Google Scholar]
- Cid MC, Hernández-Rodríguez J, Robert J, del Río A, Casademont J, Coll-Vinent B, Grau JM, et al (1999) Interferon-α may exacerbate cryoglobulinemia-related ischaemic manifestations: an adverse effect potentially related to its anti-angiogenic activity. Arthritis Rheum 42:1051–1055 [DOI] [PubMed] [Google Scholar]
- Creutzig A, Freund M (1996) Severe Raynaud's syndrome associated with interferon therapy: a case history. Angiology 47:185–187 [DOI] [PubMed] [Google Scholar]
- Diament AJ, Machado LR, Cypel S, Ramos JLA (1986) Syndrome of calcifications of basal ganglia, leukodystrophy and chronic lymphomonocytic pleocytosis of the cerebrospinal fluid: report of a case. Arq Neuropsiquiatr 44:185–190 [DOI] [PubMed] [Google Scholar]
- Fauré S, Bordelais I, Marquette C, Rittey C, Campos-Castello J, Goutières F, Ponsot G, et al (1999) Aicardi-Goutières syndrome: monogenic recessive disease, genetically heterogeneous disease, or multifactorial disease? Clin Genet 56:149–153 [DOI] [PubMed] [Google Scholar]
- Foster GR, Finter NB (1998) Are all type I human interferons equivalent? J Viral Hepat 5:143–152 [DOI] [PubMed] [Google Scholar]
- Giroud M, Gouyon JB, Chaumet F, Cinquin AM, Chevalier-Nivelon A, Alison M, Dumas R (1986) A case of progressive familial encephalopathy in infancy with calcification of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Childs Nerv Syst 2:47–48 [DOI] [PubMed] [Google Scholar]
- Goutières F (2000) Aicardi-Goutières syndrome: neurobase 2000, 1st ed. Arbor Publishing, San Diego [Google Scholar]
- Goutières F, Aicardi J, Barth PG, Lebon P (1998) Aicardi-Goutières syndrome: an update and results of interferon-α studies. Ann Neurol 44:900–907 [DOI] [PubMed] [Google Scholar]
- Ishitsu T, Chikazawa S, Matsuda I (1985) Two siblings with microcephaly associated with calcification of cerebral white matter. Jinrui Idengaku Zasshi 30:213–217 [DOI] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Rittey C, Cameron AH, Variend S (1998) Recognizable inherited syndrome of progressive central nervous system degeneration and generalized intracranial calcification with overlapping phenotype of the syndrome of Aicardi and Goutières. Am J Med Genet 75:508–515 [PubMed] [Google Scholar]
- Lathrop GM, Lalouel J-M, Julier C, Ott J (1984) Strategies for multilocus analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon P, Badoual J, Ponsot G, Goutières F, Hémeury-Cukier F, Aicardi J (1988) Intrathecal synthesis of interferon-α in infants with progressive familial encephalopathy. J Neurol Sci 84:201–208 [DOI] [PubMed] [Google Scholar]
- McEntagart M, Kamel H, Lebon P, King MD (1998) Aicardi-Goutières syndrome: an expanding phenotype. Neuropediatrics 29:163–167 [DOI] [PubMed] [Google Scholar]
- Mehta L, Trounce JQ, Moore JR, Young ID (1986) Familial calcification of the basal ganglia with cerebrospinal fluid pleocytosis. J Med Genet 23:157–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastiri K, Salem N, Korbi S, Snoussi N (1997) Microcephaly and intracranial calcification: two new cases. Clin Genet 51:142–143 [DOI] [PubMed] [Google Scholar]
- Morton NE (1956) The detection and estimation of linkage between the genes for elliptocytosis and the Rh blood type. Am J Hum Genet 8:80–96 [PMC free article] [PubMed] [Google Scholar]
- O'Connell JR, Weeks DE (1998) PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 63:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard JR, Christensen T, Nehen AM (1999) A distinct difference in clinical expression of two siblings with Aicardi-Goutières syndrome. Neuropediatrics 30:38–41 [DOI] [PubMed] [Google Scholar]
- Ott J (1991) Analysis of human genetic linkage. Johns Hopkins University Press, Baltimore [Google Scholar]
- Reardon W, Hockey A, Silberstein P, Kendall B, Farag TI, Swash M, Stevenson R, et al (1994) Autosomal recessive congenital intrauterine infection-like syndrome of microcephaly, intracranial calcification, and CNS disease. Am J Med Genet 52:58–65 [DOI] [PubMed] [Google Scholar]
- Schindler C (1999) Cytokines and JAK-STAT signalling. Exp Cell Res 253:7–14 [DOI] [PubMed] [Google Scholar]
- Stephenson JBP, Tolmie J, Heckmatt J, Tang S, Tatnall F, Lebon P (1997) Aicardi-Goutières syndrome: autoimmune clues to diagnosis and pathogenesis, and link to microcephaly intracranial calcification syndrome. Dev Med Child Neurol 39 (Suppl 77), paper 15 [Google Scholar]
- Terwilliger JD, Ott J (1994) Handbook of human genetic linkage. Johns Hopkins University Press, Baltimore [Google Scholar]
- Tolmie JL, Shillito P, Hughes-Benzie R, Stephenson JBP (1995) The Aicardi-Goutières syndrome (familial, early onset encephalopathy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis). J Med Genet 32:881–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrips A, Hiel JAP, Gabreëls FJ, Wesseling P, Rotteveel JJ (1997) The Aicardi-Goutières syndrome: variable clinical expression in two siblings. Pediatr Neurol 16:323–325 [DOI] [PubMed] [Google Scholar]