Abstract
We have evaluated the clinical potential of stavudine-5′-(p-bromophenyl methoxyalaninyl phosphate(stampidine [STAMP]), a novel aryl phosphate derivative of stavudine, as a new anti-human immunodeficiency virus (anti-HIV) agent, by examining its acute, subacute, and chronic toxicity profile in mice as well as by testing its antiviral activity in a surrogate human peripheral blood lymphocyte (Hu-PBL)-SCID mouse model of human AIDS. STAMP was very well tolerated in BALB/c and CD-1 mice, without any detectable acute or subacute toxicity at single intraperitoneal or oral bolus doses as high as 500 mg/kg of body weight. Notably, daily administration of STAMP intraperitoneally or orally for up to 8 consecutive weeks was not associated with any detectable toxicity at cumulative dose levels as high as 6.4 g/kg. Micromolar concentrations of the active STAMP metabolite in plasma were rapidly achieved and maintained for more than 4 h after parenteral as well as oral administration of a nontoxic 100-mg/kg bolus dose of STAMP. In accordance with its favorable pharmacokinetic profile and in vitro potency, STAMP exhibited dose-dependent and potent in vivo anti-HIV activity in Hu-PBL-SCID mice against a genotypically and phenotypically nucleoside analog reverse transcriptase inhibitor (NRTI)-resistant clinical HIV type 1 (HIV-1) isolate (BR/92/019; D67N, L214F, T215D, K219Q) at nontoxic dose levels. The remarkable in vivo safety and potency of STAMP warrants the further development of this promising new antiretroviral agent for possible clinical use in patients harboring NRTI-resistant HIV-1.
Combination antiretroviral therapy has become the standard of care for patients with human immunodeficiency virus (HIV) infection in the United States (19, 20, 23, 25). Antiretroviral treatment regimens usually employing combinations of drugs from at least two of the three classes of antiretroviral therapy, namely, nucleoside analog reverse transcriptase (RT) inhibitors (NRTI), nonnucleoside analog RT inhibitors (NNRTI), and protease inhibitors, exhibit a potent and sustained antiviral effect and confer consistent long-term viral suppression in patients with HIV infection (19, 20, 23, 25). However, each of these drugs can select for drug-resistant viruses, and the emergence of antiviral drug resistance limits their clinical benefit (3, 4, 6, 8, 10, 11, 14, 15, 17, 18, 21, 22, 24, 26, 31). The transmission of drug-resistant HIV is a serious problem that merits further attention by public health officials as well as virologists and clinicians. There is an urgent need for new anti-HIV agents capable of inhibiting the replication of NRTI- as well as NNRTI-resistant HIV.
Stavudine (STV) (also known as “d4T”) is a pyrimidine nucleoside analogue used in the treatment of HIV infection. It inhibits HIV RT, as do zidovudine (ZDV), didanosine, zalcitabine, and lamivudine (3TC), which comprise the family of NRTI. The 5′-triphosphates of these NRTI, which are generated intracellularly by the action of nucleoside and nucleotide kinases, are potent inhibitors of HIV type 1 (HIV-1) RT (20, 27). The rate-limiting step for the generation of the bioactive STV metabolite STV-triphosphate is the conversion of STV to its monophosphate derivative (27). In an attempt to overcome the dependence of STV on intracellular nucleoside kinase activation, we prepared STV-5′-(p-bromophenyl methoxyalaninyl phosphate(stampidine [STAMP])/HI-113, STV-5′-[p-bromophenyl methoxyalaninyl phosphate], a novel aryl phosphate derivative of STV (27, 30). In preliminary studies, we found that STAMP is substantially more potent than STV in inhibiting HIV-1 replication in thymidine kinase-deficient T cells (27, 30, 32).
Recently, we completed a detailed analysis of the in vitro activity profile of STAMP against HIV-1 isolates with B and non-B envelope subtypes (28). STAMP exhibited potent anti-HIV activity against the HIV-1 strain HTLVIIIB in human peripheral blood mononuclear cells with nanomolar 50% inhibitory concentration (IC50) and IC90 values and a selectivity index of >100,000. The IC50 (mean ± standard error of the mean [SEM]) of STAMP (0.001 ± 0.000 μM) against HTLV-IIIB was 20 times lower than the IC50 of STV (0.023 ± 0.008 μM; P < 0.001), 3 times lower than the IC50 of ZDV (0.003 ± 0.001 μM; P < 0.001), 40 times lower than the IC50 of 3TC (0.040 ± 0.025 μM; P < 0.001), 6 times lower than the IC50 of nelfinavir (0.006 ± 0.003 μM; P < 0.001), and 20 times lower than the IC50 of nevirapine (0.024 ± 0.007 μM; P < 0.001) (28) (all values are means ± SEMs). Similarly, in a side-by-side comparison against 10 ZDV-sensitive primary clinical HIV-1 isolates, 9 of which had a non-B envelope subtype, STAMP (IC50 = 0.002 ± 0.001 μM) was 100-fold more potent than STV (IC50 = 0.24 ± 0.07 μM, P < 0.0001) and twice as effective as ZDV (IC50 = 0.004 ± 0.001 μM, P = 0.04) (28). STAMP was also active against phenotypically and/or genotypically NRTI-resistant HIV and inhibited the replication of 20 ZDV-resistant primary clinical HIV-1 isolates (average ZDV IC50 = 1.6 ± 0.5 μM) with low-nanomolar to subnanomolar IC50s (average STAMP IC50 = 8.7 ± 2.8 nM) (28). Similarly, STAMP inhibited the replication of laboratory HIV-1 strains and primary clinical HIV-1 isolates (n = 9) with NNRTI binding site mutations (K103N, V106N, Y179I, Y181C, Y188L) and/or phenotypic NNRTI-resistant profile with an average IC50 of 11.2 ± 6.5 nM (28).
The marked potency of STAMP against primary clinical HIV-1 isolates with genotypic and/or phenotypic NRTI or NNRTI resistance warrants the further development of this promising new antiviral agent for possible clinical use. We recently investigated the in vivo pharmacokinetics and metabolism of STAMP in mice (2). STAMP was found to form two active metabolites, namely, alaninyl-STV-monophosphate (ala-d4T-MP) and STV, with favorable pharmacokinetics after systemic administration (2). These encouraging findings prompted us to further evaluate the clinical potential of STAMP as a new anti-HIV agent. The goal of the present study was to examine the in vivo toxicity profile and anti-HIV activity of STAMP. Our findings demonstrate that i.p. or oral (p.o.) bolus doses of STAMP are very well tolerated in mice, without any detectable acute or subacute toxicity at single-dose levels as high as 500 mg/kg of body weight. Daily i.p. or p.o. administration of STAMP for up to 8 consecutive weeks was not associated with any detectable acute, subacute, or chronic toxicity at cumulative dose levels as high as 6.4 g/kg. We evaluated the in vivo antiviral activity of STAMP in a surrogate severe combined immunodeficient (SCID) mouse model of human AIDS. Notably, STAMP exhibited potent in vivo anti-HIV activity against a genotypically and phenotypically NRTI-resistant clinical HIV-1 isolate at nontoxic dose levels.
MATERIALS AND METHODS
Anti-HIV drugs.
The synthetic procedures for preparation of STAMP and ala-d4T-MP (Fig. 1A) have been previously described in detail (27, 30, 32). Synthetic ala-d4T-MP that was used as a standard in the high-performance liquid chromatography (HPLC) procedures for detection of the STAMP metabolite ala-d4T-MP was synthesized as follows: STAMP (2.00 g; 3.58 mmol) was dissolved in a 1:1 mixture of triethylamine-water (40 ml). After stirring overnight at room temperature, the reaction mixture was concentrated with a rotary evaporator under reduced pressure below 50°C and then under high vacuum at room temperature. The residue was purified by flash chromatography, using a mixture of CHCl3-methanol (50:50) as a mobile phase. Pooling appropriate fractions and concentrating provided the desired compound as a triethylammonium salt (1.05 g; 1.82 mmol; 51%). The physicochemical analysis of the compound yielded the following results. 1H nuclear magnetic resonance (CDCl3, 300 MHz): δ 7.71 (d, J = 1.2 Hz, 1H), 7.02 (q, J = 1.8 Hz, 1H), 6.39 (dt, J = 6.0,1.5 Hz, 1H), 5.77 (dq, J = 6.0, 1.5 Hz, 1H), 4.96 (s, 1H), 4.16-3.98 (m, 2H), 3.68 (dd, J = 9.6, 6.9 Hz, 1H), 2.92 (q, J = 7.5 Hz, 12H), 1.96 (d, J = 0.9 Hz, 3H), 1.34 (d, J = 6.9 Hz, 3H), 1.22 (t, J = 7.2 Hz, 18H). HPLC (HP 1100 system): retention time, 9.46 min; purity, 99%; Zorbax SB C-3 (4.6 ′ 150 mm, 5 μm) column (50 mM NH4H2PO4/H3PO4, pH 2.5); CH3CN = 95:5, 1 ml/min; λmax, 266 nm; UV/visible, 266/192 nm.
FIG. 1.
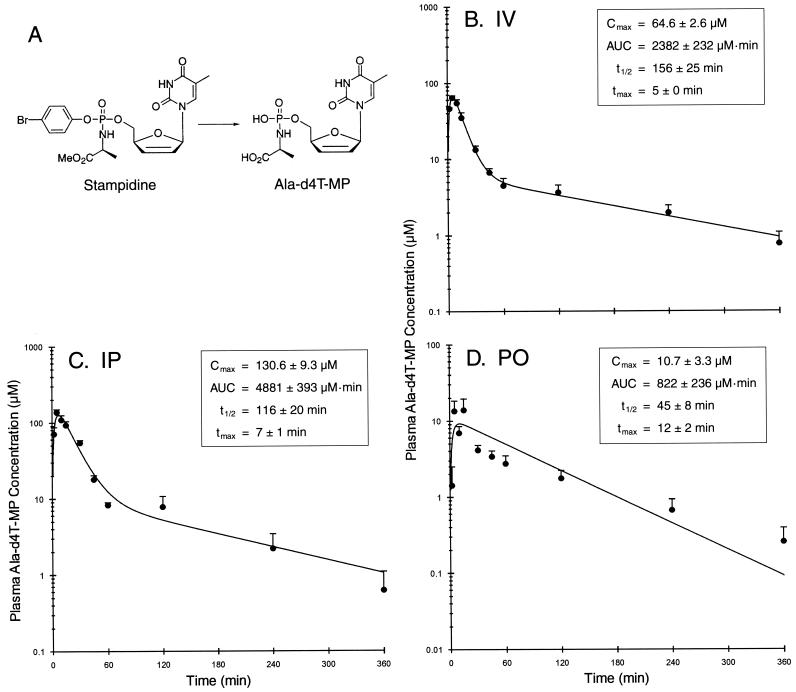
Pharmacokinetics of the active STAMP metabolite ala-d4T-MP in STAMP-treated mice. Shown are the chemical structures of STAMP and ala-d4T-MP (A) and ala-d4T-MP levels in plasma as a function of time after i.v. administration (B), i.p. administration (C), and p.o. gavage administration (D) of a 100-mg/kg bolus dose of STAMP. Data points are the means + SEMs (error bars) for the levels in plasma for each time point (four BALB/c mice and four CD-1 mice [five in panel B]). The pharmacokinetic parameters estimated from individual experiments are given in the insets with means ± SEMs. tmax, time to Cmax
Neither STAMP (in three independent experiments) nor its metabolites ala-d4T-MP and d4T (in two independent experiments) exhibited RT inhibitory activity in cell-free recombinant RT inhibition assays at concentrations as high as 50 μM, whereas d4T-triphosphate inhibited recombinant RT at nanomolar concentrations (IC50 in experiment 1, 0.021 μM; IC50 in experiment 2, 0.019 μM) (F. M. Uckun, unpublished data). ZDV (GlaxoWellcome) and 3TC (Epivir; GlaxoWellcome), for the in vivo experiments, were obtained from the Parker Hughes Institute Investigational Pharmacy Department.
HIV-1 isolate.
The genotypically and phenotypically NRTI-resistant clinical HIV-1 isolate BR/92/019 (catalog no. 1778) was obtained through the AIDS Research and Reference Reagent Program, National Institutes of Allergy and Infectious Diseases. The RT gene of this isolate harbors four mutations—namely, D67N, L214F, T215D, and K219Q—which are associated with resistance to NRTI. The in vitro IC50 of STAMP against BR/92/019 is 2 nM (28).
Animals.
Female BALB/c mice from Taconic (Germantown, N.Y.) and CD-1 mice from Charles River Laboratory (Hartford, Ct.) were housed in a controlled specific-pathogen-free environment (12-h light-12-h dark photoperiod; 22 ± 1°C; 60% ± 10% relative humidity), which is fully accredited by the U.S. Department of Agriculture. All CB-17 SCID mice used in the present study were purchased from Taconic Labs at 6 to 8 weeks of age and maintained in our Level 3 (BL-3) Containment Facility for Preclinical Research. All husbandry and experimental contact made with the mice maintained specific pathogen-free conditions. All mice were housed in microisolator cages (Allentown Caging Equipment Co., Inc., Allentown, N.J., or Lab Products, Inc., Maywood, N.Y.) containing autoclaved food, water, and bedding. Trimethoprim-sulfamethoxazole (Bactrim; Roche) was added to the drinking water of the SCID mice once a week. Animal studies were approved by Parker Hughes Institute Animal Care and Use Committee, and all animal care procedures conformed to the Guide for the Care and Use of Laboratory Animals (15).
Toxicity studies of STAMP in mice.
In acute toxicity studies using standard procedures (29), mice were treated with single i.p. bolus injections or p.o. or gavage boluses of vehicle or STAMP at multiple dose levels. Mice were allowed free access to autoclaved standard pellet food and tap water throughout the experiments and monitored daily for morbidity and mortality. Mice were electively sacrificed on day 3, 7, 14, or 30 to determine the acute toxicity of STAMP by examining their blood chemistry profiles and blood counts and evaluating multiple organs for the presence of toxic lesions. In subacute- and chronic-toxicity experiments, mice were treated with STAMP or its vehicle daily via i.p. injections, gavage, or addition of STAMP to the food for 1 to 8 weeks. Mice were monitored daily for morbidity and mortality. At the indicated time points, mice were electively sacrificed to determine the toxicity profile of STAMP by examining their blood chemistry profiles and blood counts and evaluating multiple organs for the presence of toxic lesions. Blood was collected by intracardiac puncture following anesthesia with ketamine-xylazine and was immediately heparinized. The blood chemistry profiles were examined using a Synchron CX5CE chemical analyzer (Beckman Instruments, Inc., Fullerton, Calif.). Blood counts were determined using a HESKA Vet ABC-Diff hematology analyzer (HESKA Corporation, Fort Collins, Colo.). At the time of necropsy, 22 selected tissues (bone, bone marrow, brain, spinal cord, cecum, heart, kidney, large intestine, liver, lung, lymph node, ovary, pancreas, skeletal muscle, skin, small intestine, spleen, stomach, thymus, thyroid gland, urinary bladder, and uterus, as available) were immediately collected from mice for histopathologic examination. For histopathologic studies, tissues were fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin by routine methods. Glass slides with affixed 4- to 5-μm-thick tissue sections were prepared and stained with hematoxylin and eosin.
Pharmacokinetic studies in mice.
STAMP dissolved in dimethyl sulfoxide was administered intravenously (i.v.) via the tail vein or intraperitoneally (i.p.) at a dose of 100 mg/kg. Four to five mice per strain (BALB/c or CD-1) per time point were used for pharmacokinetic studies. Blood samples (∼500 μl) were obtained from the ocular venous plexus by retro-orbital venipuncture at 0, 2, 5, 10, 15, 30, 45, 60, 120, 240, and 360 min after i.v. or i.p. injection. In order to determine the pharmacokinetics of STAMP following its p.o. administration, 12-h-fasted mice were given a bolus dose of 100-mg/kg STAMP via gavage using a 21-gauge stainless-steel ball-tipped feeding needle. Blood sampling time points were 0, 2, 5, 10, 15, 30, 45, 60, 120, 240, and 360 min after the gavage. All collected blood samples were heparinized and centrifuged at 7,000 × g for 5 min to separate the plasma fraction from the whole blood. The plasma samples were then processed immediately using the extraction procedure described below.
Determination of plasma ala-d4T-MP concentrations by quantitative HPLC.
The plasma ala-d4T-MP levels were determined by using a previously established and validated HPLC method (2). The details of the HPLC method were previously reported (2). In brief, each plasma sample (200 μl) was mixed 1:4 with acetone (800 μl) and vortexed for at least 30 s. Following centrifugation, the supernatant was transferred into a clean tube and dried under nitrogen. A 50-μl solution of 50% methanol in 200 mM HCl was used to reconstitute the extraction residue, and 40 μl of the reconstituted sample was subjected to analytical HPLC. The HPLC system used for these studies was a Hewlett-Packard (Palo Alto, Calif.) series 1100 instrument equipped with a quaternary pump, an autosampler, an automatic electronic degasser, an automatic thermostatic column compartment, a diode array detector, and a computer with Chemstation software for data analysis (2). The analytical column was a Zorbax SB-phenyl (5 μm; Hewlett-Packard, Inc.) column attached to a guard column (Hewlett-Packard, Inc.). The column was equilibrated prior to data collection. The employed linear gradient mobile phase (flow rate = 1.0 ml/min) was as follows: 100% A-0% B at 0 min, 88% A-12% B at 20 min, and 8% A-92% B at 30 min (where A is 10 mM ammonium phosphate buffer, pH 3.7, and B is acetonitrile). The detection wavelength was 268 nm. The peak width, response time, and slit were set at >0.03 min, 0.5 s, and 4 nm, respectively. We used HPLC-grade reagents and deionized distilled water. Acetonitrile was purchased from Burdick & Jackson (Allied Signal Inc., Muskegon, Mich.). Hydrochloric acid was purchased from Fisher Chemicals (Fair Lawn, N.J.). Ammonium phosphate and phosphoric acid were purchased from Sigma-Aldrich (St. Louis, Mo.). The metabolite of STAMP eluting at a retention time of 15.3 min was previously discovered to have the same retention time and UV spectrum as synthetically prepared authentic ala-d4T-MP (2). Therefore, synthetic ala-d4T-MP (retention time in spiked plasma samples = 15.3 ±0.2 min; n = 30) was used as a standard in the HPLC procedure. The lowest limit of detection was 0.25 μM, at a signal-to-noise ratio of ∼4. Good linearity (r > 0.995) was observed between concentrations ranging from 0.5 to 12.5 μM and from 12.5 to 100 μM in 200 μl of plasma. Intra- and interassay variabilities were less than 8%.
Pharmacokinetic analyses.
Pharmacokinetic modeling and parameter calculations were carried out using the WinNonlin (professional version 3.0; Pharsight, Inc., Mountain View, Calif.) pharmacokinetics software (2). Noncompartmental analysis and parameter calculations were carried out using a weight of 1/y (observed). The predicted area under the concentration-time curve (AUCpredicted) was calculated based on the linear trapezoidal method. The predicted clearance (CLpredicted) was calculated as the ratio of AUCpredicted to the dose. For compartmental analysis, an appropriate model was chosen on the basis of the lowest sum of weighted squared residuals, the lowest Schwartz criterion value, the lowest Akaike's information criterion value, the lowest standard errors of the fitted parameters, and the dispersion of the residuals. The elimination half-life (t1/2) was estimated by linear regression analysis of the terminal phase of the plasma concentration-time profile. The systemic CL was determined by dividing the dose by the estimated value of the AUC. A two-compartment model was fit to the plasma ala-d4T-MP concentration-versus-time data obtained following either i.v. or i.p. injection of 100-mg/kg STAMP. The same model also seemed to fit the pooled data obtained following p.o. administration of STAMP; however, the coefficient of variance was greater than 100% when it was used to fit the data from the individual experiments. Therefore, a one-compartment model was chosen to estimate the pharmacokinetic parameters of ala-d4T-MP after p.o. administration of STAMP.
SCID mouse model of human AIDS.
Human peripheral blood lymphocyte (Hu-PBL)-SCID mice were generated by reconstituting SCID mice by i.p. injection of 107 peripheral blood mononuclear cells from seronegative volunteer donors, as previously reported (29). Two weeks after inoculation of the cells, mice were anesthetized with isoflurane and then challenged by i.p. injection of 105 experimentally determined median 50% tissue culture infectious doses of cell-free BR/92/019, a genotypically and phenotypically NRTI-resistant HIV-1 isolate. SCID mice were infected with BR/92/019 in a biosafety-level-3 containment facility, and all manipulations were performed in a biosafety cabinet. Drugs were administered daily for 14 days either by i.p. injections or gavage. In some experiments, drugs were added to the food. Treatments were initiated on the day of virus inoculation. Throughout the experimental period, mice were monitored daily for morbidity and mortality. Two weeks after infection, Hu-PBL-SCID mice were electively killed, and their peritoneal lavage cells as well as spleen cells were examined for evidence of infection by an HIV-1 coculture assay (7) and determination of the viral RNA load (log10[RNA copies per milligram of spleen tissue or per milliliter of peritoneal lavage fluid]) using an Organon Teknika Nuclisens HIV-1 QT assay kit (bioMerieux, Durham, N.C.). Extraction of RNA was done with silica (50 μl) utilizing standard Boom technology and a NucliSens extractor (bioMerieux). Standard amplification (nucleic acid sequence-based amplification [NASBA]) and detection assay was performed according to the manufacturer's recommendations. Detection was based on electrochemiluminescent labels that emit light due to chemical reactions occurring on the surface of an electrode. For histopathologic studies, tissues were fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin by routine methods. Glass slides with affixed 4- to 5-μm-thick tissue sections were prepared, stained with hematoxylin-eosin, and submitted to the veterinary pathologist for examination.
Differences in the proportional response rate with drug treatment were analyzed using a chi-square test of independence (contingency analysis). The data were organized using a two-way classification table; all drug treatments, including the control; and the response (number positive or negative). The null hypothesis tested was that there was no difference between the observed and expected distributions in the classification table. The likelihood chi-square value was computed to test the null hypothesis (a P of <0.05 was deemed significant). In addition, a logistic regression was fitted to obtain the dose level at which the incidence of HIV positivity in tissue was reduced by 50%. The logistic regression measures the response probability as a smooth function of the drug dose [y = ex/(1 + ex), where y is the percentage response and x is the drug dose]. The 95% confidence range was computed using Fieller's theorem (JMP software). Values were reported if both the contingency analysis and the logistic regression analysis showed significant effects.
To test the effect of STAMP on the tissue viral load, a linear regression model was used to fit log10 viral load values versus STAMP dose. The significance of the fit was assessed by comparing the F ratio of the line with no response (gradient zero about the mean) and the least-squares linear fit of the data (a P of <0.05 was deemed significant). A Dunnett's post hoc test was performed to test differences between the control and each of the STAMP dose levels. All statistical calculations were performed using JMP software (SAS Institute, Cary, N.C.).
RESULTS
Toxicity and pharmacokinetics of STAMP in mice.
We first examined the acute-toxicity profile of STAMP in BALB/c mice when it was administered in a single i.p. bolus injection (n = 75) at dose levels of 6, 60, and 600 mg/kg or via gavage (n = 75) in a single p.o. bolus at dose levels of 25, 50, and 100 mg/kg. STAMP was nontoxic to BALB/c mice even at the highest i.p. bolus dose of 600 mg/kg and p.o. bolus dose of 100 mg/kg. Control mice (n = 50; 25 mice for the i.p. group and 25 mice for the p.o. group) were treated with vehicle alone. All 150 STAMP-treated mice remained healthy and gained weight throughout the 30-day observation period, with no evidence of morbidity. No toxic lesions were found in any of the 22 organs from 133 STAMP-treated mice (or 44 vehicle-treated control mice) sacrificed at 3, 7, 14, or 30 days. Thus, single-bolus doses of STAMP are nontoxic to BALB/c mice at dose levels as high as 600 mg/kg administered i.p. and 100 mg/kg administered p.o.
We next examined the cumulative subacute- and chronic-toxicity of i.p. or p.o. administered STAMP in BALB/c mice. To this end, 220 7-week-old female BALB/c mice were treated with a daily i.p. bolus dose of STAMP in 0.2 ml of 20% dimethyl sulfoxide-phosphate-buffered saline at dosages of 25 mg/kg/day (n = 55), 50 mg/kg/day (n = 55), or 100 mg/kg/day (n = 55) for 1 to 8 consecutive weeks. Control mice (n = 55) were treated with i.p. injections of the STAMP-free vehicle solution. Similarly,220 7-week-old female BALB/c mice were administered an p.o. dose of STAMP mixed with lactose in powdered food at one of three different dose levels—25 mg/kg/day (n = 55), 50 mg/kg/day (n = 55), or 100 mg/kg/day (n = 55)—for a period of 1 to 8 weeks. Control mice were treated with lactose alone in powdered food (n = 55) according to experimental protocol. All 165 mice treated with i.p.-administered STAMP and all 165 mice treated with p.o.-administered STAMP remained healthy and gained weight throughout the 1- to 8-week observation period, with no evidence of morbidity. No toxic lesions were found in any of the organs from 270 STAMP-treated mice (or 90 vehicle-treated control mice) sacrificed at weeks 1 to 8. Taken together, these experiments demonstrated that STAMP administered i.p. or p.o. daily for up to 8 weeks is nontoxic to BALB/c mice at cumulative dose levels as high as 5.6 g/kg (=100 mg/kg/day for 8 weeks).
Blood tests done on days 3, 7, 14, or 30 of the acute-toxicity experiments or weeks 1 to 8 in the subacute-toxicity experiments did not suggest any significant systemic toxicity at any of the dose levels. In particular, even at the highest dose levels, STAMP did not cause (i) anemia, neutropenia, lymphopenia, or thrombocytopenia suggestive of hematologic toxicity; (ii) elevations of blood urea nitrogen or creatinine or electrolyte disturbances suggestive of renal toxicity; (iii) elevations of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, or bilirubin suggestive of hepatotoxicity; or (iv) elevation of amylase suggestive of pancreas toxicity. The combined data sets for the 150 STAMP-treated mice versus 50 vehicle-treated mice from the acute-toxicity experiments as well as for the 330 STAMP-treated mice versus 110 vehicle-treated mice from the subacute-toxicity experiments are shown in Table 1. Thus, STAMP treatments were not associated with any subclinical systemic toxicity detectable by laboratory tests.
TABLE 1.
Acute and subacute toxicity of STAMPa
| Laboratory parameterb | Acute toxicity
|
Subacute toxicity
|
||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle (n = 50)
|
STAMP (n = 150)
|
Vehicle (n = 110)
|
STAMP (n = 330)
|
|||||
| Mean ± SE (range) | Median (geo mean) | Mean ± SE (range) | Median (geo mean) | Mean ± SE (range) | Median (geo mean) | Mean ± SE (range) | Median (geo mean) | |
| Hematology | ||||||||
| WBC (109/liter) | 4.9 ± 0.3 (1.8-12.3) | 4.7 (4.5) | 5.2 ± 0.2 (1.2-18.9) | 4.6 (4.6) | 6.6 ± 0.6 (2-66.8) | 5.8 (5.7) | 5.7 ± 0.1 (1-17.6) | 5.2 (5.3) |
| ANC (109/liter) | 0.9 ± 0.1 (0.3-2.7) | 0.8 (0.8) | 1 ± 0 (0.1-2.9) | 0.8 (0.8) | 1.5 ± 0.1 (0.2-8.7) | 1.2 (1.1) | 1.5 ± 0.1 (0-6.1) | 1.3 (1.2) |
| ALC (109/liter) | 3.9 ± 0.3 (1.4-11.6) | 3.8 (3.6) | 4.1 ± 0.2 (1-17.2) | 3.5 (3.6) | 4.9 ± 0.5 (0.4-54.1) | 4.1 (4.2) | 4 ± 0.1 (0.8-15.1) | 3.6 (3.7) |
| RBC (1012/liter) | 8.6 ± 0.3 (5.1-12.1) | 8.3 (8.3) | 8.5 ± 0.2 (0.9-16.1) | 8.4 (7.9) | 8.7 ± 0.3 (1.9-19.3) | 8.6 (8.1) | 8.5 ± 0.2 (1-18.4) | 8.5 (8) |
| Renal function or metabolism | ||||||||
| BUN (mg/dl) | 19.7 ± 0.5 (14.1-28) | 19.9 (19.5) | 21 ± 0.4 (13.5-42) | 20 (20.4) | 21.5 ± 0.4 (13.8-34.3) | 20.5 (21.1) | 22.4 ± 0.2 (12.3-46.4) | 21.9 (22.1) |
| Creatinine (mg/dl) | 0.4 ± 0 (0.1-1) | 0.3 (0.3) | 0.3 ± 0 (0-0.9) | 0.3 (0.3) | 0.4 ± 0.1 (0-5) | 0.3 (0.3) | 0.4 ± 0 (0-1.8) | 0.3 (0.3) |
| Albumin (g/dl) | 1.1 ± 0 (1-1.4) | 1.1 (1.1) | 1.1 ± 0 (0.9-1.3) | 1.1 (1.1) | 1.1 ± 0 (0.7-1.6) | 1.1 (1.1) | 1.1 ± 0 (0.8-1.5) | 1.1 (1.1) |
| Total protein (g/dl) | 3.4 ± 0.1 (2.8-4.2) | 3.3 (3.3) | 3.4 ± 0 (2.7-4.3) | 3.3 (3.4) | 3.5 ± 0 (2.7-5.5) | 3.5 (3.5) | 3.5 ± 0 (2.7-4.8) | 3.5 (3.5) |
| Triglycerides (mg/dl) | 94.1 ± 4.9 (49-244) | 88 (89.1) | 104.8 ± 3.6 (43-282) | 95 (97.1) | 91.7 ± 3.8 (38-244) | 87 (84.6) | 102.9 ± 3.1 (10-448) | 90 (91.2) |
| Calcium (mg/dl) | 5.7 ± 0.3 (2-8.2) | 7 (5) | 5.7 ± 0.2 (2-8.3) | 6.9 (5) | 6.6 ± 0.1 (2-8.3) | 6.8 (6.4) | 6.7 ± 0.1 (2-8.6) | 6.9 (6.5) |
| Phosphate (mg/dl) | 6.3 ± 0.1 (4.3-9.4) | 6.4 (6.2) | 6.5 ± 0.1 (3.7-9.3) | 6.5 (6.5) | 5.7 ± 0.1 (2.7-8.6) | 5.6 (5.5) | 5.8 ± 0.1 (1-9.2) | 5.8 (5.6) |
| Liver function | ||||||||
| ALT (IU/liter) | 12 ± 0.5 (4.6-21.9) | 11.3 (11.4) | 12.6 ± 0.4 (2.8-27) | 12.4 (11.8) | 11.4 ± 0.6 (1.5-55.5) | 10.4 (9.9) | 12.5 ± 0.4 (0.6-87.2) | 11 (11.1) |
| AST (IU/liter) | 110.1 ± 8.9 (25-270) | 104 (94.1) | 112.8 ± 5.7 (43-399) | 93 (98.2) | 131.1 ± 10 (19-646) | 91 (105.6) | 138.2 ± 6.3 (5-952) | 104 (110.9) |
| Alkaline phosphatase (IU/liter) | 84.3 ± 2.4 (31.8-115.8) | 84.4 (82.4) | 85.6 ± 1.2 (41.7-121.8) | 85.5 (84.3) | 66 ± 1.8 (5-120.3) | 67.1 (62.5) | 64.8 ± 1 (5-107) | 65.5 (61.6) |
| Total bilirubin (mg/dl) | 0.5 ± 0 (0-1.2) | 0.4 (0.4) | 0.4 ± 0 (0-1.7) | 0.4 (0.4) | 0.4 ± 0 (0.2-1.7) | 0.4 (0.3) | 0.4 ± 0 (0-1.9) | 0.4 (0.3) |
| Pancreas function: amylase (U/liter) | 777.2 ± 15.6 (558.8-1,049.9) | 787.1 (769.3) | 799.4 ± 9.4 (487-1,332.7) | 791 (791.3) | 829.3 ± 31.5 (459-3,058) | 782.6 (792.5) | 811.5 ± 12.9 (462.1-2,442.9) | 764 (787) |
In acute-toxicity experiments, BALB/c mice were treated with a single p.o. bolus of STAMP administered via gavage or an i.p. bolus injection of STAMP at multiple dose levels as detailed in the text. Five mice from each dose group were sacrificed on days 3, 7, and 14, and the remaining 10 mice from each group were sacrificed on day 30. Combined laboratory results from all time points and data points for the 150 STAMP-treated test mice and 50 vehicle-treated control mice are presented as the values for mean ± standard error geometric mean, median, and range (minimum to maximum) of laboratory parameters. Tissues from 133 STAMP-treated and 44 vehicle-treated mice in these experiments were subjected to histopathologic examinations and did not reveal any lesions. In subacute-toxicity experiments, BALB/c mice were treated daily with p.o.-administered STAMP added to their food or with i.p. bolus injections of STAMP at multiple dose levels, for 8 consecutive weeks, as detailed in the text. Five to ten mice per dose group were sacrificed weekly while on therapy. Combined laboratory results from all time points and data points for the 330 STAMP-treated test mice and 110 vehicle-treated control mice are presented as the values for mean ± standard error, geometric mean, median, and range (minimum to maximum) of laboratory parameters. Tissues from 270 STAMP-treated and 90 vehicle-treated mice in these experiments were subjected to histopathologic examinations and did not reveal any lesions.
Abbreviations: WBC, white blood count; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; RBC, red blood count; BUN, blood urea nitrogen; ALT, alanine aminotransferase (glutamic pyruvic transaminase); AST, aspartate aminotransferase (glutamic oxaloacetic transaminase).
We next set out to determine if therapeutic concentrations of the active STAMP metabolite ala-d4T-MP (IC50 = 10 nM) (29) can be achieved in plasma of BALB/c and CD-1 mice after i.v., i.p., or p.o. administration of a 100-mg/kg nontoxic bolus dose of STAMP. Ala-d4T-MP was present at micromolar concentrations in the plasma samples of STAMP-treated mice. The concentration-time curves and the estimated values for the pharmacokinetic parameters obtained by compartmental analysis are depicted in Fig. 1. A two-compartment pharmacokinetic model was used to analyze the time-dependent concentration changes of ala-d4T-MP after i.v. or i.p. administration of STAMP, whereas a one-compartment pharmacokinetic model was used to analyze the time-dependent concentration changes of ala-d4T-MP after p.o. administration of STAMP. The maximum concentration of drug in serum (Cmax) for ala-d4T-MP was 64.6 ± 2.6 μM after i.v. administration (Fig. 1B), 130.6 ± 9.3 μM after i.p. administration (Fig. 1C), and 10.7 ± 3.3 μM after p.o. administration (Fig. 1D). The time to Cmax was 5 ± 0 min after i.v. administration, 7 ± 1 min after i.p. administration, and 12 ± 2 min after p.o. administration. The AUC values were 2,382 ± 232 μM · min after i.v. administration, 4,881 ± 393 μM · min after i.p. administration, and 822 ± 236 μM · min after p.o. (gavage) administration. The elimination t1/2s were 156 ± 25 min after i.v. administration, 116 ± 20 min after i.p. administration, and 45 ± 8 min after p.o. administration. Similarly, the results from noncompartmental analyses indicated that ala-d4T-MP had Cmax values of 67.9 ± 4.8, 147.3 ± 8.1, 16.6 ±5.4 μM; AUC values of 2,539 ± 219, 4,790 ± 391, 823 ± 200 μM · min; t1/2 values of 137 ± 14, 96 ± 14, 101 ± 20; and times to Cmax of 7 ± 2, 6 ± 1, and 11 ± 2 min, respectively, after i.v., i.p., and p.o. administration of 100-mg/kg STAMP. Thus, micromolar concentrations of the active STAMP metabolite in plasma were rapidly achieved and maintained for more than 4 h after parenteral as well as p.o. administration of the nontoxic 100-mg/kg bolus dose of STAMP (Fig. 1).
Similarly, micromolar concentrations of the intact STAMP in plasma were achieved after i.v. or i.p administration of 100-mg/kg STAMP. By comparison, the plasma levels of STAMP after p.o. administration were too close to the detection limit (∼0.25 μM), which prevented an accurate assessment of the levels in plasma as a function of time and an accurate estimation of the pharmacokinetic parameters. The estimated Cmax for STAMP according to compartmental analyses was 172.3 ± 25.8 μM after i.v. administration and 14.0 ± 4.9 μM after i.p. administration. The AUC values were 846 ± 117 μM · min after i.v. administration and 309 ± 53 μM · min after i.p. administration. The elimination t1/2s and CL were 3.4 ± 0.2 min and 249 ± 30 ml/min/kg, respectively, after i.v. administration and 8.5 ± 1.5 min and 768 ± 182 ml/min/kg, respectively, after i.p. administration. Similarly, noncompartmental analysis of the same pharmacokinetic data yielded an estimated Cmax of 217.5 ± 51.5 μM after i.v. administration and 23.0 ± 3.1 μM after i.p. administration, an AUCpredicted of 919 ± 138 μM · min after i.v. administration and 344 ± 32 μM · min after i.p administration, a t1/2 of 5.1 ± 0.8 min after i.v. administration and 11.8 ± 1.5 min after i.p. administration, and a CL of 231 ± 27 ml/kg after i.v. administration and 574 ± 63 ml/kg after i.p. administration of 100-mg/kg STAMP.
In vivo activity of STAMP against NRTI-resistant HIV-1 in a surrogate SCID mouse model of human AIDS.
We studied the in vivo anti-HIV activity of STAMP against the NRTI-resistant clinical HIV-1 isolate BR/92/019 in a surrogate Hu-PBL-SCID mouse model of human AIDS at nontoxic dose levels. STAMP exhibited significant and dose-dependent anti-HIV activity against BR/92/019 in Hu-PBL-SCID mice, when it was administered i.p. daily for 14 days (Table 2). Spleen specimens from STAMP-treated mice showed coculture evidence of HIV infection less frequently than vehicle-treated control mice, and the observed differences were statistically significant (χ2 = 28.3; P < 0.0001). Whereas spleen specimens from 16 of 16 vehicle-treated mice showed coculture evidence of HIV infection, none of seven spleen specimens from mice treated with 100-mg/kg STAMP showed evidence of HIV infection in HIV coculture assays. Very similar results were obtained in HIV-NASBA assays of the spleen specimens (Table 2). Spleen specimens from STAMP-treated mice showed NASBA evidence of HIV infection less frequently than vehicle-treated control mice, and the observed differences were statistically significant (χ2 = 18.5; P = 0.002). The calculated 50% effective dose (ED50) of STAMP was 47 mg/kg (27 to 83 mg/kg [logistic regression fit]) for its impact on NASBA positivity of spleens. Furthermore, the spleen viral loads of the NASBA-positive STAMP-treated mice were lower than those of NASBA-positive control mice (F ratio = 6.862; P = 0.013). Whereas spleen specimens from 15 of 16 vehicle-treated control mice showed NASBA evidence of HIV infection, with an average viral load of 4.5 ± 0.2 logs, spleen specimens from only 2 of 8 test mice treated with 100-mg/kg STAMP showed PCR evidence of HIV infection, with an average viral load of 3.5 ± 0.3 logs (Table 2). Similarly, peritoneal lavage specimens from STAMP-treated mice showed NASBA evidence of HIV infection less frequently than vehicle-treated control mice, and the observed differences were statistically significant (χ2 = 45.9; P < 0.0001). The calculated ED50 of STAMP was 25 mg/kg (15 to 38 mg/kg [logistic regression fit]) for its impact on NASBA positivity of peritoneal lavage specimens. Furthermore, the peritoneal lavage viral loads of the NASBA-positive STAMP-treated mice were lower than those of NASBA-positive control mice (F ratio = 15.6; P = 0.0002). Whereas peritoneal lavage specimens from 14 of 14 vehicle-treated control mice showed NASBA evidence of HIV infection, with an average viral load of 4.6 ± 0.2 logs, peritoneal lavage specimens from only 1 of 15 test mice treated with 50-mg/kg STAMP and none of the 9 test mice treated with 100-mg/kg STAMP showed NASBA evidence of HIV infection (Table 2). STAMP treatment was not associated with any morbidity or mortality. Histopathologic examination of multiple organs from 34 SCID mice treated with STAMP at dosages of 10 mg/kg/day (cumulative dose = 140 mg/kg; n = 5), 25 mg/kg/day (cumulative dose = 350 mg/kg; n = 5), 50 mg/kg (cumulative dose = 700 mg/kg, n = 15), or 100 mg/kg (cumulative dose = 1.4 g/kg; n = 9) did not reveal any toxic lesions (Table 2).
TABLE 2.
Anti-HIV activity of STAMP against BR/92/019 in Hu-PBL-SCID micea
| Treatment | No. of specimens positive for HIV by NASBA/no. tested (no. Cx positive/no. tested)
|
Mean viral load ± SE (range) in:
|
No. with toxic lesions/no. tested | ||
|---|---|---|---|---|---|
| Spleen | PL | Spleen | PL | ||
| i.p. route | |||||
| Control (vehicle) (n = 16) | 15/16 (16/16) | 14/14 | 4.5 ± 0.2 (3.2-5.5) | 4.6 ± 0.2 (3.3-5.9) | 0/10 |
| STAMP dose (mg/kg) | |||||
| 10 (n = 10) | 4/5 (4/5) | 2/10 | 3.9 ± 0.3 (3.1-4.3) | 3.8 ± 0.3 (2.9-4.3) | 0/5 |
| 25 (n = 5) | 2/5 (2/5) | 2/5 | 3.8 ± 0.6 (3.2-4.4) | 3.5 ± 0.7 (2.8-4.2) | 0/5 |
| 50 (n = 15) | 6/15 (4/15) | 1/15 | 4.2 ± 0.1 (4.0-4.7) | 4.1 | 0/15 |
| 100 (n = 9) | 2/8 (0/7) | 0/9 | 3.5 ± 0.3 (3.3-3.8) | NAb | 0/9 |
| Gavage | |||||
| Control (vehicle) (n = 23) | 20/22 | 23/23 | 4.4 ± 0.1 (3.3-5.6) | 5.9 ± 0.2 (4.2-7.2) | 0/16 |
| STAMP dose (mg/kg) | |||||
| 50 (n = 10) | 4/10 | 7/10 | 4.4 ± 0.3 (3.8-5.3) | 5.0 ± 0.3 (3.8-5.7) | NDc |
| 100 (n = 20) | 7/20 | 12/20 | 4.0 ± 0.3 (3.1-5.1) | 4.8 ± 0.3 (3.1-6.2) | 0/10 |
| 200 (n = 20) | 7/20 | 12/20 | 3.7 ± 0.2 (3.2-4.5) | 4.8 ± 0.2 (3.2-5.9) | 0/9 |
| 400 (n = 9) | 0/9 | 1/9 | NA | 4.8 | 0/9 |
| Combivir (n = 10)d | 3/10 | 6/10 | 3.2 ± 0.3 (1.5-3.4) | 4.0 ± 0.4 (3.0-5.2) | ND |
| Added to food | |||||
| Control (vehicle) (n = 20) | 15/20 | 17/20 | 4.4 ± 0.2 (3.0-6.4) | 4.3 ± 0.2 (2.9-5.5) | 0/10 |
| STAMP dose (mg/kg) | |||||
| 200 (n = 10) | 3/10 | 5/8 | 3.8 ± 0.5 (3.2-4.8) | 4.3 ± 0.3 (3.4-5.1) | 0/10 |
| 400 (n = 10) | 1/10 | 1/9 | 3.0 | 3.1 | 0/10 |
Drugs were administered i.p. or via gavage twice daily for 7 days/week for 2 weeks. All mice were reconstituted with 107 PBMC and infected with 105 50% tissue culture infective dose of the HIV-1 isolate BR/92/019 2 weeks after reconstitution. Treatments were started immediately after the inoculation of the HIV-1 isolate. Results are presented as the fractions of HIV-NASBA positive and HIV coculture-positive mice as well as the arithmetic mean values of the log10-transformed spleen and peritoneal lavage (PL) viral loads with (ranges are indicated in parentheses). The median values and geometric means for the log10-transformed spleen (peritoneal lavage) viral loads in i.p.-treated mice were, respectively, 4.5 and 4.4 (4.6 and 4.5) for vehicle controls, 4.1 and 3.9 (4.1 and 3.8) for 10-mg/kg STAMP, 3.8 and 3.8 (3.5 and 3.4) for 25-mg/kg STAMP, 4.0 and 4.0 for 50-mg/kg STAMP, and 3.6 and 3.5 for 100-mg/kg STAMP. The median values and geometric means for the log10-transformed spleen (peritoneal lavage) viral loads in gavage-treated mice were, respectively, 4.5 and 4.4 (6.0 and 5.9) for vehicle controls, 4.4 and 4.4 (5.3 and 5.0) for 50-mg/kg STAMP, 3.9 and 3.9 (5.0 and 4.7) for 100-mg/kg STAMP, and 3.7 and 3.7 (4.9 and 4.8) for 200-mg/kg STAMP. The median values and geometric means for the log10 transformed spleen (peritoneal lavage) viral loads in p.o. (food supplement)-treated mice were, respectively, 4.0 and 4.3 (4.5 and 4.3) for vehicle controls and 3.5 and 3.8 (4.6 and 4.3) for 200-mg/kg STAMP.
NA, not applicable.
ND, not determined.
Combivir is 400-mg/kg ZDV plus 200-mg/kg 3TC.
As shown in Table 2, STAMP also exhibited statistically significant antiviral activity against the BR/92/019 isolate when it was administered p.o., either via gavage or by addition to the food. The twice-daily administration of STAMP via gavage resulted in a dose-dependent reduction in the incidence of NASBA positivity of the spleen specimens (χ2 = 33.3; P < 0.0001), with an ED50 of 99 mg/kg (51 to 150 mg/kg [logistic regression fit]), and peritoneal lavage specimens (χ2 = 31.6; P < 0.0001), with an ED50 of 203 mg/kg (150 to 303 mg/kg [logistic regression fit]). Whereas spleen specimens from 20 of 22 vehicle-treated control mice and peritoneal lavage specimens from 23 of 23 vehicle-treated control mice showed NASBA evidence of HIV infection, spleen specimens from none and peritoneal lavage specimens from only 1 of 9 test mice treated with 400-mg/kg/day STAMP were NASBA positive for HIV. By comparison, of the 10 control mice treated with 400-mg/kg ZDV plus 200-mg/kg 3TC, 3 had spleens that were NASBA positive for HIV and 6 had peritoneal lavage specimens that were NASBA positive for HIV (Table 2). Reminiscent of the results obtained with the i.p. administration route, the spleen (F ratio = 6.9; P = 0.001) and peritoneal lavage (F ratio = 15.6; P = 0.0002) viral loads of the NASBA-positive STAMP-treated mice were lower than those of NASBA-positive control mice. STAMP treatment was not associated with morbidity or mortality. Histopathologic examination of multiple organs from 28 SCID mice treated with STAMP at dosages of 100 mg/kg/day (cumulative dosage = 1.4 g/kg/day; n = 10), 200 mg/kg (cumulative dosage = 2.8 g/kg; n = 9), or 400 mg/kg (cumulative dosage = 5.6 g/kg; n = 9) did not reveal any toxic lesions. Addition of STAMP to the food also resulted in significant anti-HIV activity against BR/92/019, as measured by lower incidence of NASBA positivity of spleens (χ2 = 14.6; P = 0.005) as well as peritoneal lavage specimens (χ2 = 24.8; P < 0.0001) for HIV. The estimated ED50s were 120 mg/kg (12 to 271 mg/kg) for the effect on the spleen involvement and 286 mg/kg (191 to 470 mg/kg) for the effect on the peritoneal cavity involvement. This treatment was not associated with morbidity or mortality. Histopathologic examination of multiple organs from 20 SCID mice treated with STAMP at dosages of 200 mg/kg/day (cumulative dosage = 2.8 g/kg/day; n = 10) or 400 mg/kg (cumulative dosage = 5.6 g/kg; n = 9) did not reveal any toxic lesions (Table 2).
DISCUSSION
Our previous studies established the novel NRTI compound STAMP as an anti-HIV agent with potent activity against genotypically and phenotypically NRTI-resistant primary clinical HIV-1 isolates (28) and a favorable pharmacokinetics profile (2). In the present study of STAMP, we examined its acute-, subacute-, and chronic-toxicity profiles in mice. STAMP was very well tolerated in BALB/c, CD-1, and CB17-SCID mice, without any detectable acute or subacute toxicity at single i.p. or p.o. bolus dose levels as high as 500 mg/kg. Notably, daily administration of STAMP i.p. or p.o. for up to 8 consecutive weeks was not associated with any detectable toxicity at cumulative dose levels as high as 6.4 g/kg. In particular, STAMP did not cause gastrointestinal toxicity, hematologic toxicity, hepatotoxicity, neurotoxicity, or pancreas toxicity.
Recent studies of the in vivo pharmacokinetics and metabolism of STAMP in mice revealed that it forms two active metabolites, namely, ala-d4T-MP and STV, with favorable pharmacokinetics after systemic administration (2). Ala-d4T-MP exhibits potent anti-HIV activity at nanomolar concentrations (2). In the present study, micromolar concentrations of the active STAMP metabolite in plasma were rapidly achieved and maintained for more than 4 h after parenteral as well as p.o. administration of a nontoxic 100-mg/kg-bolus dose of STAMP. In accordance with its favorable pharmacokinetic profile and its in vitro potency, STAMP was active against the NRTI-resistant primary clinical HIV-1 isolate BR/92/019 in Hu-PBL-SCID mice at nontoxic cumulative dose levels. Spleen specimens as well as peritoneal lavage specimens from mice treated with STAMP twice daily either via the i.p. route or p.o. for 14 days showed NASBA evidence of HIV infection less frequently than vehicle-treated control mice. Furthermore, the spleen and peritoneal lavage viral loads of the NASBA-positive STAMP-treated mice were lower than those of NASBA-positive control mice. STAMP treatment of SCID mice was not associated with any morbidity or mortality. Histopathologic examination of multiple organs from STAMP-treated SCID mice did not reveal any toxic lesions. We have previously reported that STV is one of the in vivo metabolites of STAMP (2). The seemingly more favorable toxicity profile of STAMP when compared to STV despite the production of STV as a metabolite could be due to altered pharmacokinetics and disposition of STV in the presence of STAMP. This notion is supported by the fact that STAMP-derived STV has a much longer plasma t1/2 (2). It is also possible that STV yields lower amounts of the active d4T-triphosphate metabolite in the presence of ala-d4T-MP, which might serve as a better substrate for the kinases. The interactions between STAMP and its metabolites, especially STV, and the differences between STAMP and STV will be the subject of our future investigations.
We have previously reported that the para-bromine group in the phenyl moiety of STAMP contributes to its ability to undergo rapid hydrolysis, yielding the key active metabolite ala-d4T-MP in a thymidine kinase-independent fashion (27, 30, 32). Hence, the potency of STAMP against STV-resistant and ZDV-resistant HIV-1 isolates may be due to the rapid kinetics of the generation of its active triphosphate metabolite, yielding much higher inhibitor concentrations at the catalytic site, sufficient to overcome the binding restrictions imposed by the ZDV or STV resistance-associated RT mutations. It is also possible that the presence of an alaninyl side chain may promote the binding and/or incorporation of the triphosphate metabolite of STAMP. This possibility as well as explanation of the intracellular metabolic pathways of STAMP will be the subject of our future investigations aimed at elucidating the molecular mechanisms contributing to the remarkable potency of STAMP against ZDV-resistant HIV-1 isolates, which is unprecedented for an NRTI compound.
There are at least 10 distinct group M (major) subtypes of HIV-1 strains that are endemic to distinct geographical sites (5, 9). Currently available anti-HIV agents have been traditionally developed against subtype B HIV-1 strains, which are the predominant strains in the United States and Europe although worldwide the majority of HIV-infected individuals are infected with non-subtype B strains and the vast majority of new infections are caused by non-subtype B HIV-1 strains (5, 9, 16). Therefore, there is an urgent need to identify anti-HIV agents with potent activity against non-subtype B HIV-1. Notably, STAMP was active against each of nine primary non-subtype B HIV-1 isolates originating from South America, Asia, and sub-Saharan Africa, with IC50s ranging from 0.5 to 7 nM (2). By comparison, the recently reported in vitro IC50s of the active nucleotide analog anti-HIV agents tenofovir and adefovir against these same isolates were 100- to 20,000-fold higher (13).
A high proportion of all HIV-1-infected patients receiving contemporary antiretroviral therapies experience virologic failure initially due to RT mutations associated with NRTI resistance (10, 15, 18; Briones et al., letter). Numerous studies have also documented reduced drug susceptibility among persons with recently acquired HIV-1 infection (1, 12). Furthermore, recent studies have demonstrated wide cross-resistance between the thymidine analogs ZDV and STV, which form the foundation for most NRTI combinations in clinical use (3, 4, 6, 8, 10, 11, 14, 15, 17-26, 31). Thus, for an increasing percentage of both treatment-experienced and treatment-naïve HIV-1-infected patients, available NRTI offer limited therapeutic options. There is an urgent need for new NRTI that are active against HIV-1 that has developed resistance to the available drugs within the NRTI class. Our recent results demonstrated that STAMP is a highly potent inhibitor of NRTI-resistant HIV-1 isolates (28). To date, we have not been able to identify a single primary HIV-1 isolate or laboratory HIV-1 strain that is resistant to STAMP. The present study provides evidence that STAMP exhibits potent in vivo antiretroviral activity against NRTI-resistant HIV-1 at nontoxic doses. The favorable cytotoxicity profile of STAMP along with the observed absence of animal toxicity support expanded study of STAMP in large animal species in preparation for its initial use in humans. The remarkable potency of STAMP against primary clinical HIV-1 isolates with genotypic and/or phenotypic NRTI or NNRTI resistance warrants the further development of this promising new antiviral agent for possible clinical use in both treatment-naïve and treatment-experienced HIV-infected persons.
REFERENCES
- 1.Boden, D., A. Hurley, L. Zhang, Y. Cao, Y. Guo, E. Jones, J. Tsay, J. Ip, C. Farthing, K. Limoli, N. Parkin, and M. Markowitz. 1999. HIV-1 drug resistance in newly infected individuals. JAMA 282:1135-1141. [DOI] [PubMed] [Google Scholar]
- 2.Chen, C., T. Venkatachalam, Z. Zhu, and F. M. Uckun 2001. In vivo pharmacokinetics and metabolism of stampidine, a novel aryl phosphate derivative of d4T with potent anti-HIV activity. Drug Metab. Dispos. 29:1035-1041. [PubMed] [Google Scholar]
- 3.Coakley, E. P., J. M. Gillis, and S. M. Hammer. 2000. Phenotypic and genotypic resistance patterns of HIV-1 isolates derived from individuals treated with didanosine and stavudine. AIDS 14:F9-F15. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez, F.,J. Molto, C. Escolano, A. Mora, F. Pasquau, J. Gregori, and E. Nogueira. 2000. Genotypic resistance to antiretroviral drugs in patients with therapeutic failure to highly active antiretroviral therapy. Med. Clin. (Barcelona) 115:401-404. [DOI] [PubMed] [Google Scholar]
- 5.Hu, D. J., T. J. Dondero, M. A. Rayfield, J. R. George, G. Schochetman, H. W. Jaffe, C. C. Luo, M. L. Kalish, B. G. Weniger, C. P. Pau, C. A. Schable, and J. W. Curran 1996. The emerging genetic diversity of HIV: the importance of global surveillance for diagnostics, research and prevention. JAMA 275:210-216. [PubMed] [Google Scholar]
- 6.Izopet, J., A. Bicart-See, C. Pasquier, K. Sandres, E. Bonnet, B. Marchou, J. Puel, and P. Massip. 1999. Mutations conferring resistance to zidovudine diminish the antiviral effect of stavudine plus didanosine. J. Med. Virol. 59:507-511. [PubMed] [Google Scholar]
- 7.Jackson, J. B., S. Y. Kwok, J. J. Sninsky, J. S. Hopsicker, K. J. Sannerud, F. S. Rhame, K. Henry, M. Simpson, and H. H. Balfour. 1990. Human immunodeficiency virus type 1 detected in all seropositive symptomatic and asymptomatic individuals. J. Clin. Microbiol. 28:16-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson, V. A. 1995. Nucleoside reverse transcriptase inhibitors and resistance of human immunodeficiency virus type 1. J Infect. Dis 171(Suppl. 2):S140-S149. [DOI] [PubMed] [Google Scholar]
- 9.Kuiken, C. L., B. Foley, and B. Hahn. 1999. Human retroviruses and AIDS: A compilation and analysis of nucleic acid and amino acid sequences. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 10.Kuritzkes, D. R., A. Sevin, B. Young, M. Bakhtiari, H. Wu, M. St. Clair, E. Connick, A. Landay, J. Spritzler, H. Kessler, and M. M. Lederman. 2000. Effect of zidovudine resistance mutations on virologic response to treatment with zidovudine-lamivudine-ritonavir: genotypic analysis of human immunodeficiency virus type 1 isolates from AIDS clinical trials group protocol 315.ACTG Protocol 315 Team. J. Infect. Dis. 181:491-497. [DOI] [PubMed] [Google Scholar]
- 11.Lin, P. F., C. J. Gonzalez, B. Griffith, G. Friedland, V. Calvez, F. Ferchal, R. F. Schinazi, D. H. Shepp, A. B. Ashraf, M. A. Wainberg, V. Soriano, J. W. Mellors, and R. J. Colonno. 1999. Stavudine resistance: an update on susceptibility following prolonged therapy. Antivir. Ther. 4:21-28. [PubMed] [Google Scholar]
- 12.Little, S. J., E. S. Daar, R. T. D'Aquila, P. H. Keiser, E. Connick, J. M. Whitcomb, N. S. Hellmann, C. J. Petropoulos, L. Sutton, J. A. Pitt, E. S. Rosenberg, R. A. Koup, B. D. Walker, and D. D. Richman. 1999. Reduced antiretroviral drug susceptibility among patients with primary HIV infection. JAMA 282:1142-1149. [DOI] [PubMed] [Google Scholar]
- 13.Miller, M. D., N. A. Margot, P. D. Lamy, M. D. Fuller, K. E. Anton, A. S. Mulato, and J. M. Cherrington. 2001. Adefovir and tenofovir susceptibilities of HIV-1 after 24 to 48 weeks of adefovir dipivoxil therapy: genotypic and phenotypic analyses of study GS-96-408. J. Acquir. Immune Defic. Syndr. 27:450-458. [DOI] [PubMed] [Google Scholar]
- 14.Miller, V., M. Ait-Khaled, C. Stone, P. Griffin, D. Mesogiti, A. Cutrell, R. Harrigan, S. Staszewski, C. Katlama, G. Pearce, and M. Tisdale. 2000. HIV-1 reverse transcriptase (RT) genotype and susceptibility to RT inhibitors during abacavir monotherapy and combination therapy. AIDS 14:163-171. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien, W. A. 2000. Resistance against reverse transcriptase inhibitors. Clin. Infect. Dis. 30(Suppl. 2):S185-S192. [DOI] [PubMed] [Google Scholar]
- 15a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 16.Palmer, S., A. Alaeus, J. Albert, and S. Cox. 1998. Drug susceptibility of subtypes A, B, C, D, and E human immunodeficiency virus type 1 isolates. AIDS Res. Hum. Retrovir. 14:157-162. [DOI] [PubMed] [Google Scholar]
- 17.Picard, V., E. Angelini, A. Maillard, E. Race, F. Clavel, G. Chene, F. Ferchal, and J. M. Molina. 2001. Comparison of genotypic and phenotypic resistance patterns of human immunodeficiency virus type 1 isolates from patients treated with stavudine and didanosine or zidovudine and lamivudine. J. Infect. Dis. 184:781-784. [DOI] [PubMed] [Google Scholar]
- 18.Pillay, D., S. Taylor, and D. D. Richman. 2000. Incidence and impact of resistance against approved antiretroviral drugs. Rev. Med. Virol. 10:231-253. [DOI] [PubMed] [Google Scholar]
- 19.Rey, D., M. P. Schmitt, M. Partisani, G. Hess-Kempf, V. Krantz, E. de Mautort, C. Bernard-Henry, M. Priester, C. Cheneau, and J. M. Lang. 2001. Efavirenz as a substitute for protease inhibitors in HIV-1-infected patients with undetectable plasma viral load on HAART: a median follow-up of 64 weeks. J. Acquir. Immune Defic. Syndr. 27:459-462. [DOI] [PubMed] [Google Scholar]
- 20.Richman, D. D. 2001. HIV chemotherapy. Nature 410:995-1001. [DOI] [PubMed] [Google Scholar]
- 21.Ross, L., A. Scarsella, S. Raffanti, K. Henry, S. Becker, R. Fisher, Q. Liao, A. Hirani, N. Graham, M. St. Clair, and J. Hernandez. 2001. Thymidine analog and multinucleoside resistance mutations are associated with decreased phenotypic susceptibility to stavudine in HIV type 1 isolated from zidovudine-naive patients experiencing viremia on stavudine-containing regimens. AIDS Res. Hum. Retrovir. 17:1107-1115. [DOI] [PubMed] [Google Scholar]
- 22.Rousseau, M. N., L. Vergne, B. Montes, M. Peeters, J. Reynes, E. Delaporte, and M. Segondy. 2001. Patterns of resistance mutations to antiretroviral drugs in extensively treated HIV-1-infected patients with failure of highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 26:36-43. [DOI] [PubMed] [Google Scholar]
- 23.Shafer, R. W., and D. A. Vuitton. 1999. Highly active antiretroviral therapy (HAART) for the treatment of infection with human immunodeficiency virus type 1. Biomed. Pharmacother. 53:73-861. [DOI] [PubMed] [Google Scholar]
- 24.Shulman, N. S., R. A. Machekano, R. W. Shafer, M. A. Winters, A. R. Zolopa, S. H. Liou, M. Hughes, and D. A. Katzenstein. 2001. The AIDS Clinical Trials Group 302 Study Team. Genotypic correlates of a virologic response to stavudine after zidovudine monotherapy. J. Acquir. Immune Defic. Syndr. 27:377-380. [DOI] [PubMed] [Google Scholar]
- 25.Starr, S. E., C. V. Fletcher, S. A. Spector, F. H. Yong, T. Fenton, R. C. Brundage, D. Manion, N. Ruiz, M. Gersten, M. Becker, J. McNamara, L. M. Mofenson, L. Purdue, S. Siminski, B. Graham, D. M. Kornhauser, W. Fiske, C. Vincent, H. W. Lischner, W. M. Dankner, P. M. Flynn, et al.. 1999. Combination therapy with efavirenz, nelfinavir, and nucleoside reverse-transcriptase inhibitors in children infected with human immunodeficiency virus type 1. N. Engl. J. Med. 341:1874-1881. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki, K., G. R. Kaufmann, M. Mukaide, P. Cunningham, C. Harris, L. Leas, M. Kondo, M. Imai, S. L. Pett, R. Finlayson, J. Zaunders, A. Kelleher, and D. A. Cooper. 2001. Novel deletion of HIV type 1 reverse transcriptase residue 69 conferring selective high-level resistance to nevirapine. AIDS Res. Hum. Retrovir. 17:1293-1296. [DOI] [PubMed] [Google Scholar]
- 27.Uckun, F. M., and R. Vig. February 2000. Aryl phosphate derivatives of d4T having anti-HIV activity. U.S. patent 6,030,957.
- 28.Uckun, F. M., S. Pendergrass, T. K. Venkatachalam, S. Qazi, and D. Richman. 2002. Stampidine is a potent inhibitor of zidovudine- and nucleoside analog reverse transcriptase inhibitor-resistant primary clinical human immunodeficiency virus type 1 isolates with thymidine analog mutations. Antimicrob. Agents Chemother. 46:3613-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uckun, F. M., L. M. Chelstrom, L. Tuel-Ahlgren, I. Dibirdik, J. D. Irvin, M. Chandan-Langlie, and D. E. Myers. 1998. TXU (anti-CD7)-pokeweed antiviral protein as a potent inhibitor of human immunodeficiency virus. Antimicrob. Agents Chemother. 42:383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkatachalam, T. K., H.-L. Tai, R. Vig, C.-L. Chen, S.-T. Jan, and F. M. Uckun. 1998. Enhancing effects of a mono-bromo substitution at the para position of the phenyl moiety on the metabolism and anti-HIV activity of D4T-phenyl methoxyalaninyl phosphate derivatives. Bioorgan. Med. Chem. Lett. 8:3121-3126. [DOI] [PubMed] [Google Scholar]
- 31.Venturi, G., L. Romano, M. Catucci, M. L. Riccio, A. De Milito, A. Gonnelli, M. Rubino, P. E. Valensin, and M. Zazzi. 1999. Genotypic resistance to zidovudine as a predictor of failure of subsequent therapy with human immunodeficiency virus type-1 nucleoside reverse-transcriptase inhibitors. Eur. J. Clin. Microbiol. Infect. Dis. 18:274-282. [DOI] [PubMed] [Google Scholar]
- 32.Vig, R., T. K. Venkatachalam, and F. M. Uckun. 1998. D4T-5′-[p-bromophenyl methoxyalaninyl phosphate] as a potent and non-toxic anti-human immunodeficiency virus agent. Antivir. Chem. Chemother. 9:445-448. [PubMed] [Google Scholar]