Abstract
While genetic influences in schizophrenia are substantial, the disorder's molecular genetic basis remains elusive. Progress has been hindered by lack of means to detect nonpenetrant carriers of the predisposing genes and by uncertainties concerning the extent of locus heterogeneity. One approach to solving this complexity is to examine the inheritance of pathophysiological processes mediating between genotype and disease phenotype. Here we evaluate whether deficits in neurocognitive functioning covary with degree of genetic relationship with a proband in the unaffected MZ and DZ co-twins of patients with schizophrenia. Twin pairs discordant for schizophrenia were recruited from a total population cohort and were compared with a demographically balanced sample of control twin pairs, on a comprehensive neuropsychological test battery. The following four neuropsychological functions contributed uniquely to the discrimination of degree of genetic loading for schizophrenia and, when combined, were more highly correlated within MZ pairs than within DZ pairs, in both discordant and control twins: spatial working memory (i.e., remembering a sequence of spatial locations over a brief delay), divided attention (i.e., simultaneous performance of a counting and visual-search task), intrusions during recall of a word list (i.e., “remembering” nonlist items), and choice reaction time to visual targets. Together with evidence from human and animal studies of mediation of these functions by partially distinct brain systems, our findings suggest that there are multiple independently inherited dimensions of neural deficit in schizophrenia and encourage a search for genes contributing to quantitative variation in discrete aspects of disease liability. On tests of verbal and visual episodic memory, but not on the liability-related measures, patients were more impaired than their own MZ co-twins, suggesting a preferential impact of nongenetic influences on long-term memory systems.
Introduction
Twin and adoption studies have demonstrated a substantial genetic contribution to schizophrenia (i.e., on the order of 65%–85% [Kendler and Diehl 1993; Cannon et al. 1998a] [MIM 604906]), but the molecular genetic basis of the disorder remains elusive (Karayiorgou and Gogos 1997). These efforts have been hindered in part by lack of means to detect non–clinically penetrant carriers of the predisposing genes and by uncertainties concerning both the nature of the nongenetic etiologic influences and the extent of locus heterogeneity. Reduced penetrance is indicated by <100% concordance for schizophrenia and related disorders in MZ twins (Cannon et al. 1998a) and by equivalent morbid risks in offspring of affected and unaffected MZ co-twins (Fisher 1971; Gottesman and Bertelsen 1989). Heterogeneity is suggested by the absence of a common chromosomal region linked to schizophrenia in all or a majority of families, even those sampled from isolated gene pools (Karayiorgou and Gogos 1997; Hovatta et al. 1999). This complexity encourages a search for phenotypic indicators that reflect discrete components of pathophysiological processes mediating between particular sets of predisposing genes and clinical diagnosis (“endophenotypes” [Gottesman et al. 1987; Cannon 1996; Freedman et al. 1997]). Such indicators should be sensitive to the degree of genetic liability in clinically unaffected pedigree members and could be used to select more-homogeneous subgroups of families for linkage analysis.
Promising endophenotypes for schizophrenia include indicators of disturbances in prefrontal and temporo-limbic systems and their interconnections (Kremen et al. 1994; Cannon 1996). Impairments in these systems are relatively prominent against a background of generalized cerebral dysfunction (Saykin et al. 1994; Cannon et al. 1998b), and individuals with acquired lesions in these areas show many of the symptoms characteristic of schizophrenia (Luria 1966; Halgren 1982; Roberts et al. 1990). Several studies have demonstrated familial aggregation of schizophrenia with deficits in neuropsychological tests sensitive to prefrontal and temporal lobe damage, including tests of working memory, mental sequencing, and episodic learning and memory (Goldberg et al. 1990; Cannon et al. 1994; Kremen et al. 1994; Faraone et al. 1995; Byrne et al. 1999). Deficits on these tests have been observed in patients during both active and remitted phases of illness and both before and during treatment with antipsychotic drugs (Cannon et al. 1994; Saykin et al. 1994; Censits et al. 1997). Similar deficits appear in a portion of their nonschizophrenic first-degree relatives, even after those with substance-use disorders and other psychiatric diagnoses are excluded (Cannon et al. 1994; Faraone et al. 1999).
It remains to be determined whether the neuropsychological deficits in relatives of patients with schizophrenia could be explained by nongenetic influences shared in common by individuals reared in the same home. Although shared environmental influences are not likely to have a major impact on the transmission of schizophrenia overall (Cannon et al. 1998a), such factors could play a role in the transmission of impaired neurocognition, given their contribution, in the general population, to familial resemblance in intellectual functioning (Bouchard et al. 1990). If the deficits in relatives of patients with schizophrenia reflect a genetic rather than an environmental source, one would expect a greater degree of deficit the closer the genetic relationship to a proband, when environmental similarity among relative pairs is held constant. One such test is a comparison of the nonschizophrenic MZ and DZ co-twins of patients with schizophrenia. Both types of twins share gestational and postgestational rearing environments with a future patient but differ by a ratio of 2:1 in genetic liability to the disease. Heritability of an indicator would be supported also if the degree of deficit is correlated within pairs of discordant co-twins and more highly so among MZ than among DZ pairs. Although a prior study reported evidence of impaired neurocognition in the MZ co-twins of patients with schizophrenia, compared with controls (Goldberg et al. 1990), the possibility of a shared environmental contribution to these deficits was not addressed. To our knowledge, no previous published study has evaluated twin resemblance in neurocognitive functioning among both MZ and DZ twins discordant for schizophrenia, as compared with control pairs of both zygosities.
Here we have applied a comprehensive neuropsychological test battery to samples of MZ and DZ twins discordant for schizophrenia who were ascertained from a total-population cohort and to demographically similar control twins without a personal or family history of schizophrenia or related disorders. Multiple cognitive domains were evaluated to assess whether two or more dimensions of neurocognitive dysfunction meet the criteria for an endophenotypic indicator after individual differences in the other domains and in general ability have been controlled, a pattern that would be consistent with their determination by at least partially independent sets of genes. We also sought to clarify whether genetic and nongenetic influences affect the same or at least partially different neural systems, both by comparing the neurocognitive-deficit profiles of patients with schizophrenia and those of their co-twins and by comparing, between discordant and control pairs, the magnitude of the heritability estimate on each measure.
Subjects and Methods
The study protocol was reviewed and approved by the institutional review boards (IRBs) of the University of California (Los Angeles), the University of Pennsylvania, and the National Public Health Institute of Finland, and all participants signed IRB-approved informed-consent forms.
Subjects
Subjects were drawn from a twin cohort consisting of all of the same-sex twins born in Finland during 1940–57, inclusive, and in which both members of each pair were alive and residing in Finland as of 1967 (N=9,562 pairs: 2,495 MZ, 5,378 DZ, and 1,689 of unknown zygosity, according to a questionnaire-based classification [Kaprio et al. 1990]). This cohort was screened, for the period 1969–91, for a history of hospitalization, medicine prescriptions, and/or work disability due to a psychiatric indication, in three national computerized databases—the Hospital Discharge Register, the Free Medicine Register, and the Pension Register (Cannon et al. 1998a). These searches identified a total of 335 pairs containing at least one member with a diagnosis of schizophrenia or schizoaffective disorder according to any of the three sources. Discordant MZ and DZ pairs were recruited randomly from this population, along with demographically balanced samples of control pairs of each zygosity. Discordant pairs in which, on direct interview, either the proband had a diagnosis of schizoaffective disorder–affective type or the co-twin had a psychotic-disorder diagnosis were excluded. Control pairs were excluded either if there was a history of psychosis-related treatment or work disability in any of their first-degree relatives or if either co-twin was found, on direct interview, to meet diagnostic criteria for a psychotic disorder or schizotypal, paranoid, or schizoid personality disorder. The final samples consisted of 48 pairs discordant for schizophrenia (18 MZ and 30 DZ) and 55 control pairs (28 MZ and 27 DZ). In addition, we evaluated 8 pairs concordant for schizophrenia, to test for comparability, in clinical and neuropsychological measures, between probands from concordant and discordant pairs.
Procedures
Clinical evaluation
Each co-twin was interviewed by a different examiner who was blind to zygosity and to diagnostic status of the other co-twin. All subjects were interviewed using the Structured Clinical Interview for DSM-III-R Disorders, Patient or Non-Patient edition (Spitzer et al. 1989). Any subject with a psychotic condition was also rated using the Scale for the Assessment of Positive Symptoms (SAPS [Andreasen 1984]) and Scale for the Assessment of Negative Symptoms (SANS [Andreasen 1983]). All other subjects were interviewed using the Personality Disorders Examination (Loranger et al. 1985). A standardized medical-record coding form was used to summarize details of the illness and treatment history of any subject with a history of inpatient admissions. The interviewer assigned diagnoses according to Diagnostic and Statistical Manual of Mental Disorders, Version IV (DSM-IV [American Psychiatric Association 1994]) criteria, utilizing all of the information available on each case. For each subject, a detailed case report summarizing the clinical, social, occupational, and medical history was generated and subsequently stripped of identifying and diagnostic information. Another diagnostician, who was not involved in the initial interviews, read the case summaries and independently assigned a primary DSM-IV diagnosis to each case. Reliability (Cohen 1960) of the primary diagnosis was excellent (i.e., κ=.96±.02). Diagnostic disagreements were flagged, and another independent diagnostician rated those cases for consensus diagnoses.
Zygosity
For all pairs, zygosity was determined by DNA analysis using the following markers: DIS80 (20 alleles), DI7S30 (13 alleles), apoB (20 alleles), COL2A1 (10 alleles), vWA (9 alleles), and HUMTH01 (6 alleles). Assuming an average heterozygosity rate of 70% per marker, we estimate that this procedure will falsely classify a DZ pair as MZ in ∼1/482 cases.
Neuropsychological testing
A comprehensive neuropsychological test battery was administered to each subject by pre- and postdoctoral examiners who worked under the supervision of neuropsychology faculty and who, prior to the assessments, had extensive training with the battery. Each co-twin was tested by a different examiner who was blind to both zygosity and diagnostic status of the other co-twin. All subjects received the same battery of tests, in a fixed order. Total time for the test battery was ∼3–4 h, including rest breaks as needed.
Table 1 provides a description of the measures and corresponding cognitive functions that they were designed to assess. The test instruments were selected to measure, with approximately equivalent reliability, a broad range of cognitive functions, the neural mechanisms of which have been at least partially dissociated from each other in animal and/or human studies. A well-established distinction within the domain of memory is that between short-term, or “working,” memory and long-term, or “episodic,” memory, the former depending critically on prefrontal cortical regions (e.g., see Funahashi et al. 1989) and the latter depending on medial temporal lobe structures and adjacent temporal cortex (e.g., see Squire and Zola 1996). A further distinction may be made in terms of mnemonic and other types of processing (e.g., reasoning) in relation to verbal versus nonverbal information, reflecting the general tendency for specialized processing of the two types of information, in the left and right hemispheres, respectively (Benson and Zaidel 1985). Within the domain of verbal episodic memory, depending on lesion size and the relative involvement of prefrontal and temporal lobe structures, amnestic syndromes may involve a preferential impact on the retrieval of previously learned information from long-term storage, the rate of learning and recall of newly presented information, organizational strategies during recall (such as semantic clustering or logical reconstruction, as in memory of stories), or recall errors such as intrusions (“remembering” items that were not in the learning set) (Schacter et al. 1998). In the visuospatial domain there are separate areas specialized for processing faces versus other types of complex visual stmuli (Farah 1996). A number of complex cognitive operations require integration of different types of information or orchestration among different processes, as in the division of attention when two tasks must be performed simultaneously, sequencing of stimuli into an appropriate order, conceptual grouping of stimuli on the basis of their physical properties, and selective processing of a target in the presence of distraction, all involving “executive” processes that appear to be supported by partially distinct areas in the prefrontal cortex as well as by other regions (Posner and Petersen 1990; Koechlin et al. 1999; Corbetta et al. 1991). Finally, reaction time in speeded tasks may be affected by a motor-speed deficit that is separable from other cognitive operations that may be involved, such as stimulus detection, feature matching, and response selection (Sakai et al., in press). In addition to assessing these specific domains of functioning, we obtained a measure of general ability, in the form of an overall intelligence quotient (IQ), as prorated from scores on the Vocabulary, Similarities, Block Design, and Digit Symbol subtests of the Weschler Adult Intelligence Scale-Revised (WAIS-R [Wechsler 1981]).
Table 1.
Description of Measures Employed in the Neuropsychological Test Battery
| Label | Description (Measure and Instrument) | Reference |
| Verbal working memory | Immediate recall of strings of digits, either in original or reverse order of presentation (Digit Span subtest of Weschler Memory Scale–Revised) | Russell (1975) |
| Spatial working memory | Immediate recall of sequences of spatial locations, in original or reverse order of presentation (Visual Span subtest of Weschler Memory Scale–Revised) | Russell (1975) |
| Divided attention | Simultaneously counting backward and performing spatial cancellation (percentage decrement, from single-task performance in a Brown-Petersen dual-task paradigm) | Vilkki et al. (1996) |
| Sequencing | Alternating connection of letters and numbers in ascending sequence in a random spatial display (Trails B subtest of Halstead-Reitan Battery) | Reitan and Wolfson (1985) |
| Categorization | Conceptual grouping of items, on the basis of their shape, color, and number (categories achieved on Wisconsin Card Sort Test) | Heaton (1981) |
| Verbal fluency | Generation of animal names (no. of items generated in 1 min, on the Verbal Fluency subtest of the Multilingual Aphasia Examination) | Benton and Hamsher (1976) |
| Choice reaction time | Pressing the appropriate button when a target appears on either the left or the right side of fixation (average time to respond, in a Posner paradigm) | Finkelstien (1998) |
| Selective attention | Pressing a button when a target appears in a continuous series mixed with foils and in the presence of flanking distractors (false alarms on a Continuous Performance Task) | Finkelstien (1998) |
| Verbal episodic memory | Learning and recall of a word list (average no. of items recalled, on learning and on 5-min– and 20-min–delay trials of California Verbal Learning Test) | Delis et al. (1983) |
| Recall intrusions | “Recall” of items not on the learning list (California Verbal Learning Test) | Delis et al. (1983) |
| Semantic clustering | Proximal recall of semantically related items on the learning list (California Verbal Learning Test) | Delis et al. (1983) |
| Story memory | Learning and recall of prose passages (Weschler Memory Scale–Revised) | Russell (1975) |
| Visual episodic memory | Learning and recall of complex visual objects (Weschler Memory Scale–Revised–R) | Russell (1975) |
| Face recognition | Recognition of previously presented faces in a continuous series with foils | Gur et al. (1993) |
| Verbal knowledge and ability | Defining words and solving verbal analogies (Vocabulary and Similarities subtests of the WAIS-R) | Wechsler (1981) |
| Visuospatial ability | Assembling blocks to match a presented pattern, and rapid substitution of series of digits with symbols (Block Design and Digit Symbol subtests of WAIS-R) | Wechsler (1981) |
| Motor speed | Rapidity of index-finger tapping (Tapping subtest of the Halstead-Reitan Battery) | Reitan and Wolfson (1985) |
Statistical Analyses
The primary goals of the data analyses were (1) to identify neurocognitive deficits that increase linearly with genetic relationship to a proband, (2) to isolate the nongenetic contributions to neurocognitive deficits in patients with schizophrenia, and (3) to determine whether, on the test measures, co-twins show significant resemblance commensurate with their genetic relatedness.
-
1.
The neuropsychological test data were analyzed for association with genetic liability status (i.e., MZ co-twins vs. DZ co-twins vs. controls), by means of canonical discriminant analysis. This approach derives the combination of predictor variables that best discriminates among the different levels of the classification variable, controlling for redundancy (i.e., intercorrelation) in the predictors by evaluating their unique contributions to the discrimination. As an optimization procedure, discriminant analysis is sensitive both to actual group differences and to random variation that, by chance, differs between groups. It is thus generally recommended that investigators split their samples into halves, in order to estimate the replicability of findings. Although the sample sizes of co-twins of patients with schizophrenia are too small to permit statistically meaningful split-half analysis, the sample size of control twins is sufficiently large for this purpose. We therefore randomly sampled one control twin from each pair and used that group as controls in a calibration analysis, and we used the remaining control twins in a replication analysis. We also performed the analysis by using test data averaged within pairs of control twins, such that all control subjects were to some degree “represented” in the same analysis. This approach also preserves the assumption of independence of observations. Because there were significant variance differences, on the test measures, between the risk groups and controls (which were accentuated by 5%–15% in the analysis using within-pair averaged data), the within-class covariance matrices were used instead of the pooled covariance matrices (Cliff 1987). If two predictors share most of their criterion-related variation, neither may show an independent association with the criterion when both are included in the prediction model simultaneously. We therefore also repeated the analyses by using a stepwise elimination algorithm, excluding, in successive steps, predictors with insignificant (P>.10) contributions to the discrimination. A standardized canonical variate score was computed, representing the combination of predictors that best discriminated liability status.
-
2.
The nongenetic contributions to neurocognitive deficits in patients with schizophrenia were isolated by computing the average patient minus co-twin differences among discordant MZ pairs and evaluating these differences for statistical deviation from zero by using the matched-pair t-statistic. To reduce the chance of type I error, the test measures used in these comparisons were restricted to those which contributed uniquely to the discrimination either of liability groups or of patients with schizophrenia from (unrelated) control twins. The latter measures were identified in canonical discriminant analyses paralleling those described above in relation to liability status. It is important to emphasize that neurocognitive differences between patients and unrelated controls can reflect both genetic and nongenetic sources of variation—and therefore are of secondary importance in this context.
-
3.
Twin resemblance on the liability-related and disease-specific test measures was evaluated using the intraclass correlation coefficient (ICC). Genetic involvement is supported when the ICC for a trait in MZ pairs is significantly larger than that in DZ pairs. In most contexts, the ICC of a trait in MZ pairs can be taken as an index of its broad heritability (i.e., when both additive and nonadditive genetic effects are taken into account [Lynch and Walsh 1998]). Although, in relation to the neurocognitive variables, it would be desirable to derive separate estimates for additive and dominance genetic effects as well as for unique and shared environmental effects, testing for and quantifying the magnitude of such effects requires sample sizes that are much larger than those in the present study (Neale and Cardon 1992).
Results
Sample Characteristics and Tests of Bias
Table 2 gives demographic information on the patient, MZ co-twin, DZ co-twin, and control groups. The groups were equivalent in terms of age, gender, handedness, duration of cohabitation, parental social class, and current or lifetime recurrent affective disorder. Patients and MZ co-twins had significantly less educational attainment than did DZ co-twins and controls, and patients and their co-twins had significantly lower overall IQs than did controls. Overall IQ was highly correlated with both educational attainment (r=.56, P=.0001) and parental social class (r=.20, P=.005). Schizophrenia probands had a higher rate of substance-use disorders than did the other three groups, who did not differ from one another. The mean ± SD age at onset in the patient group was 25.3±6.1 years; their mean ± SD positive and negative symptom-severity scores (i.e., on the SAPS and SANS global items) were 2.3±0.8 and 1.9±1.1, respectively.
Table 2.
Demographic Characteristics of the Four Comparison Groups
|
Co-Twins |
||||||
| Measure | Patients (N=48) | MZ (N=18) | DZ (N=30) | Controls(N=110) | Statistic | P |
| No. (%) of females | 24 (50) | 9 (50) | 15 (50) | 52 (47) | χ2 = .2 | NS |
| No. (%) of left-/mixed-handed individuals | 4 (8) | 3 (17) | 3 (10) | 6 (6) | χ2 = 3.0 | NS |
| No. (%) of substance disorders | 11 (23) | 3 (17) | 3 (10) | 5 (5) | χ2 = 12.6 | .006 |
| No. (%) of affective disorders | 7 (15) | 3 (17) | 5 (17) | 10 (9) | χ2 = 2.2 | NS |
| Mean ± SD age (years) | 48.5 ± 4.9 | 48.9 ± 4.9 | 48.5 ± 5.0 | 49.1 ± 4.2 | F = .3 | NS |
| Mean ± SD parental socioeconomic statusa | 4.5 ± 1.3 | 4.8 ± 1.0 | 4.4 ± 1.5 | 4.4 ± 1.2 | F = .7 | NS |
| Mean ± SD cohabitation (years) | 19.6 ± 3.4 | 20.7 ± 2.9 | 18.9 ± 3.6 | 20.5 ± 4.3 | F = 1.5 | NS |
| Mean ± SD educationb | 2.9 ± 1.6 | 2.8 ± 1.7 | 3.7 ± 1.7 | 4.2 ± 1.6 | F = 8.8 | .01 |
| Mean ± SD overall IQc | 87.8 ± 15.6 | 97.1 ± 17.1 | 99.8 ± 17.4 | 107.4 ± 12.6 | F = 14.5 | .0001 |
The studied probands were comparable to the remainder of the discordant-twin proband population, in terms of year of birth (t298=-0.9, P=.34), sex (χ21=1.5, P=.21), age at first hospital admission (t264=0.32, P=.74), number of hospital admissions (t298=0.75, P=.45), and eligibility for disability pension (χ21=0.1, P=.71). Furthermore, studied MZ probands were equivalent to studied DZ probands, in terms of age at evaluation (t46=-0.6, P=.54), sex (χ21=0.0, P=.91), age at onset (t46=0.91, P=.34), positive-symptom severity (t46=1.2, P=.24), and negative-symptom severity (t46=0.1, P=.92). Finally, probands from discordant pairs did not differ from probands from concordant pairs, either in neuropsychological functioning overall (F(1,55)=0.6, P=.43) or on any of the specific measures (P>.20 in each case) or in severity of positive (t55=0.2, P=.82) or negative (t55=1.3, P=.23) symptoms.
Genetic Liability Status and Neuropsychological Functioning
The results of the analyses predicting genetic liability class are given in table 3; there was only minor variation between the calibration, replication, and whole-sample analyses. Our discussion of the results therefore focuses on the whole-sample analyses, which should be most broadly representative. The canonical correlation of the 17 cognitive test measures with liability status was highly significant (R=.64±.06, 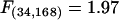 , P=.003), but only two measures contributed a significant proportion of unique variance to the discrimination: spatial working memory and divided attention. Along with choice reaction time and recall intrusions, these two variables were the only variables retained in the stepwise reduction of the prediction model to large-effects–only terms, resulting in a canonical correlation nearly identical to that in the all-effects model (R=.59±.06, F(8,194)=6.12, P=.0001).
, P=.003), but only two measures contributed a significant proportion of unique variance to the discrimination: spatial working memory and divided attention. Along with choice reaction time and recall intrusions, these two variables were the only variables retained in the stepwise reduction of the prediction model to large-effects–only terms, resulting in a canonical correlation nearly identical to that in the all-effects model (R=.59±.06, F(8,194)=6.12, P=.0001).
Table 3.
Results of Canonical Discriminant Analyses of Neuropsychological Test Measures Predicting Liability Status
|
Whole Sample |
||||||||||||
|
Calibration Samplea |
Replication Samplea |
All-Effects Model |
Large-Effects Model |
|||||||||
| Variable | Partial R2 | Pb | Weightc | Partial R2 | Pb | Weightc | Partial R2 | Pb | Weightc | Partial R2 | Pb | Weightc |
| Verbal working memory | .009 | .66 | .17 | .004 | .85 | .11 | .004 | .82 | .09 | |||
| Spatial working memory | .084 | .02 | .59 | .041 | .17 | .44 | .082 | .03 | .57 | .087 | .01 | .53 |
| Divided tion | .097 | .01 | −.55 | .076 | .04 | −.52 | .118 | .005 | −.60 | .115 | .002 | −.60 |
| Sequencing | .014 | .55 | .01 | .011 | .63 | −.08 | .015 | .54 | .05 | |||
| Verbal fluency | .005 | .81 | −.08 | .011 | .63 | −.05 | .008 | .73 | −.01 | |||
| Categorization | .010 | .65 | .18 | .005 | .81 | .13 | .006 | .76 | .13 | |||
| Choice reaction time | .058 | .08 | −.41 | .041 | .17 | −.32 | .062 | .06 | −.41 | .054 | .06 | −.38 |
| Selective attention | .022 | .39 | .18 | .002 | .93 | .06 | .014 | .55 | .17 | |||
| Verbal episodic memory | .005 | .82 | −.06 | .006 | .78 | .03 | .012 | .61 | −.15 | |||
| Recall intrusions | .040 | .18 | −.42 | .057 | .08 | −.49 | .063 | .06 | −.51 | .075 | .02 | −.49 |
| Semantic clustering | .016 | .51 | .31 | .018 | .46 | .19 | .031 | .27 | .36 | |||
| Story memory | .001 | .94 | .07 | .011 | .64 | .05 | .006 | .79 | .07 | |||
| Visual episodic memory | .029 | .28 | −.08 | .004 | .83 | −.09 | .013 | .58 | −.11 | |||
| Face recognition | .023 | .38 | −.07 | .023 | .38 | −.21 | .033 | .25 | −.24 | |||
| Verbal ability | .004 | .85 | .15 | .019 | .45 | .36 | .021 | .41 | .39 | |||
| Spatial ability | .007 | .75 | −.16 | .007 | .76 | −.18 | .014 | .55 | −.28 | |||
| Motor speed | .005 | .82 | .05 | .007 | .76 | −.04 | .004 | .83 | −.00 | |||
Results are for all-effects models.
Probability that the partial R2=0.
Standardized canonical weights.
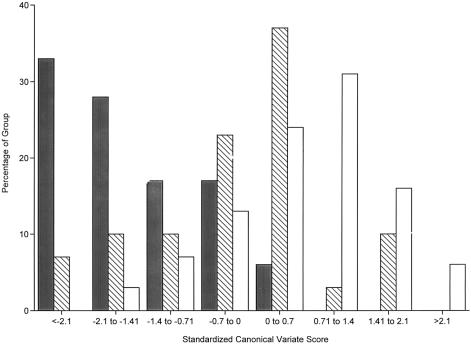
Figure 1 gives the frequency distribution of the standardized canonical variate scores, by risk group. All but one of the MZ co-twins of patients with schizophrenia have a negative score, and 78% have scores of ⩾0.7 SD units below the mean, compared with 27% of the DZ co-twins and 10% of the control twins (χ2=30.6, df=2, P=.001). The mean ± standard error of the mean (SEM) standardized canonical variate scores of the MZ co-twin, DZ co-twin, and control groups were -1.61±0.25, -0.21±0.19, and 0.65±0.13, respectively. In an analysis of variance (ANOVA) controlling for overall IQ, age, gender, handedness, and substance-use history, the linear decrease in scores with increasing genetic loading for schizophrenia was highly significant (F(1,95)=51.5, P=.0001), and each level of genetic risk differed significantly from the others (MZ co-twins vs. controls, t72=-7.3, P=.0001; DZ co-twins vs. controls, t84=-2.9, P=.003; MZ co-twins vs. DZ co-twins, t47=-4.6, P=.0002). This linear decrease in performance with increasing genetic risk for schizophrenia also was observed on each of the variables that contributed uniquely to the canonical discrimination: spatial working memory (P=.0003), divided attention (P=.0003), recall intrusions (P=.005), and choice reaction time (P=.05).
Figure 1.
Frequency distribution (in %) of MZ co-twin (blackened bars), DZ co-twin (hatched bars), and control twin (unmarked bars) groups, according to their standardized scores on the liability-related canonical neuropsychological variate. The canonical variate represents the linear combination of neuropsychological scores that best discriminates among liability groups; the measures contributing uniquely to the discrimination are (in order of magnitude) divided attention, spatial working memory, recall intrusions, and choice reaction time. For statistical information, see table 3.
In 5 (28%) of the 18 MZ and 2 (7%) of the 30 DZ pairs discordant for schizophrenia, the co-twin met criteria for a schizophrenia-related personality disorder (schizotypal, paranoid, or schizoid; χ2=4.1, df=1, P=.05). When co-twins with schizophrenia-spectrum–disorder diagnoses were excluded, there continued to be a linear effect of genetic liability class on the canonical variate scores (F(1,88)=49.8, P=.0001), and each risk group continued to differ significantly from the others (MZ co-twins vs. controls, t67=-6.9, P=.0001; DZ co-twins vs. controls, t82=-3.0, P=.003; and MZ co-twins vs. DZ co-twins, t40=-4.4, P=.0001). Furthermore, 77% of MZ co-twins and 29% of DZ co-twins had canonical variate scores ⩽−0.7, which continued to be in excess of the corresponding rate of 10% in the control group (χ2=24.7, df=2, P=.001).
Nongenetic and Diagnosis-Related Effects
The results of the canonical discriminant analyses predicting schizophrenia diagnosis are given in table 4. Again, the results showed only minor variations between the calibration, replication, and whole-sample analyses, and we therefore focus our discussion on the whole-sample analyses, which should be most representative. The canonical correlation of the 17 cognitive test measures with presence versus absence of schizophrenia was highly significant (R=.78±.04, F(17,85)=7.88, P=.0001). Only one measure—visual episodic memory—contributed a significant proportion of unique variance to this discrimination, although several other measures bordered on significance. Visual episodic memory was retained, along with spatial working memory, divided attention, verbal episodic memory, and motor speed, in the stepwise reduction of the prediction model to large-effects–only terms, resulting in a canonical correlation nearly identical to that in the all-effects model (R=.76±.04, F(5,97)=26.9, P=.0001).
Table 4.
Results of Canonical Discriminant Analyses of Neuropsychological Test Measures Predicting Disease Status[Note]
|
Whole Sample |
||||||||||||
|
Calibration Sample |
Replication Sample |
All-Effects Model |
Large-Effects Model |
|||||||||
| Variable | Partial R2 | P | Weight | Partial R2 | P | Weight | Partial R 2 | P | Weight | Partial R2 | P | Weight |
| Verbal working memory | .013 | .29 | −.21 | .009 | .38 | −.17 | .008 | .39 | −.17 | |||
| Spatial working memory | .047 | .04 | .38 | .023 | .15 | .28 | .040 | .06 | .37 | .031 | .08 | .29 |
| Divided attention | .014 | .27 | −.17 | .035 | .08 | −.28 | .032 | .09 | −.26 | .034 | .06 | −.26 |
| Sequencing | .013 | .28 | −.18 | .002 | .65 | −.08 | .003 | .59 | −.09 | |||
| Verbal fluency | .002 | .67 | .08 | .009 | .38 | .17 | .009 | .37 | .17 | |||
| Categorization | .004 | .57 | .09 | .009 | .36 | .15 | .004 | .55 | .10 | |||
| Choice reaction time | .013 | .30 | −.19 | .010 | .35 | −.17 | .014 | .27 | −.20 | |||
| Selective attention | .013 | .29 | −.17 | .014 | .27 | −.18 | .013 | .28 | −.18 | |||
| Verbal episodic memory | .017 | .23 | .39 | .055 | .02 | .72 | .028 | .12 | .53 | .13 | .0002 | .71 |
| Recall intrusions | .000 | .88 | −.02 | .015 | .26 | −.19 | .004 | .55 | −.09 | |||
| Semantic clustering | .012 | .32 | .27 | .009 | .38 | −.24 | .000 | .83 | .07 | |||
| Story memory | .001 | .79 | .06 | .007 | .45 | .20 | .001 | .73 | .09 | |||
| Visual episodic memory | .063 | .01 | .68 | .028 | .12 | .47 | .046 | .04 | .62 | .028 | .09 | .39 |
| Face recognition | .019 | .21 | −.26 | .007 | .44 | −.16 | .018 | .21 | −.26 | |||
| Verbal ability | .003 | .62 | −.11 | .006 | .47 | −.19 | .002 | .68 | −.10 | |||
| Spatial ability | .007 | .43 | −.19 | .000 | .82 | −.07 | .009 | .36 | −.26 | |||
| Motor speed | .022 | .17 | .29 | .012 | .32 | .21 | .017 | .23 | .26 | .055 | .02 | .39 |
Note.— Data are as described in the footnotes to table 3.
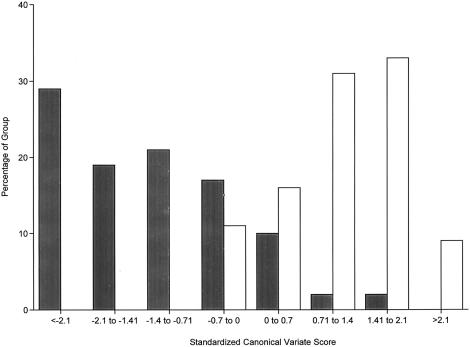
Figure 2 gives a frequency distribution of the canonical variate scores, by diagnostic group. All but three of the patients with schizophrenia have a negative score, and 70% have scores ⩾0.7 SD below the mean, compared with none of the controls (χ2=55.6, df=1, P=.001). The mean ± SEM standardized canonical variate scores of the patient and control groups were -1.33±0.17 and 1.16±0.11, respectively. In the ANOVA controlling for overall IQ, age, gender, handedness, and substance-use history, the diagnostic difference was highly significant (F(1,96)=68.6, P=.0001). The diagnosis effect also was significant for each of the cognitive test variables that contributed uniquely to the canonical discrimination: verbal episodic memory (P=.0001), visual episodic memory (P=.0001), motor speed (P=.003), divided attention (P=.003), and visuospatial working memory (P=.03).
Figure 2.
Frequency distribution (in %) of the schizophrenia (blackened bars) and control (unblackened bars) groups, according to their standardized scores on the disease-related canonical neuropsychological variate. The canonical variate represents the linear combination of neuropsychological scores that best discriminates among diagnostic groups; the measures contributing uniquely to the discrimination are (in order of magnitude) verbal episodic memory, visual episodic memory, motor speed, spatial working memory, divided attention, and choice reaction time. For statistical information, see table 3.
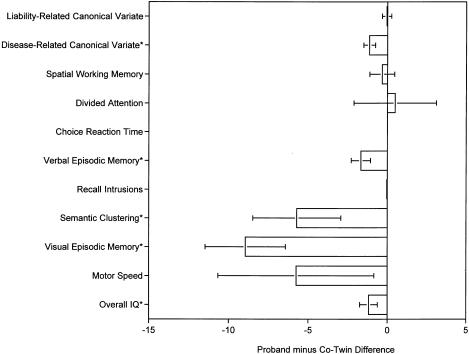
Figure 3 plots the differences, in terms of mean ± SEM, between patients and their MZ co-twins, on both the liability- and the disease-related test measures. Patients performed significantly worse than their own MZ co-twins, on the disease-related canonical variate and, at the univariate level, on verbal episodic memory, semantic clustering, visual episodic memory, and overall IQ, but they did not do so on either the liability-related canonical variate or, at the univariate level, on spatial working memory, divided attention, choice reaction time, recall intrusions, or motor speed.
Figure 3.
Mean ± SEM differences between patients and their own MZ co-twins, with regard to the liability- and disease-related measures. Canonical variates are in SD units; all other variables are in original units. *P<.05.
Intraclass Correlations
Table 5 shows the ICCs for the liability- and disease-related measures in discordant and control pairs, by zygosity. The twin correlation on the liability-related canonical variate is marginally to significantly greater among MZ pairs than among DZ pairs, in both discordant and control twins, and the ICCs in MZ pairs are comparable between discordant and control twins, suggesting equivalent degrees of broad heritability. In contrast, although, in control twins, the twin correlation on the disease-related canonical variate was greater in MZ pairs than in DZ pairs, this difference was not observed in pairs discordant for schizophrenia, and the ICC in MZ pairs was significantly lower in discordant than in control twins, a result consistent with a reduction in broad heritability in this canonical variate in discordant pairs. In general, the pattern of findings at the univariate level paralleled that at the level of the canonical variables, with variables having large liability-related canonical weights showing greater MZ than DZ correlations, in both discordant and control pairs, and with variables having large disease-related canonical weights showing reduced MZ correlations in discordant compared with control pairs. Only in the case of recall intrusions was the ICC significantly higher among discordant than among control MZ pairs.
Table 5.
ICCs on Liability- and Disease-Related Measures
|
Discordant Pairs |
Control Pairs |
||||||
| Variable | ICCDZ(r ± SE) | ICCMZ(r ± SE) | t MZ>DZ | ICCDZ (r ± SE) | ICCMZ(r ± SE) | tMZ>DZ | tCMZ>DMZa |
| Liability canonical variate | .18 ± .18 | .43 ± .19* | 1.31** | .05 ± .19 | .62 ± .12* | 3.59* | 1.05 |
| Disease canonical variate | .00 ± .18 | .11 ± .23 | .55 | .30 ± .18* | .65 ± .11* | 2.23* | 3.06* |
| Spatial working memory | .16 ± .18 | .42 ± .20* | 1.31** | .05 ± .19 | .40 ± .16* | 2.17* | −.11 |
| Divided attention | .42 ± .15* | .32 ± .21** | −.51 | .09 ± .19 | .26 ± .18** | 1.11** | −.29 |
| Choice reaction time | .00 ± .18 | .02 ± .24 | .12 | .00 ± .19 | .49 ± .14* | 3.08* | 2.63* |
| Verbal episodic memory | .00 ± .18 | .23 ± .22 | 1.18** | .58 ± .13* | .69 ± .10* | .72 | 2.57* |
| Recall intrusions | .00 ± .18 | .64 ± .14* | 3.25* | .14 ± .19 | .15 ± .18 | .05 | −2.73* |
| Semantic clustering | .03 ± .18 | .18 ± .23 | .78 | .21 ± .18** | .43 ± .15* | 1.34** | 1.36** |
| Visual episodic memory | .09 ± .18 | .00 ± .23 | −.44 | .01 ± .19 | .49 ± .14* | 2.98* | 2.75* |
| Motor speed | .17 ± .18 | .00 ± .24 | −.86 | .27 ± .18** | .36 ± .18* | .55 | 2.02* |
| Overall IQ | .17 ± .18 | .40 ± .20* | 1.20** | .44 ± .16* | .79 ± .07* | 2.19* | 2.17* |
CMZ = control MZ; DMZ = discordant MZ.
P<.05.
P<.10.
Discussion
Genetic Influences
In this study we have used a discordant-twin design to evaluate 17 different neuropsychological test measures as endophenotypic indicators of genetic risk for schizophrenia. Measures of four neurocognitive functions—spatial working memory, divided attention, choice reaction time, and recall intrusions—were independently sensitive to genetic loading for schizophrenia when all 17 measures were controlled simultaneously. These differences scaled linearly with degree of genetic loading for schizophrenia, such that MZ co-twins were significantly more impaired than were DZ co-twins, who, in turn, were significantly more impaired than were controls. Converging evidence of a genetic determination of these performance differences was provided by a larger intrapair correlation, in MZ compared with DZ pairs, on the combination of these measures, a pattern that was also present, at the univariate level, on measures of spatial working memory and recall intrusions.
Because there were no differences, in parental social class or in duration of cohabitation, either between discordant and control pairs or between discordant MZ and discordant DZ pairs, and because the liability-related differences in test performance were independent of the effects of general ability level, differences in the similarity of shared postnatal environmental experiences do not appear to compete with a genetic explanation of the neurocognitive deficits observed in the co-twins of patients with schizophrenia. Factors associated with the shared gestational and perinatal environment could play a role, but, to compete with a genetic explanation of the performance differences, obstetric complications would have to be more frequent in histories of discordant MZ pairs than in those of discordant DZ pairs, a pattern that was not observed in relation to prenatal or perinatal complications coded blindly from the original obstetric records on approximately half the studied twin pairs (T. D. Cannon, unpublished data).
There were no differences, in the rates of substance-use–disorder or affective-disorder diagnoses, between co-twins of patients with schizophrenia and control twins, and the group differences in performance were independent of the presence of these diagnoses. Thus, we can rule out the possibility that the liability-related differences in neuropsychological test performance are due to an excess of nonspecific mental illness. However, consistent with the results reported in previous work (Kendler and Diehl 1993), schizophrenia-related personality disorders were significantly more prevalent in the MZ co-twins than in the DZ co-twins of patients with schizophrenia. In view of this pattern, it could be argued that the neuropsychological differences between MZ and DZ co-twins are secondary to the manifestation of schizophrenia-spectrum symptomatology, rather than reflective of an inherited diathesis to it. However, when co-twins with spectrum personality disorders were excluded, the liability-related differences in measures of spatial working memory, divided attention, choice reaction time, and recall intrusions remained significant. This result demonstrates that, with regard to genetic liability status in relatives of patients with schizophrenia, performance on these neuropsychological tests is more sensitive than schizophrenia-spectrum diagnostic categories.
Because, in demographic and clinical characteristics, the studied probands from discordant MZ and DZ pairs did not differ either from each other or from the remainder of the discordant-twin proband population, we also can rule out the possibility that the liability-related differences in neuropsychological functioning are due to an unusual clinical or demographic profile in the probands. Furthermore, there were no differences, in either neuropsychological test performance or symptom severity, between probands from discordant pairs and those from concordant pairs. Taken together, these results suggest that the discordant pairs evaluated in the present study adequately represent the degree and variety of genetic predisposition to schizophrenia in the Finnish twin population. Although Finland is a rather isolated gene pool (de la Chapelle 1993), it has, for common diseases such as schizophrenia, nearly the same genetic variation as is seen in other populations (Terwilliger and Weiss 1988).
In view of evidence that lateral prefrontal (D’Esposito et al. 1995) and frontal polar (Koechlin et al. 1999) regions are critical for performance on working-memory and divided-attention paradigms, respectively, and since many of the cognitive operations involved in choice-reaction-time paradigms (e.g., sustained attention, target detection, and response selection) are associated with frontal lobe functioning (e.g., see Posner and Petersen 1990; Corbetta et al. 1991), the present study's findings suggest that genetic liability to schizophrenia may impact prefrontal cortical systems preferentially. This interpretation is also supported by evidence that patients with schizophrenia, as well as their siblings, show reduced gray matter in the frontal lobes, but not in posterior cortical regions, compared with controls (Cannon et al. 1998b). Genetic liability to schizophrenia was also associated with a tendency to make recall intrusions (and, to a lesser extent, to fail to cluster semantically related items together during recall) on a verbal list–learning task. Although, in general, learning and recall of episodic information depend on medial temporal lobe structures and adjacent temporal cortex (Squire and Zola 1996), recall intrusions are not common in amnesia secondary to temporal lobe damage but are characteristic of amnestic syndromes associated with frontal lobe lesions (Schacter et al. 1998). In addition, a number of functional neuroimaging studies have found that episodic-memory retrieval is associated with activation of prefrontal cortex (Buckner 1996). Taken together, these considerations suggest that the deficits on verbal list–learning tasks in relatives of patients with schizophrenia may be explained by an inherited disturbance in prefrontal cortical circuits involved in memory retrieval.
It could be questioned whether specificity of the liability-related differences to working-memory, divided-attention, choice-reaction-time, and episodic-memory–retrieval paradigms results from differential reliability and difficulty (i.e., true score variance) of the test measures employed (Chapman and Chapman 1989). Although this possibility cannot be ruled out entirely, reliability estimates for the test measures that were and were not independently correlated with genetic liability to schizophrenia are roughly comparable (i.e., in the .7–.8 range), as are estimates of the degree of variation on these test measures in the control group. Furthermore, because, compared with controls, patients were impaired on every measure at the univariate level, and because there were moderate to large intrapair correlations on all measures in control MZ pairs, the differential sensitivity of the neuropsychological test measures to genetic loading for schizophrenia is not due to either a lack of sensitivity of these measures to schizophrenia or to a lack of heritable influence in the general population. In any case, because the liability-related variance on tests in other domains (e.g., categorization and selective attention) was not independent of differences in spatial working memory, divided attention, choice reaction time, and episodic-memory retrieval, the marginal utility of these other measures in genetic research on schizophrenia can be questioned.
If different susceptibility loci for schizophrenia are expressed in a final common pathway in the nervous system, covarying a cognitive indicator of this system should remove the liability-related variation in cognitive indicators of any related neural system. Contrary to this prediction, in the present study the liability-related differences in spatial working memory, divided attention, choice reaction time, and episodic-memory retrieval were independent of each other, as well as of deficits in other abilities and in overall IQ, indicating that, in different subsets of co-twins, there were different profiles of deficit on these measures. This pattern suggests that at least partially distinct sets of susceptibility genes for schizophrenia may contribute to variation in these domains of neurocognitive functioning, an implication that requires confirmation by admixture or segregation analyses in a large sample of families with multiple members affected with schizophrenia.
These findings should facilitate the search for susceptibility genes for schizophrenia, in at least two major ways. First, specification of phenotypic affection, based on degree of neurocognitive deficit, will allow genetically liable but clinically unaffected relatives to become informative for linkage and will enable linkage analyses to search for genes that contribute to quantitative variation in disease liability. Second, assessment of the neurocognitive profiles of patients and their relatives will allow investigators to determine the degree to which deficits in each functional domain are segregating in each of the families under study, thus permitting both data-driven specification of heterogeneity parameters in whole-sample analyses and selection of phenotypically more homogeneous groups of families in subsample analyses.
Nongenetic and Disease-Specific Influences
The test measures that uniquely discriminated patients with schizophrenia from controls include two—spatial working memory and divided attention—that uniquely discriminated among genetic liability groups, but patients performed comparably to their own MZ co-twins on these measures (as well as on the other measures that contributed significantly to the liability contrast). Three additional measures—verbal episodic memory, visual episodic memory, and motor speed—uniquely discriminated patients from controls, and, on two of these (i.e., the episodic-memory measures), patients performed significantly worse than did their own MZ co-twins. Thus, although patients’ deficits in spatial working memory, divided attention, recall intrusions, and choice reaction time appear to be accounted for completely by genetic influences, nongenetic influences must contribute to their deficits in verbal and visual episodic memory. Also supporting this conclusion is evidence of significantly reduced heritability (i.e., lower MZ-twin correlation) on the episodic-memory measures, but not on the liability-related measures, in discordant compared with control twins. Taken together, this pattern indicates that nongenetic influences affect some neural systems that are not affected by genetic predisposition to schizophrenia and suggests that the nongenetic effects are particularly pronounced in the verbal and visuospatial episodic-memory systems.
The design of the present study does not allow us to differentiate etiologically relevant nongenetic influences from influences secondary to illness expression or treatment. One reason to suspect that the nongenetic component of verbal and visual episodic-memory deficits in schizophrenia reflects an etiologic influence is that such deficits appear in recent-onset patients prior to treatment with antipsychotic drugs and do not progress beyond the deterioration associated with normal aging (Cannon et al. 1994; Saykin et al. 1994; Censits et al. 1997). Downward social drift and comorbid substance use represent additional secondary influences that could account for the memory impairments in the patients. However, the schizophrenia-related differences on measures of verbal and visual episodic-memory functioning persisted after control for attained socioeconomic class (whose influence was completely redundant with respect to overall IQ) and substance-use history statistically (P=.0001 and .0004, respectively) and were present even after exclusion of subjects with a history of substance disorder (in both cases, P=.0001). Furthermore, anatomical changes in the medial temporal lobe structures that are critical for episodic-memory functioning are more severe among patients with schizophrenia who have a history of hypoxia-associated obstetric complications (Stefanis et al. 1999). In contrast, because antidopaminergic drugs have marked effects on the extrapyramidal motor pathways (Gerlach 1991), the nongenetic contribution to motor slowing in schizophrenia could be accounted for fully or in part by exposure to neuroleptic medications.
Given the comparability, in degree of deficit, between patients and their own MZ co-twins, on measures of spatial working memory, divided attention, choice reaction time, and recall intrusions, such deficits may be necessary but clearly are not sufficient for the manifestation of schizophrenic symptomatology. Deficits on tests of verbal and visual episodic learning and memory are, however, greater in patients than in their MZ co-twins. Thus, if the nongenetic component of episodic learning and memory deficits in schizophrenia does not reflect a secondary influence, such deficits may help to elucidate the factors potentiating the formation of psychotic symptoms among those who are genetically predisposed.
Acknowledgments
This research was supported by National Institute of Mental Health grant MH52857 (to T.D.C.). The authors wish to thank Ulla Mustonen, Pirjo Keki, and Eila Viopio for their contributions to subject recruitment and evaluation, Antti Tanskanen for his contributions to the register searches, and Kauko Heikkila for his contributions to data management of the Finnish Twin Cohort Study material.
Electronic-Database Information
The accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for schizophrenia [MIM 604906])
References
- American Psychiatric Association (1994) DSM IV: diagnostic and statistical manual of mental disorders. American Psychiatric Association, Washington, DC [Google Scholar]
- Andreasen N (1983) The scale for the assessment of negative symptoms (SANS). University of Iowa, Iowa City [Google Scholar]
- ——— (1984) The scale for the assessment of positive symptoms (SAPS). University of Iowa, Iowa City [Google Scholar]
- Benson DF, Zaidel E (1985) The dual brain. Guilford Press, New York [Google Scholar]
- Benton A, Hamsher K (1976) Multilingual aphasia examination. University of Iowa, Iowa City [Google Scholar]
- Bouchard TJ, Lykken DT, McGue M, Segal NL, Tellegan A (1990) Sources of human psychological differences: the Minnesota study of twins reared apart. Science 250:223–228 [DOI] [PubMed] [Google Scholar]
- Buckner RL (1996) Beyond HERA: contributions of specific prefrontal brain areas to long-term memory retrieval. Psychonomics Bull Rev 3:149–158 [DOI] [PubMed] [Google Scholar]
- Byrne M, Hodges A, Grant E, Owens DC, Johnstone EC (1999) Neuropsychological assessment of young people at high genetic risk for developing schizophrenia compared with controls: preliminary findings of the Edinburgh High Risk Study (EHRS). Psychol Med 29:1161–1173 [DOI] [PubMed] [Google Scholar]
- Cannon TD (1996) Abnormalities of brain structure and function in schizophrenia: implications for aetiology and pathophysiology. Ann Med 28:533–539 [DOI] [PubMed] [Google Scholar]
- Cannon TD, Kaprio J, Lonnqvist J, Huttunen M, Koskenvuo M (1998a) The genetic epidemiology of schizophrenia in a Finnish twin cohort: a population-based modeling study. Arch Gen Psychiatry 55:67–74 [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TGM, Huttunen M, Lonnqvist J, Salonen O, Valanne L, Poutanen V-P, et al (1998b) Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry 55:1084–1091 [DOI] [PubMed] [Google Scholar]
- Cannon TD, Zorrilla LE, Shtasel D, Gur RE, Gur RC, Marco E, Moberg P, et al (1994) Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch Gen Psychiatry 51:651–661 [DOI] [PubMed] [Google Scholar]
- Censits DM, Ragland JD, Gur RC, Gur RE (1997) Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: a longitudinal study. Schizophr Res 24:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP (1989) Strategies for resolving the heterogeneity of schizophrenics and their relatives using cognitive measures. J Abnorm Psychol 98:357–366 [DOI] [PubMed] [Google Scholar]
- Cliff N (1987) Analyzing multivariate data. Harcourt Brace Jovanovich, New York [Google Scholar]
- Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Measurement 20:37–46 [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE (1991) Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci 11:2383–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Chapelle A (1993) Disease gene mapping in isolated human populations: the example of Finland. J Med Genet 30:857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, Kramer JH, Kaplan E, Ober B (1983) California verbal learning test, res ed. Psychological Corporation, Cleveland [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M (1995) The neural basis of the central executive system of working memory. Nature 378:279–81 [DOI] [PubMed] [Google Scholar]
- Farah M (1996) Is face recognition “special”? evidence from neuropsychology. Behav Brain Res 76:181–189 [DOI] [PubMed] [Google Scholar]
- Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Lyons MJ, Tsuang MT (1995) Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a diagnostic efficiency analysis. J Abnorm Psychol 104:286–304 [DOI] [PubMed] [Google Scholar]
- Faraone SV, Seidman LJ, Kremen WS, Toomey R, Pepple JR, Tsuang MT (1999) Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a 4-year follow-up study. J Abnorm Psychol 108:176–181 [DOI] [PubMed] [Google Scholar]
- Finkelstien J (1998) Attention deficits in schizophrenia: endophenotypic markers of vulnerability to the disorder? PhD thesis, University of Pennsylvania, Philadelphia [Google Scholar]
- Fisher M (1971) Psychoses in the offspring of schizophrenic monozygotic twins and their normal co-twins. Br J Psychiatry 118:43–52 [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, et al (1997) Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA 94:587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS (1989) Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol 61:331–349 [DOI] [PubMed] [Google Scholar]
- Gerlach J (1991) New antipsychotics: classification, efficacy, and adverse effects. Schizophr Bull 17:289–309 [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Ragland JD, Torrey EF, Gold JM, Bigelow LB, Weinberger DR (1990) Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry 47:1066–1072 [DOI] [PubMed] [Google Scholar]
- Gottesman II, Bertelsen A (1989) Confirming unexpressed genotypes for schizophrenia: risks in the offspring of Fischer's Danish identical and fraternal discordant twins. Arch Gen Psychiatry 46:867–872 [DOI] [PubMed] [Google Scholar]
- Gottesman II, McGuffin P, Farmer AE (1987) Clinical genetics as clues to the “real” genetics of schizophrenia (a decade of modest gains while playing for time). Schizophr Bull 13:23–47 [DOI] [PubMed] [Google Scholar]
- Gur RC, Jaggi JL, Ragland JD, Resnick SM, Shtasel D, Muenz L, Gur RE (1993) Effects of memory processing on regional brain activation: cerebral blood flow in normal subjects. Int J Neurosci 72:31–44 [DOI] [PubMed] [Google Scholar]
- Halgren E (1982) Mental phenomena induced by stimulation in the limbic system. Hum Neurobiol 1:251–260 [PubMed] [Google Scholar]
- Heaton R, Cfhelune G, Talley J, Kay G, Curtiss G (1993) Wisconsin card sorting test manual: revised and expanded. Psychological Assessment Resources, Odessa, FL [Google Scholar]
- Hovatta I, Varilo T, Suvisaari J, Terwilliger JD, Ollikainen V, Arajarvi R, Juvonen H, et al (1999) A genomewide screen for schizophrenia genes in an isolated Finnish subpopulation suggesting multiple susceptibility loci. Am J Hum Genet 65:1114–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprio J, Koskenvuo M, Rose RJ (1990) Population-based twin registries: illustrative twin cohort study. Acta Genet Med Gemellol 39:427–439 [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Gogos JA (1997) A turning point in schizophrenia genetics. Neuron 19:967–979 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Diehl SR (1993) The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr Bull 19:261–285 [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J (1999) The role of the anterior prefrontal cortex in human cognition. Nature 399:148–151 [DOI] [PubMed] [Google Scholar]
- Kremen WS, Seidman LJ, Pepple JR, Lyons MJ, Tsuang MT, Faraone SV (1994) Neuropsychological risk indicators for schizophrenia: a review of family studies. Schizophr Bull 20:103–119 [DOI] [PubMed] [Google Scholar]
- Loranger AW, Sussman VL, Oldham JM, Russakoff LM (1985) Personality disorder examination: a structured interview for making diagnosis of DSM-III-R personality disorders. Cornell Medical College, White Plains, NY [Google Scholar]
- Luria AR (1966) Higher cortical functions in man. Basic Books, New York [Google Scholar]
- Lynch M, Walsh B (1998) Genetics and analysis of quantitative traits. Sinauer Associates, Sunderland, MA [Google Scholar]
- Neale MC, Cardon LR (1992) Methodology for genetic studies of twins and families. Kluwer Academic, Boston [Google Scholar]
- Posner M, Petersen SE (1990) The attention system of the human brain. Annu Rev Neurosci 13:25–42 [DOI] [PubMed] [Google Scholar]
- Reitan R, Wolfson D (1985) The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Neuropsychology Press, Tucson [Google Scholar]
- Roberts GW, Done DJ, Bruton C, Crow TJ (1990) A “mock up” of schizophrenia: temporal lobe epilepsy and schizophrenia-like psychosis. Biol Psychiatry 28:127–143 [DOI] [PubMed] [Google Scholar]
- Russell E (1975) A multiple scoring method for assessment of complex memory functions. J Consult Clin Psychol 43:800–809 [Google Scholar]
- Sakai K, Hikosaka O, Takino R, Miyauchi S, Nielsen M, Tamada T (2000) What and when: parallel and convergent processing in motor control. J Neurosci 20:2691–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC (1994) Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry 51:124–131 [DOI] [PubMed] [Google Scholar]
- Schacter DL, Norman KA, Koutstaal W (1998) The cognitive neuroscience of constructive memory. Annu Rev Psychol 49:289–318 [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB (1989) Instruction manual for the structured clinical interview for DSM-III-R SCID. Biometrics Research, New York [Google Scholar]
- Squire LR, Zola SM (1996) Memory, memory impairment, and the medial temporal lobe. Cold Spring Harb Symp Quant Biol 61:185–195 [PubMed] [Google Scholar]
- Stefanis N, Frangou S, Yakeley J, Sharma T, O'Connell P, Morgan K, Sigmudsson T, et al (1999) Hippocampal volume reduction in schizophrenia: effects of genetic risk and pregnancy and birth complications. Biol Psychiatry 46:697–702 [DOI] [PubMed] [Google Scholar]
- Terwilliger JD, Weiss KM (1998) Linkage disequilibrium mapping of complex disease: fantasy or reality? Curr Opin Biotechnol 9:578–594 [DOI] [PubMed] [Google Scholar]
- Vilkki J, Virtanen S, Surma-Aho O, Servo A (1996) Dual task performance after focal cerebral lesions and closed head injuries. Neuropsychologia 34:1051–1056 [DOI] [PubMed] [Google Scholar]
- Wechsler D (1981) Wechsler adult intelligence scale-revised (WAIS-R) manual. Psychological Corporation, Cleveland [Google Scholar]