Abstract
Skin fibroblast cells from two unrelated male infants with a chromosome-instability disorder were analyzed for their response to colcemid-induced mitotic-spindle checkpoint. The infants both had severe growth and developmental retardation, microcephaly, and Dandy-Walker anomaly; developed Wilms tumor; and one died at age 5 mo, the other at age 3 years. Their metaphases had total premature chromatid separation (total PCS) and mosaic variegated aneuploidy. Mitotic-index analysis of their cells showed the absence of mitotic block after the treatment with colcemid, a mitotic-spindle inhibitor. Bromodeoxyuridine-incorporation measurement and microscopic analysis indicated that cells treated with colcemid entered G1 and S phases without sister-chromatid segregation and cytokinesis. Preparations of short-term colcemid-treated cells contained those cells with chromosomes in total PCS and all or clusters of them encapsulated by nuclear membranes. Cell-cycle studies demonstrated the accumulation of cells with a DNA content of 8C. These findings indicate that the infants’ cells were insensitive to the colcemid-induced mitotic-spindle checkpoint.
We (Kajii et al. 1998) recently reported a chromosomal instability syndrome in two infants, characterized by (1) premature separation of chromatids (PCS [MIM 176430]) in all chromosomes, called “total premature chromatid separation” (total PCS), in >50% of their lymphocytes, and (2) a variety of mosaic aneuploidies, especially trisomies, double trisomies, and monosomies, a finding called “mosaic variegated aneuploidy.” The clinical findings in these two infants included severe pre- and postnatal growth retardation, profound developmental retardation, severe microcephaly, hypodysplasia of the brain, Dandy-Walker anomaly, bilateral cataracts, and uncontrollable clonic seizures. One of them developed Wilms tumor, and both died at age <2 years. In the two families, the parents and three other relatives showed 2.5%–47% of lymphocytes with total PCS but without either mosaic variegated aneuploidy or phenotypic abnormalities. The total-PCS trait thus was transmitted in an autosomal dominant fashion, and the two affected infants were homozygous for the trait. The clinical findings for the disorder, on the other hand, appear to be autosomal recessive. We reasoned that, in view of the presence of chromosomal instability in the somatic cells of the affected infants, the basis of the chromosomal instability disorder that we had described might be a mitotic-spindle checkpoint defect, similar to those in the colorectal cancer cells described by Cahill et al. (1998).
We established skin fibroblast cell lines from another two unrelated Japanese infants with the homozygous total-PCS trait—cell line MY1sk from a male infant (patient 1) who had not previously been reported and cell line PCS1 from a male infant (patient 2) reported by Kawame et al. (1999). These two infants both had severe pre- and postnatal growth retardation, profound developmental retardation, severe microcephaly, hypodysplasia of the brain, Dandy-Walker anomaly, and hypoplasia of the corpus callosum. Patient 2, in addition, had bilateral cataracts with corneal opacities, hypospadias, and bilateral cryptorchidism. Both of these infants developed intractable clonic seizures—patient 1 at age 4 mo, patient 2 at age 1 wk. Patient 1 developed a right-kidney tumor at age 9 wk and underwent right nephrectomy. Pathological examination showed stage I Wilms tumor with favorable histology. Patient 2 developed, at age 7 mo, bilateral cystic kidney tumors. Left nephrectomy and right-tumor excision were performed, with a resulting diagnosis of Wilms tumor (nephroblastoma type). The two infants had 48.5%–87.5% of their lymphocytes, and 48%–50% of their cultured skin fibroblasts, in total PCS and had mosaic variegated aneuploidy in both lymphocytes and cultured skin fibroblasts. One died at age 5 mo, the other at age 3 years. Their four phenotypically normal parents had 3.5%–42.5% lymphocytes in total PCS but no mosaic variegated aneuploidy.
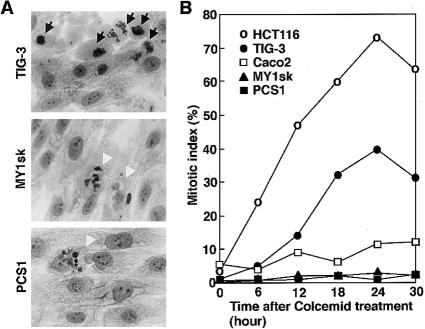
Using these and reference cell lines, we analyzed their cell cycle, according to the method described by Cahill et al. (1998). In brief, cells were grown on a glass slide, treated with 1 μg of colcemid/ml (a microtubule-disrupting agent), harvested at 6-h intervals, fixed with 3:1 methanol:acetic acid, stained with Hoechst 33258, and analyzed under a fluorescence microscope. TIG-3 (normal fibroblasts) and HCT116 (a colorectal cancer cell line with microsatellite instability) both accumulated mitotic cells, with a peak at 24 h after treatment with colcemid (fig. 1A and B), an indication that these cells have intact mitotic checkpoints. On the other hand, MY1sk and PCS1 (fibroblast cell lines from the two infants that we have described above) both showed an abnormal response, with fewer mitotic cells and no clear peak in mitotic index (fig. 1B). Instead, cells with multiple small nuclei appeared (fig. 1A) and accumulated, accounting for 27.0%–27.9% of the cells after 24 h of treatment with colcemid. Caco2 (a colorectal cancer cell line with chromosomal instability) showed a response of mitotic indices that was similar to that of MY1sk and PCS1.
Figure 1 .
A, top, Giemsa-staining of normal fibroblasts (TIG-3) 24 h after treatment with 1 μg of colcemid/ml. Cells with tightly condensed chromosomes characteristic of longstanding mitotic arrest are indicated (arrows). A, middle, Cells from patient 1 (MY1sk), treated in the same manner and including multinucleated cells (arrowheads) but without those with mitotic figures. A, bottom, Cell from patient 2 (PCS1), with multiple small nuclei (arrowhead). B, Mitotic indices after treatment with colcemid. Normal fibroblasts (TIG-3) and a colorectal cancer cell line with microsatellite instability (HCT116) both showed mitotic block, with a peak at 24 h of treatment with colcemid; on the other hand, the cell lines from the two infants described in the present report (MY1sk and PCS1) showed no appreciable increase of mitotic cells with increasing duration of treatment with colcemid. Caco2, a colorectal cancer cell line with well-demonstrated chromosomal instability (Cahill et al. 1998), behaved in a similar manner.
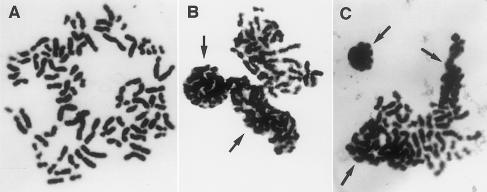
Chromosome preparations from MY1sk and PCS1, treated with 0.2 μg of colcemid/ml for 3.5 h, had, respectively, 65% and 55% metaphases in total PCS (fig. 2A), whereas those from Caco2 had no such cells. Of the cells in total PCS, 25%–27% showed all or clusters of chromosomes tightly packed and covered by nuclear membranes (fig. 2B and C). These abnormal cells were interpreted to have escaped mitotic arrest by colcemid and to have entered telophase without going through sister-chromatid segregation.
Figure 2 .
A, Metaphase in total PCS from skin fibroblasts (MY1sk) of patient 1, treated with 0.2 μg of colcemid/ml for 3.5 h. B, Another cell from the same preparation, with two groups of chromosomes in total PCS, covered by nuclear membranes and in the process of formation of micronuclei. C, Metaphase from skin fibroblasts (PCS1) of patient 2, with three groups of chromosomes encapsulated by nuclear membranes.
DNA synthesis in these cell lines was studied using bromodeoxyuridine (BrdU) incorporation into replicating chromosomes as an indicator. In brief, cells were grown on a glass slide and were treated with 1 μg of colcemid/ml for 24 h, and BrdU was added at 10 μM 2 h prior to harvest. Cells were fixed with cold methanol for 10 min, air-dried onto a glass slide, immersed in 2 M HCl at 37°C for 1 h, neutralized with 0.1 M borate buffer pH 8.5 for 10 min, washed three times with PBS, and treated in a humidified chamber with 50 μg anti-BrdU antibody conjugated with fluorescein/ml for 1 h. The slides thus treated were washed three times with PBS, counterstained with Hoechst 33258, and screened under a fluorescence microscope. BrdU incorporation in normal fibroblasts (TIG-3) after 24 h of treatment with colcemid was reduced by 65% of the level seen in the absence of treatment with colcemid. On the other hand, BrdU incorporation in the fibroblast cells from the two infants that we have described above (MY1sk and PCS1) did not show appreciable decreases after treatment with colcemid (data not shown). These results indicate that the fibroblast cells from the two infants escaped mitotic arrest and reentered S phase.
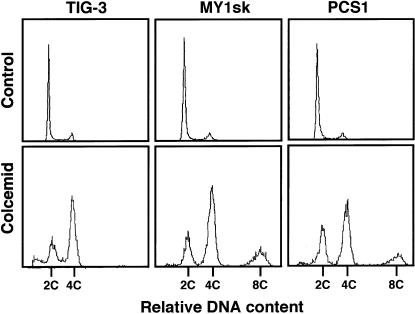
Flow-cytometric analysis was performed as described elsewhere (Ito et al. 1999), to learn about cell-cycle profiles in these cell lines. In brief, cells were treated with 1 μg of colcemid/ml for 65 h, fixed with 70% cold ethanol, stained with 50 μg of propidium iodide/ml PBS, and analyzed by flow cytometry. Normal fibroblasts (TIG-3) and the cells from the two infants that we have described above (MY1sk and PCS1), without treatment with colcemid, all showed a tall 2C peak and a small 4C peak (fig. 3, top). After treatment with colcemid, the normal fibroblasts (TIG-3) had shifted from the DNA content of 2C to 4C, an indication of mitotic block, along with a 2C cell distribution that may correspond to G0 resting cells. TIG-3 showed, in addition, a small fraction with DNA content less than 2C, which may represent apoptotic cells induced by colcemid. On the other hand, MY1sk and PCS1 both exhibited the smaller 2C peak and the taller 4C peak, similar to those in TIG-3, and a small additional peak at 8C. The accumulation of the 8C cells provided evidence that the cells escaped from mitotic arrest and entered the second G2/M phase without sister-chromatid segregation and cytokinesis. We infer that the 4C cells in MY1sk and PCS1 are not the mitotic-blocked cells but, rather, the cells entering G1 phase without going through sister-chromatid segregation, as seen in figure 2.
Figure 3 .
Flow-cytometric analysis of normal fibroblasts (TIG-3) and fibroblasts from the two infants described in the present report (MY1sk and PCS1), without and with 65 h of treatment with colcemid.
In the present study, cultured fibroblasts from the two unrelated affected infants showed total PCS with mosaic variegated aneuploidy and did not arrest in metaphase after treatment with colcemid. Instead, as measured by BrdU incorporation and microscopic analysis, the cells entered G1 and then S phases without sister-chromatid segregation and cytokinesis. Cell-cycle studies demonstrated the accumulation of large numbers of the infants’ cells with a DNA content of 8C. These results provide additional evidence that the infants’ cells are insensitive to the colcemid-induced mitotic-spindle checkpoint.
Mitotic-spindle checkpoint originally was defined by yeast mutants that failed to arrest in mitosis when treated with a microtubule-disrupting agent. By means of this strategy, MAD (mitosis-arrest deficient [Li and Murray 1991]) and BUB (budding uninhibited by benomyl [Hoyt et al. 1991]) genes have been recovered, and genetic and biochemical studies show that these products are part of a signaling cascade that, by inactivating an anaphase-promoting complex (APC) and its regulator protein Cdc20, blocks sister-chromatid separation (for a review, see Gardner and Burke 2000). Some of these proteins localize to kinetochores that are not attached to the mitotic spindle, suggesting that the checkpoint proteins sense and respond to the unattached kinetochore. Human counterparts (hMAD1-2, hMAD2B, hBUB1-2, hBUBR1/MAD3, hCDC20/p55CDC, and hMPS1) of the yeast components have been cloned and shown to be required for the checkpoint control (Cahill et al. 1999; for reference, see Dobie et al. 1999). Low-level expression of the hMAD2 gene was found in some of the breast cancer cells that exhibited an abnormal mitotic checkpoint (Li and Benezra 1996). The loss-of-function in hMAD1 protein, which is due to sequestration by Tax, is responsible for transformation of cells by T-cell leukemia virus (Jin et al. 1998). Mutations of the hBUB1 gene cause checkpoint defects in a small fraction of colorectal cancers (Cahill et al. 1998). In addition to these signaling components, some kinetochore proteins have been proposed to be involved in the mitotic-spindle checkpoint. Drosophila ZW10 is a kinetochore component that recruits dynein, a microtubule motor, to the kinetochore, and it plays a role in accurate chromosome segregation (Starr et al. 1998). In zw10 null mutants, sister chromatids separate prematurely in the presence of a microtubule-disrupting agent, representing malfunction of the mitotic-spindle checkpoint. Drosophila rough deal (rod) mutants also exhibit anaphase defects similar to those in zw10 mutants, suggesting that the two proteins function in the same pathway (Scaerou et al. 1999). It remains to be seen which of these numerous genes or related genes is responsible for the disorder that we have described.
Acknowledgments
We thank the families for their cooperation in the study, and we thank Drs. Yoichiro Tsuji, Masato Tsukahara, and Yoko Sugio for providing skin-biopsy specimens from the patients. This study was supported by Ministry of Education, Science and Culture of Japan grant 10877384 and by Special Coordination Funds for Promoting Science and Technology from the Science and Technology Agency, Japan.
Electronic-Database Information
The accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for PCS [MIM 176430])
References
- Cahill DP, da Costa LT, Carson-Walter EB, Kinzler KW, Vogelstein B, Lengauer C (1999) Characterization of MAD2B and other mitotic spindle checkpoint genes. Genomics 58:181–187 [DOI] [PubMed] [Google Scholar]
- Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JKV, Markowitz SD, Kinzler KW, et al (1998) Mutations of mitotic checkpoint genes in human cancers. Nature 392:300–303 [DOI] [PubMed] [Google Scholar]
- Dobie KW, Hari KL, Maggert KA, Karpen GH (1999) Centromere proteins and chromosome inheritance: a complex affair. Curr Opin Genet Dev 9:206–217 [DOI] [PubMed] [Google Scholar]
- Gardner RD, Burke DJ (2000) The spindle checkpoint: two transitions, two pathways. Trends Cell Biol 10:154–158 [DOI] [PubMed] [Google Scholar]
- Hoyt MA, Totis L, Roberts BT (1991) S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66:507–517 [DOI] [PubMed] [Google Scholar]
- Ito A, Tauchi H, Kobayashi J, Morishima K, Nakamura A, Hirokawa Y, Matsuura S, et al (1999) Expression of full-length NBS1 protein restores normal radiation responses in cells from Nijmegen breakage syndrome patients. Biochem Biophys Res Commun 265:716–721 [DOI] [PubMed] [Google Scholar]
- Jin D-Y, Spencer F, Jeang K-T (1998) Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 93:81–91 [DOI] [PubMed] [Google Scholar]
- Kajii T, Kawai T, Takumi T, Misu H, Mabuchi O, Takahashi Y, Tachino M, et al (1998) Mosaic variegated aneuploidy with multiple congenital abnormalities: homozygosity for total premature chromatid separation trait. Am J Med Genet 78:245–249 [DOI] [PubMed] [Google Scholar]
- Kawame H, Sugio Y, Fuyama Y, Hayashi Y, Suzuki H, Kurosawa K, Maekawa K (1999) Syndrome of microcephaly, Dandy-Walker malformation, and Wilms tumor caused by mosaic variegated aneuploidy with premature centromere division (PCD): report of a new case and review of the literature. J Hum Genet 44:219–224 [DOI] [PubMed] [Google Scholar]
- Li R, Murray AW (1991) Feedback control of mitosis in budding yeast. Cell 66:519–531 [DOI] [PubMed] [Google Scholar]
- Li Y, Benezra R (1996) Identification of a human mitotic checkpoint gene: hsMAD2. Science 274:246–248 [DOI] [PubMed] [Google Scholar]
- Scaerou F, Aguilera I, Saunders R, Kane N, Blottiere L, Karess R (1999) The rough deal protein is a new kinetochore component required for accurate chromosome segregation in Drosophila. J Cell Sci 112:3757–3768 [DOI] [PubMed] [Google Scholar]
- Starr DA, Williams BC, Hays TS, Goldberg ML (1998) ZW10 helps recruit dynactin and dynein to the kinetochore. J Cell Biol 142:763–774 [DOI] [PMC free article] [PubMed] [Google Scholar]