Abstract
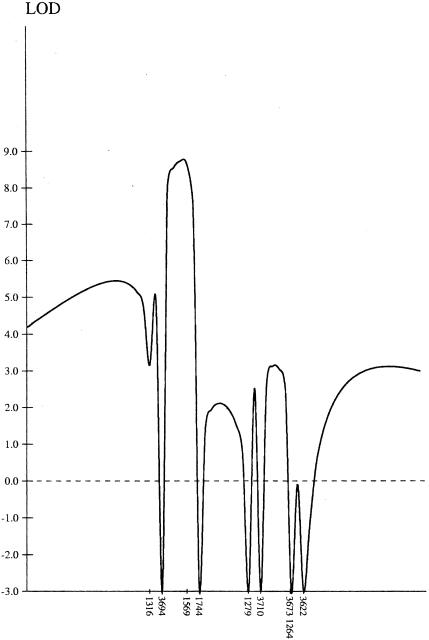
Seckel syndrome (MIM 210600) is an autosomal recessive disorder of low birth weight, severe microcephaly, and dysmorphic facial appearance with receding forehead, prominent nose, and micrognathia. We have performed a genomic screen in two consanguineous families of Pakistani origin and found that the disorder segregates with markers between loci D3S1316 and D3S3710, which map to chromosome 3q22.1-q24. Analysis using HOMOZ/MAPMAKER gave a maximum LOD score of 8.72. All five affected individuals were homozygous for the same allele, for two adjacent polymorphic markers within the region segregating with the disease, narrowing the region to 12 cM.
Seckel syndrome (MIM 210600) comprises intrauterine growth retardation, severe proportionately short stature, severe microcephaly, a “bird-headed” profile, and mental retardation. A number of Seckel-like syndromes have been identified, most notably microcephalic osteodysplastic primordial dwarfism types I–III (Bass et al. 1975; Majewski and Spranger 1976; Majewski and Goecke 1982; Majewski et al. 1982a, 1982b) and microcephalic osteodysplastic dysplasia (Hersh et al. 1994). These can be differentiated from Seckel syndrome on clinical and radiographic grounds.
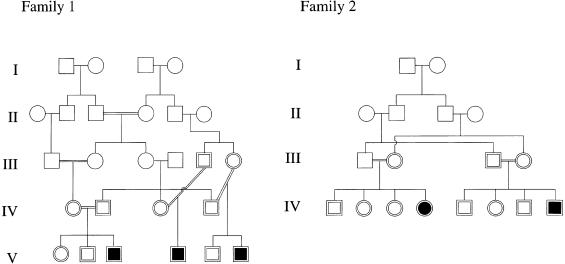
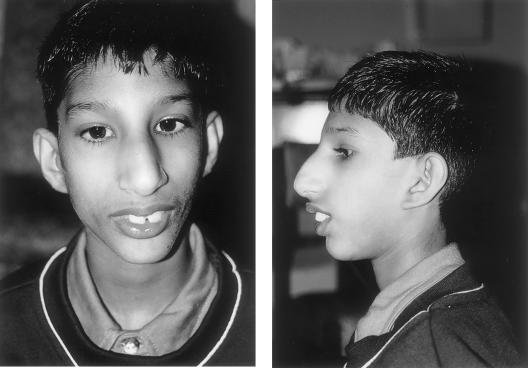
We have undertaken autozygosity mapping in two consanguineous families with Seckel syndrome that were from the same village in Pakistan but were not known to be related to each other (fig. 1). The proband in the first family, V6, was born at 35 wk gestation, weighing 1.1 kg (−3.3 SD) with a head circumference of 24 cm (−8 SD). His mother reported that the fontanelles were not palpable at birth. At age 9 years his height was 106 cm (−4.8 SD) and head circumference was 37 cm (−12 SD). He has moderate mental retardation and first walked at age 7 years. He has striking microcephaly, a receding forehead, and micrognathia with a prominent nose (fig. 2). He has crowded teeth and dental malocclusion. His ears are posteriorly rotated, with deficient lobes. He has no visual problems. He has a characteristic stance, with flexion at the hips and pronation of the forearms. The facial appearance, stature, and learning ability of his two affected cousins were very similar.
Figure 1.
A, Pedigree of family 1. B, Pedigree of family 2.
Figure 2.
Facial appearance of V6, showing microcephaly, receding forehead, micrognathia, prominent nose, and dental malocclusion. The ears are posteriorly rotated, with deficient lobes.
The radiological features in the index case included microcrania with fused sutures, a mild thoracic kyphosis with the ribs angulated posteriorly, and multiple ivory epiphyses in the hand. There was no dislocation of the radial heads. The pelvic radiographs showed narrow iliac blades, cox valga, and minor subluxation of the hips, features that were also present on pelvic radiographs of his cousin. Chromosome analysis in the index case was normal 46,XY with no evidence of increased spontaneous breakage, no increased breakage following gamma irradiation, and normal sister chromatid–exchange levels. Lymphocyte and immunoglobulin counts were normal.
The second family was seen in Pakistan, and no radiographs or accurate measurements are available. IV4 is now age 3.5 years and moderately retarded in her development. She is able to sit with support but does not crawl or have any words. She is very small, with microcephaly, and has the same facial dysmorphism as the affected children in the first family. Like them, she looks alert. IV8 is age 7 mo, small, and profoundly microcephalic.
A genomewide linkage screen was performed using a set of 367 fluorescence-labeled markers (Research Genetics set 8) at an average spacing of 10 cM. PCRs were performed in a total volume of 25 μl containing 60 ng of DNA, 0.1 μM each primer, 1.25 U of Taq DNA polymerase, 0.2 mM of each dNTP, 2 mM MgCl2, 50 mM KCL, 10 mM Tris-HCL (pH 9.0), and 0.1% Triton X. In each PCR reaction, around six primer sets in a similar size range were included, though overlapping size ranges for one dye would not be amplified or electrophoresed together. PCRs were performed as follows: initial denaturing at 94°C for 5 min, followed by 30 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min. Products were electrophoresed, alongside a TAMRA 500 standard, through a 4% polyacrylamide/6 M urea/1 × Tris borate–EDTA gel at 3,000 V for 2 h at 50°C. Data were retrieved using the ABI Genescan Analysis software package. The samples from the three affected individuals in family 1 were analyzed initially. For all markers where the affecteds were homozygous, the remaining samples from family 1 were analyzed. Extra markers from regions of interest were obtained from the Weizmann Institute's Unified Database for Human Genome Mapping, and all samples from family 1 and family 2 were analyzed for these markers. A single set of primers was used in each amplification reaction, in a total volume of 15 μl with 0.5 U of Taq polymerase and 2.5 mM MgCl2; otherwise, the PCR conditions were as described above. Multipoint analysis was performed using the HOMOZ/MAPMAKER program (Kruglyak et al. 1995).
After the initial screen, the three affected individuals were homozygous for markers at loci D2S2739 and D2S441 on chromosome 2; for D3S1764, D3S1744, D3S1763, and D3S3053 on chromosome 3q, and for single loci on chromosomes 4, 6, 10, and 17. The loci on chromosome 2, 4, 6, 10, and 17 were excluded after analysis of all the samples from family 1 and family 2 (data not shown). The genotypes of the affected children and their parents, for the chromosome 3 loci of interest, are shown in the table. D3S1316 is heterozygous in V6 (family 1) and marks the proximal limit of the homozygosity, and D3S1593 and DS3710 are heterozygous in IV4 (family 2), giving the distal limit of homozygosity. When the haplotype data are looked at, it seems likely that D3S1593 is telomeric of D3S1744, rather than centromeric—as shown in the Weizmann database. All five affected children are homozygous for the same allele size for the marker at D3S3694, for which 7 of the 10 parents were heterozygous, and D3S1569, for which 4 of the 10 parents were heterozygous. Results from the unaffected siblings were included in the data analysis; none were homozygous for loci in this region, for markers where the parental genotypes were informative. Multipoint linkage analysis of a subset of these markers using HOMOZ/MAPMAKER gave a maximum LOD score of 8.72 (fig. 3). The region of overlapping homozygosity extends over ∼15 cM, and the region for which all five affected individuals are homozygous for the same allele is ∼12 cM.
Table 1.
Genotype Data for the Affected Individuals and Their Parents for the Region of Interest on Chromosome 3
|
Genotypes in Family b |
||||||||||||||||||||||||||||||
| 1 |
2 |
|||||||||||||||||||||||||||||
| Markera | IV2 | IV1 | V3 | III5 | IV3 | V4 | IV4 | III6 | V6 | III1 | III2 | IV4 | III3 | III4 | IV8 | |||||||||||||||
| D3S3023 | 247 | 247 | 232 | 244 | 232 |
247 |
244 | 247 | 232 | 247 | 244 |
247 |
244 | 244 | 232 | 244 | 232 |
241 |
232 | 247 | 232 | 247 | ||||||||
| D3S1764 | 229 | 233 | 233 | 237 | 233 | 233 | 233 | 233 | 229 | 233 | 233 | 233 | 233 | 237 | 233 | 233 | 233 | 233 | 233 | 233 | 233 | 241 | 233 | 2330 | ||||||
| D3S3637 | 178 | 186 | 178 | 180 | 178 | 178 | 178 | 188 | 178 | 178 | 178 | 178 | 178 | 178 | 178 | 178 | 192 | 202 | 192 | 192 | 186 | 192 | 192 | 200 | 192 | 192 | ||||
| D3S1316 | 285 | 285 | 283 | 285 | 285 | 285 | 285 | 287 | 285 | 289 | 285 | 285 | 281 | 285 | 281 |
285 |
279 | 283 | 283 | 283 | 283 | 283 | 281 | 283 | 283 | 283 | ||||
| D3S3694 | 146 | 148 | 146 | 152 | 146 | 146 | 146 | 146 | 146 | 146 | 146 | 146 | 140 | 146 | 138 | 146 | 146 | 146 | 140 | 146 | 138 | 146 | 146 | 146 | 146 | 146 | 146 | 148 | 146 | 146 |
| D3S1569 | 277 | 277 | 277 | 295 | 277 | 277 | 277 | 295 | 277 | 277 | 277 | 277 | 277 | 289 | 277 | 297 | 277 | 277 | 277 | 277 | 277 | 277 | 277 | 277 | 277 | 277 | 277 | 277 | 277 | 277 |
| D3S3022 | 244 | 246 | 240 | 244 | 244 | 244 | 244 | 244 | 240 | 244 | 244 | 244 | 244 | 246 | 244 | 244 | 240 | 246 | 246 |
246 |
246 | 246 | 240 | 246 | 246 | 246 | ||||
| D3S1593 | 137 | 139 | 137 | 145 | 137 | 137 | 137 | 137 | 137 | 139 | 137 | 137 | 137 | 143 | 137 | 153 | 137 | 137 | 137 | 137 | 137 | 139 | 137 | 141 | 137 | 141 | 137 | 137 | ||
| D3S1744 | 152 | 156 | 152 | 152 | 152 | 152 | 138 | 152 | 152 | 164 | 152 | 152 | 148 | 152 | 148 | 152 | 152 | 152 | 152 | 160 | 148 | 160 | 160 | 160 | 152 | 160 | 156 | 160 | 160 | 160 |
| D3S1279 | 266 | 276 | 266 | 268 | 266 | 266 | 266 | 270 | 266 | 268 | 266 | 266 | 266 | 266 | 266 | 266 | 266 | 266 | 268 | 268 | 268 | 268 | 268 | 268 | 268 | 268 | 268 | 268 | 268 | 268 |
| D3S3710 | 269 | 273 | 269 | 273 | 273 | 273 | 273 | 273 | 269 | 273 | 273 | 273 | 269 | 273 | 271 | 273 | 273 | 273 | 269 | 271 | 271 | 273 | 271 | 273 | 269 | 273 | 267 | 273 | 273 | 273 |
| D3S3575 | 215 | 221 | 221 | 221 | 221 | 221 | 221 | 223 | 219 | 221 | 221 | 221 | 217 | 221 | 221 | 221 | 217 | 221 | 221 | 221 | 221 | 221 | 221 | 223 | 221 | 221 | ||||
| D3S1553 | 170 | 172 | 170 | 172 | 172 | 172 | 170 | 172 | 168 | 172 | 172 | 172 | 172 | 172 | 172 | 172 | 170 | 170 | 170 | 170 | 170 | 170 | 170 | 170 | 170 | 170 | ||||
| D3S3673 | 137 | 139 | 137 | 137 | 137 | 137 | 133 | 137 | 137 | 143 | 137 | 137 | 137 | 139 | 137 | 143 | 137 | 137 | 141 | 143 | 139 | 149 | 139 | 141 | 139 | 143 | 137 | 139 | 139 | 139 |
| D3S712 | 196 | 198 | 196 | 196 | 196 | 196 | 196 | 202 | 196 | 196 | 196 | 196 | 196 | 208 | 196 | 208 | 196 | 196 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | ||
| D3S3643 | 244 | 246 | 240 | 246 | 246 | 246 | 246 | 246 | 244 | 246 | 246 | 246 | 246 | 248 | 246 | 250 | 246 | 246 | 244 | 244 | 244 | 244 | 244 | 248 | 244 | 244 | 244 | 244 | ||
| D3S3682 | 225 | 225 | 225 | 225 | 225 | 225 | 225 | 227 | 225 | 225 | 225 | 225 | 225 | 225 | 225 | 225 | 223 | 225 | 225 | 225 | 225 | 225 | 225 | 225 | 225 | 225 | ||||
| D3S1264 | 261 | 263 | 261 | 261 | 261 | 261 | 253 | 261 | 257 | 261 | 261 |
261 |
261 | 261 | 257 | 261 | 261 |
261 |
259 | 263 | 261 | 261 | 259 | 261 | 261 | 263 | 259 | 261 | 261 | 261 |
| D3S3622 | 222 | 232 | 222 | 224 | 222 | 222 | 232 | 232 | 222 | 226 | 222 | 232 | 232 | 232 | 222 | 232 | 226 | 226 | 226 | 230 | 226 | 226 | 226 | 228 | 226 | 226 | ||||
| D3S1614 | 149 | 149 | 149 | 151 | 149 | 149 | 145 | 147 | 145 | 149 | 145 | 149 | 147 | 149 | 145 | 151 | 145 | 149 | 145 | 149 | 149 | 155 | 147 | 149 | 147 | 149 | 149 | 149 | ||
| D3S1282 | 143 | 143 | 143 | 147 | 143 | 143 | 143 | 145 | 143 | 145 | 143 | 143 | 145 | 145 | 143 | 145 | 143 | 149 | 143 | 143 | 143 | 143 | 143 | 143 | ||||||
| D3S1763 | 277 | 277 | 277 | 277 | 277 | 277 | 277 | 277 | 265 | 277 | 277 | 277 | 265 | 277 | 277 | 277 | 269 | 273 | 261 | 269 | 269 | 273 | 261 | 269 | 269 | 269 | ||||
| D3S3053 | 234 | 234 | 234 | 234 | 234 |
234 |
234 | 234 | 234 | 238 | 234 | 234 | 234 | 234 | 234 | 238 | 234 | 234 | 234 | 234 | 234 |
234 |
||||||||
The marker order is taken from the Weizmann database.
The regions of homozygosity in the five affected individuals are boxed.
Figure 3.
Multipoint LOD score for chromosome 3 markers.
In these families, Seckel syndrome maps to chromosome 3q22.1-3q24. Given that the families are from the same geographic region and that affected individuals are homozygous for the same allele, for two adjacent microsatellite polymorphisms, it is likely that they share a common ancestor. There are a number of candidate genes in the region segregating with the disorder. One of these is FRP1/ATR (Cimprich et al. 1996; Smith et al. 1998) which is related to ATM, the gene defective in ataxia telangiectasia (AT [MIM 208900]). ATM is a member of the phosphotidylinositol (PI)3-kinase family, which is involved in a wide variety of regulatory events, including signaling of DNA damage and control of cell cycle progression (Brown et al. 1999; Cortez et al. 1999). Although the clinical features of AT are distinct from those of Seckel syndrome, there have been reports suggesting that Seckel syndrome could be a DNA-repair disorder. Syrrou et al. (1995) described increased frequency of chromosome abnormalities in response to mitomycin C and increased frequency of sister-chromatid exchange in three siblings with Seckel syndrome. One of these siblings developed pancytopenia. Woods et al. (1995) reported an infant with a clinical diagnosis of Seckel syndrome who became pancytopenic at age 16 mo, in whom chromosome analysis of a bone marrow aspirate revealed increased chromosome breakage following mitomycin treatment. The clinical features in this child overlapped with those of Nijmegen breakage syndrome (NBS [MIM 251260]), which are growth retardation and microcephaly of pre- or postnatal onset, a characteristic facial appearance with a receding forehead, prominent midface with long nose, and receding mandible (Der Kaloustian et al. 1995, 1996). The gene defective in NBS encodes p95, a member of the MRE11/RAD50 double-strand break–repair complex (Carney et al. 1998; Matsuura et al. 1998; Varon et al. 1998). The cytogenetic abnormalities in NBS are the same as those found in AT—namely, multiple rearrangements mainly involving chromosomes 7 and 14 and increased sensitivity of lymphocytes and fibroblasts to ionizing radiation. Patients with AT have normal early development but then develop truncal ataxia, dysarthria, and cerebral deterioration, with affected individuals usually unable to walk after age 10 years. They have short stature and conjunctival telangiectasia. They are prone to infections and have increased incidences of leukemia and lymphoma. They have raised AFP levels and reduced immunoglobulins. The children in the family we studied have normal immunoglobulin profiles and no evidence of increased chromosome breakage, and they show none of the characteristic clinical features of AT. However, there is a significant clinical overlap between the DNA-repair defect NBS and Seckel syndrome. Given the indistinguishable chromosome abnormalities in NBS and AT, it is noteworthy that there is, in the region, a gene with homology to the gene defective in AT.
There is a second gene that may be implicated in DNA repair in the region segregating with Seckel syndrome in this family. SMARCA3 has DNA-dependent ATPase and helicase activities (Sheridan et al. 1995). Bloom syndrome (BLM [MIM 210900]), another of the chromosome-instability syndromes, results from mutations in RecQL, the product of which has DNA-dependent ATPase, DNA helicase, and 3′→5′ single-stranded DNA–translocation activities (Ellis et al. 1995). The clinical features of Bloom syndrome include sun sensitivity, telangiectatic skin lesions, short stature of prenatal onset, and increased incidence of lymphomas and leukemias. The chromosome abnormality in Bloom syndrome is increased sister-chromatid exchange. However, sun sensitivity was not a feature in the affected children in our family, and sister-chromatid exchange was not increased. Investigation of further families is required to determine whether Seckel syndrome is a heterogeneous or homogeneous condition and to narrow the critical region such that the defective gene can be identified.
Acknowledgments
We wish to thank Dr. Christine Hall of the Great Ormond Street Hospital for Sick Children, London, for her comments on the radiographs. We also thank the families for their help in completing this work, in particular for organizing H.G.'s trip to Pakistan and for their hospitality during the visit. A.J. was funded by the Wellcome Trust.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for Seckel syndrome [MIM 210600], AT [MIM 208900], NBS [MIM 251260], and BLM [MIM 210900])
- Unified Database for Human Genome Mapping, The http://bioinformatics.weizmann.ac.il/udb (for markers)
References
- Bass H, Smith L, Sparkes R, Gycpes M (1975) Case report 33. Syndrome Ident 3:12–14 [Google Scholar]
- Brown KD, Barlow C, Wynshaw-Boris A (1999) Multiple ATM-dependent pathways: an explanation for pleiotropy. Am J Hum Genet 64:46–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JP, Maser RS, Olivares H, Davis EM, Le Beau M, Yates JR III, Hays L, et al (1998) The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell 93:477–486 [DOI] [PubMed] [Google Scholar]
- Cimprich KA, Shin TB, Keith CT, Schreiber SL (1996) cDNA cloning and gene mapping of a candidate human cell cycle checkpoint protein. Proc Natl Acad Sci USA 93:2850–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez D, Wang Y, Qin J, Elledge SJ (1999) Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science 286:1162–1166 [DOI] [PubMed] [Google Scholar]
- Der Kaloustian VM, Elliott AM, Eydoux P (1995) Severe intrauterine growth retardation with increased mitomycin C sensitivity, or Nijmegen breakage syndrome? J Med Genet 32:998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der Kaloustian VM, Kleijer W, Booth A, Auerbach AD, Mazer B, Elliott AM, Abish S, et al (1996) Possible new variant of Nijmegen breakage syndrome. Am J Med Genet 65:21–26 [DOI] [PubMed] [Google Scholar]
- Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, Ciocci S, Proytcheva M, et al (1995) The Bloom's syndrome gene product is homologous to RecQ helicases. Cell 83:655–666 [DOI] [PubMed] [Google Scholar]
- Hersh JH, Joyce MR, Spranger J, Goatley EC, Lachman RS, Bhatt S, Rimoin DL (1994) Microcephalic osteodysplastic dysplasia. Am J Med Genet 51:194–199 [DOI] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Lander ES (1995) Rapid multipoint linkage analysis of recessive traits in nuclear families, including homozygosity mapping. Am J Hum Genet 56:519–527 [PMC free article] [PubMed] [Google Scholar]
- Majewski F, Goecke T (1982) Studies of microcephalic primordial dwarfism. I. Approach to a delineation of the Seckel syndrome. Am J Med Genet 12:7–21 [DOI] [PubMed] [Google Scholar]
- Majewski F, Ranke M, Schinzel A (1982a) Studies of microcephalic primordial dwarfism. II. The osteodysplastic type II of primordial dwarfism. Am J Med Genet 12:23–35 [DOI] [PubMed] [Google Scholar]
- Majewski F, Spranger J (1976) [A new (brachymelic) type of primordial dwarfism. Monatsschr Kinderheilkd 124:499–503 [PubMed] [Google Scholar]
- Majewski F, Stoeckenius M, Kemperdick H (1982b) Studies of microcephalic primordial dwarfism. III. An intrauterine dwarf with platyspondyly and anomalies of pelvis and clavicles—osteodysplastic primordial dwarfism type III. Am J Med Genet 12:37–42 [DOI] [PubMed] [Google Scholar]
- Matsuura S, Tauchi H, Nakamura A, Kondo N, Sakamoto S, Endo S, Smeets D, et al (1998) Positional cloning of the gene for Nijmegen breakage syndrome. Nat Genet 19:179–181 [DOI] [PubMed] [Google Scholar]
- Sheridan PL, Schorpp M, Voz ML, Jones KA (1995) Cloning of an SNF2/SWI2-related protein that binds specifically to the SPH motifs of the SV40 enhancer and to the HIV-1 promoter. J Biol Chem 270:4575–4587 [DOI] [PubMed] [Google Scholar]
- Smith L, Liu SJ, Goodrich L, Jacobson D, Degnin C, Bentley N, Carr A, et al (1998) Duplication of ATR inhibits MyoD, induces aneuploidy and eliminates radiation-induced G1 arrest. Nat Genet 19:39–46 [DOI] [PubMed] [Google Scholar]
- Syrrou M, Georgiou I, Paschopoulos M, Lolis D (1995) Seckel syndrome in a family with three affected children and hematological manifestations associated with chromosome instability. Genet Couns 6:37–41 [PubMed] [Google Scholar]
- Varon R, Vissinga C, Platzer M, Cerosaletti KM, Chrzanowska KH, Saar K, Beckmann G, et al (1998) Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell 93:467–476 [DOI] [PubMed] [Google Scholar]
- Woods CG, Leversha M, Rogers JG (1995) Severe intrauterine growth retardation with increased mitomycin C sensitivity: a further chromosome breakage syndrome. J Med Genet 32:301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]