Abstract
The multiresistance plasmid pJHCMW1, harbored by a clinical Klebsiella pneumoniae strain isolated from a neonate with meningitis, was sequenced. A circular sequence of 11,354 bp was generated, of which 7,993 bp make up Tn1331, a transposon including the antibiotic resistance genes aac(6′)-Ib, aadA1, blaOXA-9, and blaTEM-1. The gene aac(6′)-Ib is included in a gene cassette, and both aadA1 and blaOXA-9 are included in a single-gene cassette that may have arisen as a consequence of a recombination event involving two integrons. The pJHCMW1 plasmid replicates through a ColE1-like RNA-regulated mechanism, includes a functional oriT, and two loci with similarity to XerCD site-specific recombination target sites involved in plasmid stabilization by the resolution of multimers. One of these two loci, mwr, is active and has been the subject of previous studies, and the other, dxs, is not functional but binds the recombinase XerD with low affinity. Two additional open reading frames were identified, one with low similarity to two hypothetical membrane proteins from Mycobacterium tuberculosis and Mycobacterium leprae and the other with low similarity to psiB, a gene encoding a function that facilitates the establishment of the transferring plasmid in the recipient bacterial cell during the process of conjugation.
Klebsiella pneumoniae, a gram-negative rod, is a known cause of community-acquired bacterial pneumonia and other infectious diseases (7, 18, 38). This bacterium has also been identified as the causative agent of primary liver abscess, an important complication in diabetic patients in some geographical regions (12). K. pneumoniae also accounts for a substantial amount of hospital-acquired urinary tract infections, pneumonia, septicemias, meningitis, and soft tissue infections (5, 33, 39). The autoimmune disease ankylosing spondylitis is thought to be a possible sequela of K. pneumoniae infection (31). Although the molecular mechanisms of K. pneumoniae virulence are still not well understood, it has been proposed that the antiphagocytic capsule of K. pneumoniae plays a role in lung infections by preventing phagocytosis and suppressing the host immunological responses (14, 17). A recent study suggested that the capsule may induce production of interleukin 10 at the site of infection, which in turn may down-regulate the expression of other proinflammatory cytokines (40). Other putative virulence factors of pathogenic strains of K. pneumoniae could be iron acquisition systems, adhesions, serum resistance, and production of lipopolysaccharides (16). K. pneumoniae has been reported to be increasingly resistant to multiple antibiotics (28, 39), and the genetic determinants for resistance are often plasmid mediated (19, 33, 39). The plasmid pJHCMW1 was harbored by a K. pneumoniae clinical strain isolated from a neonate with meningitis during an outbreak of hospital infection (39). This plasmid contains the transposon Tn1331 which includes four antibiotic resistance genes: aac(6′)-Ib, aadA1, blaOXA-9, and blaTEM-1 (32, 36). Here we report the sequencing and describe the entire pJHCMW1 plasmid. Our analysis indicates that this plasmid can be considered the multiresistance transposon Tn1331 plus a DNA stretch carrying the functions for replication and mobilization.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Plasmid pJHCMW1 was originally isolated from K. pneumoniae JHCK1 (39). This plasmid was introduced into Escherichia coli XL1Blue (recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10]) (Stratagene), a strain also used as a host for DNA recombinant methods. Recombinant clones were generated using pUC18 as a cloning vector.
Bacterial growth medium and general procedures.
Growth of bacteria was in Lennox L broth (1% tryptone, 0.5% yeast extract, 0.5% NaCl). Plasmid DNA was prepared using the Qiagen plasmid mini kit (Qiagen, Inc.). Recombinant clones or pJHCMW1 was sequenced using BigDye (ABI) and DYEnamic ET (Amersham) chemistries on an ABI Prism (model 310 or 3100) instrument and M13 forward and reverse primers or custom-designed primers. Sequences were examined and assembled with Sequencher 4.1.2 software (Gene Codes Corp.). DNA and protein sequence analyses were performed using the Artemis program (http://www.sanger.ac.uk), the CLUSTAL W and BESTFIT programs of the Sequencing Analysis Software Package of the University of Wisconsin Genetics Computer Group (10), the HMMTOP program (http://www.enzim.hu/hmmtop/index.html) (37), and the DAS program (http://www.sbc.su.se/∼miklos/DAS/) (6).
DNA binding assays.
The oligonucleotides used had the following sequences:dif, 5′GATCCTTGGTGCGCATAATGTATATTATGTTAAATGGTACCCTGCA and 5′GGGTACCATTTAACATAATATACATTATGCGCACCAAGGATC; dxs, 5′TCGACGGTGTATGGCCATTTAAGGGATAATGTAACCTG and 5′GATCCAGGTTACATTATCCCTTAAATGGCCATACACCG; andmwr, 5′GATCCGGCGGTGCACGCAACAGATGTTATGGTAAATACG and 5′AATTCGTATTTACCATAACATCTGTTGCGTGCACCGCCG.
Approximately 10 pmol of oligonucleotide was end labeled with 50 μCi of [γ-32P]ATP and phage T4 polynucleotide kinase (5 U) in kinase buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 5 mM dithiothreitol, 0.1 mM spermidine) in a final volume of 20 μl. The labeled oligonucleotide was purified by chromatography through a Nuctrap Probe Purification column (Stratagene) and then ethanol precipitation. The radiolabeled oligonucleotide was dissolved in 15 μl of H2O and then made double stranded by annealing with 50 pmol of the complementary oligonucleotide. The mixture was heated to 75°C for a few minutes in a water bath, and then the thermostat was turned off to allow the sample to slowly cool to room temperature overnight. The annealed double-stranded radiolabeled oligonucleotides were purified by electrophoresis on an 8% polyacrylamide gel in Tris-borate buffer (100 mM Tris [pH 8], 100 mM boric acid, 2 mM EDTA) as described previously (34). The radiolabeled oligonucleotides were mixed with 0.1 mg of poly(dI-dC) per ml and the appropriate protein(s). The binding reaction was allowed to proceed for 10 min at 37°C, and then the reaction mixtures were immediately transferred to ice. The samples were analyzed by electrophoresis in a polyacrylamide gel as described above. The radioactive complexes were detected by exposure to X-ray film.
Nucleotide sequence accession numbers.
The complete circular nucleotide sequence of pJHCMW1 has been deposited in the GenBank sequence library and assigned the accession number AF479774.
RESULTS AND DISCUSSION
This project was originally initiated to focus on the resistance to amikacin found in clinical strains of K. pneumoniae isolated from diseased neonates (39). A multiresistant clinical K. pneumoniae strain isolated in a children's hospital from a neonate with meningitis was studied further (39). The resistance to amikacin and other aminoglycosides such as tobramycin and kanamycin (but not gentamicin) in this isolate was due to the presence of a plasmid, pJHCMW1, which includes the aac(6′)-Ib gene (39). Studies on the biological properties of pJHCMW1 led to the isolation and characterization of a transposon named Tn1331 (36), which includes aac(6′)-Ib as well as three more resistance genes, aadA, blaOXA-9, and blaTEM-1. Further studies characterized the transposon and the genetic organization and expression of the resistance genes (32, 35). The aac(6′)-Ib, aadA, and blaOXA-9 genes are transcribed as a polycistronic mRNA from a promoter located upstream of aac(6′)-Ib. In addition, blaOXA-9 is expressed from another promoter located immediately upstream of the structural gene (35).
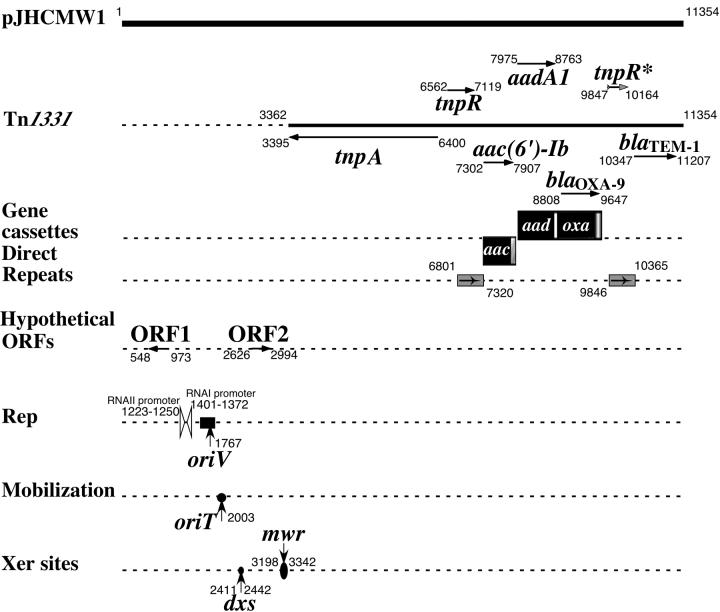
The complete sequence of pJHCMW1 was determined to be 11,354 bp, and analysis of the sequence revealed that 7,993 bp constitute the multiresistance transposon Tn1331. The remaining portion of pJHCMW1 contains sequence coding for the replication, mobilization, and possibly maintenance functions in addition to two hypothetical open reading frames (ORFs) that encode products that do not have significant matches in the databases. Figure 1 shows the locations and sizes of these genes and genetic structures. The transposon Tn1331 can be considered Tn3 with the addition of a DNA region, which has the structure of the variable portion of the integrons (32, 35) (Fig. 1). The inverted repeats at the Tn1331 ends as well as the tnpR gene are identical to those of Tn3. The Tn1331 tnpA nucleotide sequence is missing 9 nucleotides with respect to the Tn3 tnpA gene. Nucleotides 853 to 870 (coordinates as in GenBank accession number v00613) in the Tn3 tnpA gene are a 9-nucleotide tandem repeat that encodes the amino acid sequence GFHGFH. In contrast, the Tn1331 version of tnpA has only one of the 9-nucleotide repeats encoding the amino acid sequence GFH (coordinates 4214 to 4222). The Tn1331 fragment that has the genetic structure of the variable part of the integrons includes two gene cassettes harboring the antibiotic resistance genes aac(6′)-Ib, aadA1, and blaOXA-9 (35) and is flanked by 520-bp direct repeats (Fig. 1). Models for the genesis of Tn1331 as an evolutionary product of Tn3 have been described previously, and they involve a duplication of a portion of Tn3 that generated the 520-bp direct repeats (32). To determine whether the gene cassettes or the rest of the DNA in pJHCMW1 have different origins, we determined the percent G+C along the whole sequence as well as for each ORF. The results indicate that the mean G+C content of this plasmid is 48.96%, with all the ORFs located within Tn1331 displaying values similar to that of the full-length pJHCMW1. In contrast, the hypothetical ORF1 and ORF2, which were mapped outside Tn1331, displayed G+C contents of 43.63 and 42.48%, respectively. These differences in G+C content suggest that the origin of these two ORFs could be different from those contained within the Tn1331 transposable element.
FIG. 1.
Genetic map of pJHCMW1. Genes or ORFs are shown with the starting and ending coordinates. The transposon Tn1331, the direct repeats, and the replication (Rep), mobilization, and recombination elements are indicated. The gray arrowhead in tnpR∗ indicates that this is a truncated gene (the N terminus of the encoded protein is absent [32]).
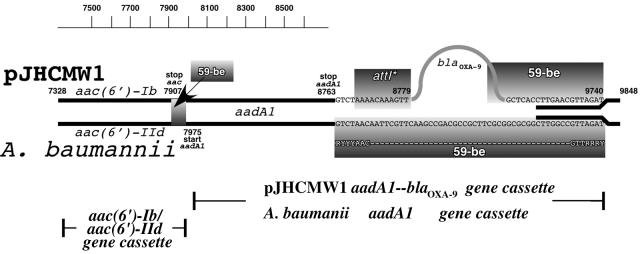
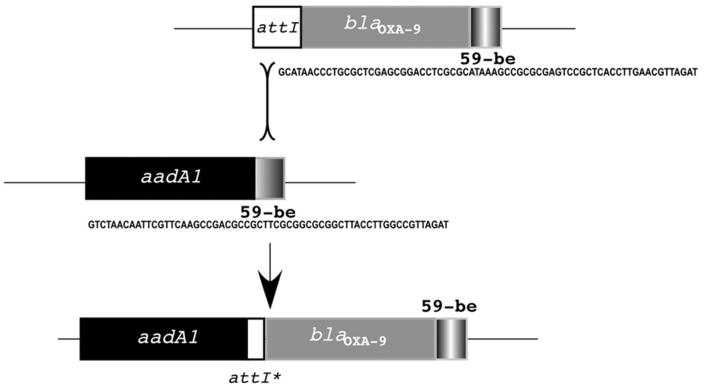
Antibiotic resistance genes are often found as part of gene cassettes, which include a coding region followed by the 59-be element, a site-specific recombination target for integron integrases (30). While aac(6′)-Ib is included in a gene cassette with the regular gene-59-be structure, the aadA1 and blaOXA-9 genes are unusual because both are included in the same gene cassette (35). Computer analysis demonstrated that the sequence of this gene cassette has very high similarity with the sequence of an integron-borne gene cassette that was recently found in Acinetobacter baumannii that carries only aadA1 (27). As is the case for pJHCMW1, the aadA1 gene cassette found in this A. baumannii integron is preceded by another one containing a gene, aac(6′)-IId, which codes for a protein that has 98% identity with AAC(6′)-Ib [the A. baumannii gene has Ser instead of Leu, which is characteristic of AAC(6′)-II enzymes (26)]. A diagram summarizing data comparing the nucleotide sequences and the structures of the gene cassettes is shown in Fig. 2. The aac(6′)-Ib/aac(6′)-IId, 59-be, and aadA1 sequences are highly similar in pJHCMW1 and the A. baumannii integron. However, while as in every regular gene cassette, the A. baumannii aadA1 is followed by a 59-be element, in pJHCMW1 following aadA1 there is a DNA region inserted that includes what may be considered a remnant of an attI site (attI∗ [Fig. 2]) and the blaOXA-9 gene followed by its 59-be (Fig. 2). This arrangement suggests that an integron containing the aadA1 gene cassette underwent an illegitimate recombination event with another integron in which the gene cassette adjacent to the 5′ conserved portion contained blaOXA-9 (Fig. 3) (35). A crossover may have occurred between the attI 5′ of blaOXA-9 and the 59-be 3′ of aadA1, resulting in the loss of the 59-be and the generation of attI∗ (Fig. 3) (35). The diagram in Fig. 3 also shows that after the insertion of blaOXA-9, the 59-be sequence has not been modified. Acquisition and modification of resistance genes through illegitimate recombination have also been reported in other systems. Analysis of Pasteurella and Mannheimia isolates carrying the sulII and strA genes showed that more than half of the isolates had an insertion of the catAIII which occurred by illegitimate recombination (15). Fusions of gene cassettes that may have occurred by other mechanisms have also been described elsewhere (11, 21, 22).
FIG. 2.
Comparison of structures of regions including aac(6′)-Ib, aadA1, and blaOXA-9 from pJHCMW1 and aac(6′)-IId and aadA1 from A. baumannii. The black lines represent DNA fragments with high homology. The numbers are the base pair coordinates in the pJHCMW1 nucleotide sequence. The nucleotide sequence of the 59-be element following aadA1 is shown. The homology between A. baumannii and pJHCMW1 DNA ends at the end of the aadA1 structural gene and starts again at coordinate 9727 (final portion of the blaOXA-9 59-be element). At the bottom of the diagram, the positions and lengths of the gene cassettes are shown.
FIG. 3.
Possible mechanism for the generation of the gene cassette containing aadA1 and blaOXA-9. Two putative integrons, one containing blaOXA-9 immediately following attI and one with a gene cassette containing aadA1 crossover (probably an illegitimate recombination event), generating the gene cassette, including both genes found in pJHCMW1. In the process, the sequence of the 59-be element located 3′ of blaOXA-9 has been conserved, but the attI site and the 59-be element 3′ of aadA1 have been lost, leaving a sequence indicated as attI∗. The attI site is located adjacent to the intI gene in the 5′ conserved region and is where gene cassettes are inserted in integrase-mediated reactions (22). The nucleotide sequences of the 59-be elements are shown.
The blaOXA-9 gene cassette has also been found in another integron structure, In40, isolated from Enterobacter aerogenes (GenBank accession number AF034958) (24). The nucleotide sequence of this blaOXA-9 gene cassette is identical to the blaOXA-9-59-be portion of the pJHCMW1 gene cassette including aadA1 and blaOXA-9 (data not shown). The homology between pJHCMW1 and In40 at the region including blaOXA-9 starts at pJHCMW1 coordinate 8777 and ends at 9848, the beginning of one of the 520-bp direct repeats (data not shown).
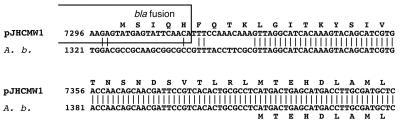
Inspection of the N-terminal portion of the aac(6′)-Ib genes from K. pneumoniae and A. baumannii shows that the gene from pJHCMW1 has the potential to encode a longer protein compared to that from A. baumannii due to the fusion to the initial portion of blaTEM-1 (32) (Fig. 4). We do not know whether the actual start codon of the pJHCMW1 AAC(6′)-Ib is at the ATG codon at coordinate 7302, 7389, or 7410. If the actual start codon were at coordinate 7389, both versions of the enzyme would have identical N termini. However, this may not be a critical question, because Casin et al. (3) recently analyzed variants of AAC(6′)-Ib from members of the family Enterobacteriaceae and found that there is a high flexibility in the structural requirements at the N terminus of this enzyme. The pJHCMW1-encoded AAC(6′)-Ib protein and other proteins in the family of the AAC(6′)-I enzymes have recently been partially characterized by mutagenesis (25, 26, 29).
FIG. 4.
Alignment of the nucleotide sequences at the N terminus of aac(6′)-Ib from pJHCMW1 and aac(6′)-IId from A. baumannii (A. b.). The proteins encoded by these genes have 98% identity [comparison starts at the AAC(6′)-Ib Met at nucleotide 7389]. Identical nucleotides are indicated by the vertical lines between the two sequences. The sequence identical to that of blaTEM-1 in the pJHCMW1 aac(6′)-Ib gene is boxed.
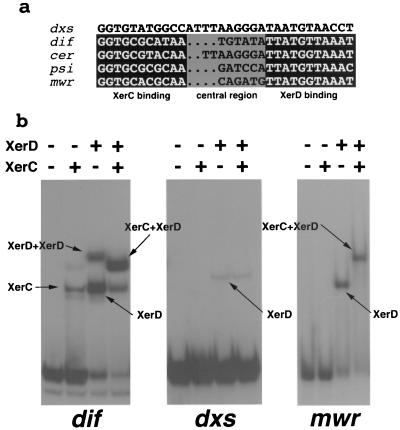
Maintenance and dissemination functions are included in a region between coordinates 1223 (the first nucleotide of the −35 sequence of the RNAII promoter) and 3342 (the last nucleotide of the mwr recombination site) (Fig. 1). The replication of pJHCMW1 occurs by an RNA-regulated mechanism similar to that described for the ColE1 plasmid (9). oriV is located at coordinate 1767 (Fig. 1). A sequence with high similarity to the ColE1 oriT is present with the nick site at coordinate 2003. This oriT has been proven functional in experiments where the ColE1 mob and RK2 tra genes were supplemented in trans (9). The Xer site-specific recombination site mwr is located at coordinates 3198 to 3342. This site shares homology with other Xer site-specific recombination sites that act as targets of the Xer site-specific recombination system to convert plasmid and chromosome dimers to monomers (1). The mwr core recombination site has 71% identity with the corresponding psi and dif sites and 75% identity with cer, although cer has 8 nucleotides in its central region (Fig. 5a). However, an important difference between mwr and other Xer site-specific recombination target sites is that the efficiency of recombination at mwr in E. coli is strongly dependent on the osmolarity of the growth medium (23). The binding of the mwr core recombination site to the recombinases XerC and/or XerD is shown in Fig. 5b, which shows the cooperative binding of XerC and XerD. At coordinates 2411 to 2442, there is another site that shares a lower degree of similarity with Xer recombination sites; this site was named dxs (for deficient Xer recombination site). Figure 5a shows a comparison of the nucleotide sequences of mwr, cer, psi, and dif with that of dxs. The percentages of identity of the dxs XerD-binding site and the corresponding region of the other sites are 64 (dif) and 54.5 (mwr, cer, and psi). Recombination analysis showed that dxs is not functional under the conditions assayed (data not shown). However, in vitro DNA-protein binding experiments demonstrated that dxs exhibits a low but detectable binding affinity to XerD. Figure 5b shows a comparison of binding of dif, mwr, and dxs to XerC, XerD, and both recombinases. As expected, dif exhibited weak binding to XerC but showed strong binding to XerD and cooperative binding to XerC and XerD (Fig. 5b). As previously known, mwr binding to XerC alone was not detected, but binding to XerD was strong and strong cooperative binding to XerC and XerD was observed (Fig. 5b). Conversely, dxs did not show binding to XerC and showed only very weak binding with XerD. Addition of both, XerC and XerD, did not enhance binding (Fig. 5b). These results suggest that this may be a remnant of a Xer recombination site. To the best of our knowledge, there are no reports of any biological function by a site with the characteristics of dxs.
FIG. 5.
Comparison of Xer recombination sites and DNA-protein binding. (a) Alignment of the nucleotide sequences of the core recombination sites mwr, cer, psi, dif, and dxs. The XerC and XerD binding sites and the central regions (gray) are shown. (b) Oligonucleotides with the dif, mwr, and dxs nucleotide sequences were end labeled and incubated with (+) and without (−) the indicated proteins. The products of the binding reactions were separated by electrophoresis in an 8% polyacrylamide gel, and the bands were detected by autoradiography. The nature of the complexes for each signal is shown.
Flanking the replication and oriT regions, there are two ORFs predicted to encode proteins of 15,705 Da and pI 10.2 (ORF1, encompassing coordinates 973 to 551), and 13,267 Da and pI 5.1 (ORF2, encompassing coordinates 2626 to 2991) (Fig. 1). The predicted ORF1 protein contains two transmembrane helices (aa 28 to 52 and 87 to 106) as determined using the HMMTOP program (http://www.enzim.hu/hmmtop/index.html) (37) and one (aa 28 to 52) as determined using the DAS program (http://www.sbc.su.se/∼miklos/DAS/) (6). No transmembrane regions were found in the amino acid sequence of the predicted ORF2 protein. The predicted ORF1 protein shows a low degree of similarity with a hypothetical protein from Mycobacterium tuberculosis (GenBank accession number Z92771, hypothetical protein Rv3278c) (4) (60% similarity and 26% identity in the amino acid sequence from amino acids [aa] 56 to 107) and a Mycobacterium leprae putative membrane protein (GenBank accession number AL583919, hypothetical protein ML0733) (50% similarity and 20% identity in the sequence from aa 56 to 133) (4). The predicted ORF2 protein has a low similarity to the psiB gene from pMK101 (61% similarity and 34% identity in the sequence from aa 8 to 61) (GenBank accession number U72482) (8). This is one of two conserved genes found in several conjugative plasmids thought to encode functions that facilitate the establishment of the transferring plasmid in the recipient bacterial cell during conjugation (2, 13). The plasmid ColIb-P9 psiB gene and the ardA and ssb genes have been shown to be expressed through zygotic induction (13) and to use single-stranded DNA transcription (2). All three genes seem to be transcribed from the same promoter, which shares similarity with that of Frpo, a single-stranded DNA promoter (20) located in the leading region of plasmid F. Plasmid pJHCMW1 carries a functional oriT (9), but no other conjugation-related gene had been identified before. Although the similarity between the ORF2 protein and PsiB is very low, experiments will be performed to determine whether the predicted ORF2 protein plays a role in establishment of pJHCMW1 in the recipient cell upon conjugation and to identify the promoter that drives transcription of this hypothetical protein.
K. pneumoniae has been reported to be increasingly resistant to multiple antibiotics, and the genetic determinants for resistance are often plasmid mediated. The plasmid pJHCMW1 is responsible for resistance to several aminoglycosides and β-lactams in the clinical K. pneumoniae JHCK1 isolate (39). This plasmid seems to have evolved to carry the multiresistance transposon Tn1331 and the minimal functions required for replication, stability, and mobilization.
Acknowledgments
This work was supported in part by Public Health grants AI47115-01 (M.E.T.) and LS Basin MIRT T37 TW00048-05 from the National Institutes of Health, a grant from Wellcome Trust (D.S.), and a grant from Miami University (L.A.A.). R.S. was supported by MSD grant R25 GM56820-03 from the National Institutes of Health.
We thank Garry Blakely and Sean Colloms for helpful discussions and suggestions.
REFERENCES
- 1.Barre, F., and D. J. Sherratt. 2002. Xer site-specific recombination: promoting chromosome segregation, p. 145-161. In N. Craig, R. Craigie, M. Gellert, and A. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, D.C.
- 2.Bates, S., R. A. Roscoe, N. J. Althorpe, W. J. Brammar, and B. M. Wilkins. 1999. Expression of leading region genes on IncI1 plasmid ColIb-P9: genetic evidence for single-stranded DNA transcription. Microbiology 145:2655-2662. [DOI] [PubMed] [Google Scholar]
- 3.Casin, I., F. Bordon, P. Bertin, A. Coutrot, I. Podglajen, R. Brasseur, and E. Collatz. 1998. Aminoglycoside 6′-N-acetyltransferase variants of the Ib type with altered substrate profile in clinical isolates of Enterobacter cloacae and Citrobacter freundii. Antimicrob. Agents Chemother. 42:209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 5.Coque, T. M., A. Oliver, J. C. Perez-Diaz, F. Baquero, and R. Canton. 2002. Genes encoding TEM-4, SHV-2, and CTX-M-10 extended-spectrum β-lactamases are carried by multiple Klebsiella pneumoniae clones in a single hospital (Madrid, 1989 to 2000). Antimicrob. Agents Chemother. 46:500-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cserzo, M., E. Wallin, I. Simon, G. von Heijne, and A. Elofsson. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10:673-676. [DOI] [PubMed] [Google Scholar]
- 7.Daza, R., J. Gutierrez, and G. Piedrola. 2001. Antibiotic susceptibility of bacterial strains isolated from patients with community-acquired urinary tract infections. Int. J. Antimicrob. Agents 18:211-215. [DOI] [PubMed] [Google Scholar]
- 8.Delver, E. P., and A. A. Belogurov. 1997. Organization of the leading region of IncN plasmid pKM101 (R46): a regulation controlled by CUP sequence elements. J. Mol. Biol. 271:13-30. [DOI] [PubMed] [Google Scholar]
- 9.Dery, K. J., R. Chavideh, V. Waters, R. Chamorro, L. S. Tolmasky, and M. E. Tolmasky. 1997. Characterization of the replication and mobilization regions of the multiresistance Klebsiella pneumoniae plasmid pJHCMW1. Plasmid 38:97-105. [DOI] [PubMed] [Google Scholar]
- 10.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubois, V., L. Poirel, C. Marie, C. Arpin, P. Nordmann, and C. Quentin. 2002. Molecular characterization of a novel class 1 integron containing blaGES-1 and a fused product of aac3-Ib/aac6′-Ib′ gene cassettes in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 46:638-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsueh, P. R., J. J. Wu, L. J. Teng, Y. C. Chen, P. C. Yang, S. W. Ho, and K. T. Luh. 2002. Primary liver abscess caused by one clone of Klebsiella pneumoniae with two colonial morphotypes and resistotypes. Emerg. Infect. Dis. 8:100-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones, A. L., P. T. Barth, and B. M. Wilkins. 1992. Zygotic induction of plasmid ssb and psiB genes following conjugative transfer of Incl1 plasmid Collb-P9. Mol. Microbiol. 6:605-613. [DOI] [PubMed] [Google Scholar]
- 14.Kabha, K., L. Nissimov, A. Athamna, Y. Keisari, H. Parolis, L. A. Parolis, R. M. Grue, J. Schlepper-Schafer, A. R. Ezekowitz, D. E. Ohman, and I. Ofek. 1995. Relationships among capsular structure, phagocytosis, and mouse virulence in Klebsiella pneumoniae. Infect. Immun. 63:847-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kehrenberg, C., and S. Schwarz. 2002. Nucleotide sequence and organization of plasmid pMVSCS1 from Mannheimia varigena: identification of a multiresistance gene cluster. J. Antimicrob. Chemother. 49:383-386. [DOI] [PubMed] [Google Scholar]
- 16.Lai, Y. C., H. L. Peng, and H. Y. Chang. 2001. Identification of genes induced in vivo during Klebsiella pneumoniae CG43 infection. Infect. Immun. 69:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai, Y. C., S. L. Yang, H. L. Peng, and H. Y. Chang. 2000. Identification of genes present specifically in a virulent strain of Klebsiella pneumoniae. Infect. Immun. 68:7149-7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liam, C. K., K. H. Lim, and C. M. Wong. 2001. Community-acquired pneumonia in patients requiring hospitalization. Respirology 6:259-264. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Martinez, L., A. Pascual, and G. A. Jacoby. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797-799. [DOI] [PubMed] [Google Scholar]
- 20.Masai, H., and K. Arai. 1997. Frpo: a novel single-stranded DNA promoter for transcription and for primer RNA synthesis of DNA replication. Cell 89:897-907. [DOI] [PubMed] [Google Scholar]
- 21.Parent, R., and P. H. Roy. 1992. The chloramphenicol acetyltransferase gene of Tn2424: a new breed of cat. J. Bacteriol. 174:2891-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partridge, S. R., G. D. Recchia, C. Scaramuzzi, C. M. Collis, H. W. Stokes, and R. M. Hall. 2000. Definition of the attI1 site of class 1 integrons. Microbiology 146:2855-2864. [DOI] [PubMed] [Google Scholar]
- 23.Pham, H., K. J. Dery, D. J. Sherratt, and M. E. Tolmasky. 2002. Osmoregulation of dimer resolution at the plasmid pJHCMW1 mwr locus by Escherichia coli XerCD recombination. J. Bacteriol. 184:1607-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ploy, M. C., P. Courvalin, and T. Lambert. 1998. Characterization of In40 of Enterobacter aerogenes BM2688, a class 1 integron with two new gene cassettes, cmlA2 and qacF. Antimicrob. Agents Chemother. 42:2557-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poirel, L., T. Lambert, S. Turkoglu, E. Ronco, J. Gaillard, and P. Nordmann. 2001. Characterization of class 1 integrons from Pseudomonas aeruginosa that contain the blaVIM-2 carbapenem-hydrolyzing 32 β-lactamase gene and of two novel aminoglycoside resistance gene cassettes. Antimicrob. Agents Chemother. 45:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rather, P. N., H. Munayyer, P. A. Mann, R. S. Hare, G. H. Miller, and K. J. Shaw. 1992. Genetic analysis of bacterial acetyltransferases: identification of amino acids determining the specificities of the aminoglycoside 6′-N-acetyltransferase Ib and IIa proteins. J. Bacteriol. 174:3196-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riccio, M. L., N. Franceschini, L. Boschi, B. Caravelli, G. Cornaglia, R. Fontana, G. Amicosante, and G. M. Rossolini. 2000. Characterization of the metallo-β-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of blaIMP allelic variants carried by gene cassettes of different phylogeny. Antimicrob. Agents Chemother. 44:1229-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shannon, K., K. Fung, P. Stapleton, R. Anthony, E. Power, and G. French. 1998. A hospital outbreak of extended-spectrum 32 β-lactamase-producing Klebsiella pneumoniae investigated by RAPD typing and analysis of the genetics and mechanisms of resistance. J. Hosp. Infect. 39:291-300. [DOI] [PubMed] [Google Scholar]
- 29.Shmara, A., N. Weinsetel, K. J. Dery, R. Chavideh, and M. E. Tolmasky. 2001. Systematic analysis of a conserved region of the aminoglycoside 6′-N-acetyltransferase type Ib. Antimicrob. Agents Chemother. 45:3287-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stokes, H. W., D. B. O'Gorman, G. D. Recchia, M. Parsekhian, and R. M. Hall. 1997. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol. Microbiol. 26:731-745. [DOI] [PubMed] [Google Scholar]
- 31.Tiwana, H., R. S. Natt, R. Benitez-Brito, S. Shah, C. Wilson, S. Bridger, M. Harbord, M. Sarner, and A. Ebringer. 2001. Correlation between the immune responses to collagens type I, III, IV and V and Klebsiella pneumoniae in patients with Crohn's disease and ankylosing spondylitis. Rheumatology (Oxford) 40:15-23. [DOI] [PubMed] [Google Scholar]
- 32.Tolmasky, M. E. 1990. Sequencing and expression of aadA, bla, and tnpR from the multiresistance transposon Tn1331. Plasmid 24:218-226. [DOI] [PubMed] [Google Scholar]
- 33.Tolmasky, M. E., R. M. Chamorro, J. H. Crosa, and P. M. Marini. 1988. Transposon-mediated amikacin resistance in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 32:1416-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tolmasky, M. E., S. Colloms, G. Blakely, and D. J. Sherratt. 2000. Stability by multimer resolution of pJHCMW1 is due to the Tn1331 resolvase and not to the Escherichia coli Xer system. Microbiology 146:581-589. [DOI] [PubMed] [Google Scholar]
- 35.Tolmasky, M. E., and J. H. Crosa. 1993. Genetic organization of antibiotic resistance genes (aac(6′)-Ib, aadA, and oxa9) in the multiresistance transposon Tn1331. Plasmid 29:31-40. [DOI] [PubMed] [Google Scholar]
- 36.Tolmasky, M. E., and J. H. Crosa. 1987. Tn1331, a novel multiresistance transposon encoding resistance to amikacin and ampicillin in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 31:1955-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tusnady, G. E., and I. Simon. 2001. The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849-850. [DOI] [PubMed] [Google Scholar]
- 38.Wang, T. K., S. S. Wong, and P. C. Woo. 2001. Two cases of pyomyositis caused by Klebsiella pneumoniae and review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 20:576-580. [DOI] [PubMed] [Google Scholar]
- 39.Woloj, M., M. E. Tolmasky, M. C. Roberts, and J. H. Crosa. 1986. Plasmid-encoded amikacin resistance in multiresistant strains of Klebsiella pneumoniae isolated from neonates with meningitis. Antimicrob. Agents Chemother. 29:315-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida, K., T. Matsumoto, K. Tateda, K. Uchida, S. Tsujimoto, and K. Yamaguchi. 2001. Induction of interleukin-10 and down-regulation of cytokine production by Klebsiella pneumoniae capsule in mice with pulmonary infection. J. Med. Microbiol. 50:456-461. [DOI] [PubMed] [Google Scholar]