Abstract
Surveillance for Streptococcus pneumoniae resistant to penicillin and other antimicrobial agents is necessary to define the optimal empirical antibiotic therapy for meningitis in resource-poor countries such as Vietnam. The clinical and microbiological features of 100 patients admitted to the Centre for Tropical Diseases in Ho Chi Minh City, Vietnam, between 1993 and 2002 with invasive pneumococcal disease were studied. A penicillin-nonsusceptible pneumococcus (MIC, ≥0.1 μg/ml) was isolated from the blood or cerebrospinal fluid of 8% of patients (2 of 24) between 1993 and 1995 but 56% (20 of 36) during 1999 to 2002 (P < 0.0001). Pneumococcal isolates resistant to penicillin (MIC, ≥2.0 μg/ml) increased from 0% (0 of 24) to 28% (10 of 36) (P = 0.002). Only one isolate was ceftriaxone resistant (MIC, 2.0 μg/ml). Penicillin-nonsusceptible pneumococci were isolated from 78% of children younger than 15 years (28 of 36) compared with 25% of adults (16 of 64) (P = 0.0001). Isolation of a penicillin-nonsusceptible pneumococcus in adults with meningitis was independently associated with referral from another hospital (P = 0.005) and previous antibiotic therapy (P = 0.025). Multilocus sequence typing showed that 86% of the invasive penicillin-resistant pneumococcus isolates tested (12 of 14) were of the Spain23F-1 clone. The serotypes of >95% of the penicillin-nonsusceptible pneumococci were included in the currently available pneumococcal vaccines. Our findings point to the recent introduction and spread of the Spain23F-1 clone of penicillin-resistant pneumococci in Vietnam. Simple clinical predictors can be used to guide empirical antibiotic therapy of meningitis. Pneumococcal vaccination may help to control this problem.
Streptococcus pneumoniae is an important cause of pneumonia and meningitis, particularly in resource-poor tropical countries such as Vietnam (34). In such countries, penicillin has been a satisfactory first-line treatment for invasive pneumococcal disease including meningitis for half a century. However, pneumococci resistant to multiple antimicrobial agents including penicillin have become significantly more common in the last 15 years and have become a worldwide problem (33). Countries in the Far East have some of the highest levels of resistance to penicillin and other commonly used antimicrobials (17, 30). There are limited data concerning this issue from Vietnam. However, two recent studies of the antimicrobial susceptibilities of nasal pneumococci in schoolchildren found that 12 to 19% of the children carried penicillin-resistant pneumococci (17, 24). Since resistant nasopharyngeal S. pneumoniae may predict resistance in clinical isolates, these results implied that penicillin resistance may also be common in invasive clinical isolates.
Treatment of penicillin-resistant pneumococcal meningitis with penicillin will result in clinical failure. Chloramphenicol is not a viable alternative in these patients because these organisms are often resistant to multiple antibiotics, and so extended-spectrum cephalosporins such as ceftriaxone are needed. However, these antibiotics are often far too expensive to use widely. Furthermore, many hospitals in countries such as Vietnam have limited facilities for isolating pneumococci from blood and cerebrospinal fluid (CSF) and performing susceptibility testing. Simple clinical predictors to determine which patients would benefit from empirical therapy with expensive extended-spectrum cephalosporins would therefore be valuable.
The emergence of penicillin-resistant pneumococci in different countries may result from the spread of particular clones or from the de novo appearance of new clones (20, 27, 37). This has been confirmed by a variety of the molecular methods available for the epidemiological typing of S. pneumoniae including multilocus sequence typing (MLST). MLST is a recently developed method which relies on comparison of the sequences of ∼450-bp internal fragments of seven housekeeping genes between strains. The combination of alleles at each locus defines an allelic profile or sequence type (5). This technique, which yields an unambiguous sequence type, has now been used to characterize isolates of S. pneumoniae from many countries.
We have studied patients with invasive pneumococcal infection admitted to an infectious- disease referral hospital in Ho Chi Minh City, Vietnam, between 1993 and 2002, and we document a significant increase in the isolation of penicillin-resistant pneumococci, predominantly the Spain23F-1 clone (20). We also report epidemiological and clinical predictors for the isolation of a penicillin-resistant pneumococcus.
MATERIALS AND METHODS
Patients and bacterial isolates.
We studied all patients (n = 100) admitted to the Centre for Tropical Diseases, Ho Chi Minh City, between January 1993 and January 2002 from whom S. pneumoniae was recovered from blood or cerebrospinal fluid. This hospital admits patients directly from the surrounding urban community as well as serving as a referral hospital for infectious- disease problems for Ho Chi Minh City and the provinces of southern Vietnam. For the MLST, an additional set of isolates (n = 18) was used which was a subset of penicillin- resistant S. pneumoniae strains that had been isolated during a previous study of nasal carriage in Vietnamese schoolchildren (24).
Blood and CSF were processed by standard microbiological methods (26). Potential S. pneumoniae strains were selected by colony morphology and alpha-hemolysis and confirmed by Gram staining and determination of susceptibility to optochin (Oxoid, Basingstoke, United Kingdom). Only a single isolate from each patient was studied. All strains were stored after primary isolation in glycerol broth at −40°C or in liquid nitrogen. MIC determinations, serotyping, and MLST were performed at a later date. Some isolates did not remain viable during storage; 80 isolates were available for MIC determinations, and 60 were available for serotyping.
Antimicrobial susceptibility testing.
S. pneumoniae organisms were screened for susceptibility to penicillin with a 1-μg oxacillin disk (Oxoid) by the Kirby-Bauer disk diffusion method. Strains with a zone of inhibition of ≥20 mm were considered penicillin susceptible, while those with a zone of <20 mm were considered potentially penicillin resistant (22).
MIC were determined by the E test (AB Biodisk, Solna, Sweden) as specified by the manufacturer. Penicillin G, ceftriaxone, chloramphenicol, erythromycin, tetracycline, trimethoprim-sulfamethoxazole, rifampin, vancomycin, azithromycin, cefepime, imipenem, and levofloxacin were tested. Suspensions of S. pneumoniae with turbidity equivalent to that of a 0.5 McFarland standard were prepared by suspension of 5 to 10 colonies from a sheep blood agar plate. The suspension was applied with a cotton swab to plates containing Mueller-Hinton agar (Oxoid) with 5% sheep blood. The E test strip was then placed on the surface of the agar. The plates were incubated at 37°C for 18 h in 5% CO 2. The concentration of antimicrobial agent inhibiting growth was taken as the MIC. S. pneumoniae ATCC 49619 was used as the quality control strain and gave values within the acceptable range.
Antimicrobial susceptibility breakpoints were defined according to NCCLS criteria (23). For penicillin, an MIC of ≤0.06 μg/ml was considered susceptible, an MIC of ≥0.1 to 1.0 μg/ml was considered intermediate, and an MIC of ≥2.0 μg/ml was considered resistant.
Serotyping.
Serotyping was performed by the quellung reaction using specific antisera (Pneumotest; Staten Serum Institute, Copenhagen, Denmark). One colony was tested from the subculture of the frozen isolate.
MLST.
MLST was performed as described previously (5, 27, 37). In brief, the nucleotide sequences of ∼ 450-bp internal regions from the aroE, gdh, gki, recP, spi, xpt, and ddl genes were amplified by PCR using previously described primers (5). The gene fragments were sequenced on both strands, using the same primers, with an ABI 3700 Prism automated sequencer with Big Dye 2.0 terminators (PE Applied Biosystems). For each gene, the sequences were compared with each other and with those in the pneumococcal MLST database (http://www.mlst.net/). Sequences were assigned to known alleles if they were identical to alleles in the database or to new alleles if they differed in sequence from any of the known alleles. No weighting was given to the degree of sequence divergence between different alleles. In the absence of knowledge of the proportion of allelic changes that are due to recombination rather than mutation, we cannot say that alleles differing at many sites are any more distantly related than those differing at a single site. The alleles at each of the seven loci defined the allelic profile, or sequence type, and the relatedness of isolates was determined by constructing a tree by the unweighted pair group method with arithmetic means (UPGMA) from the matrix of pairwise differences between the allelic profiles by using Satistica software (StatSoft, Tulsa, Okla.) as described elsewhere (5).
Clinical data.
The relationship between age of the patient and year of isolation of a penicillin-susceptible or nonsusceptible pneumococcus strain was determined for all patients. Thirty-six of the adults had been recruited to a double-blind placebo-controlled trial of dexamethasone in the treatment of bacterial meningitis. The prospectively collected clinical data from these patients were analyzed to look for factors that might predict the isolation of a penicillin- nonsusceptible isolate of S. pneumoniae.
Analysis.
Statistical analysis was performed using the Epi-Info package version 6.0 (Centers for Disease Control and Prevention, Atlanta, Ga.) and SPSS version 7.5 for Windows (SPSS Inc., Chicago, Ill.) Categorical variables were compared using the χ2 test or Fisher's exact test. Non-normally distributed continuous variables were compared using the Mann-Whitney U test. A P value of <0.05 was considered significant.
RESULTS
Between January 1993 and January 2002, S. pneumoniae was isolated from the blood and/or CSF of 100 patients admitted to the Centre for Tropical Diseases. By disk sensitivity testing, 49 isolates (49%) had an oxacillin zone of ≥20 mm and 51 (51%) had an oxacillin zone of <20 mm. A total of 56 of the isolates (56%) were penicillin susceptible by MIC determination (MIC, ≤0.06 μg/ml), 26 (26%) were penicillin intermediate (MIC ≥0.125 μg/ml and ≤1.0 μg/ml), and 18 (18%) were penicillin resistant (MIC, ≥2.0 μg/ml). All isolates with an oxacillin zone of ≥20 mm were susceptible to penicillin by MIC determination. Of 51 isolates with an oxacillin zone of <20 mm, 7 (14%) were susceptible to penicillin by MIC determination; the remainder were intermediate or resistant. The positive predictive value of oxacillin resistance for penicillin nonsusceptibility was 86%, with a negative predictive value of 100%. A total of 35 isolates had no zone of inhibition around the oxacillin disk. Of these isolates, 18 were penicillin resistant and the remainder were penicillin intermediate. The positive predictive value of no oxacillin zone for a penicillin-resistant pneumococcus was 51%, with a negative predictive value of 100%.
Predictive factors.
Between 1993 and 1995, 8% of the pneumococci (2 of 24) were penicillin nonsusceptible (MIC, ≥0.1 μg/ml) compared with 55% of strains (22 of 40) isolated between 1996 and 1998 and 56% (20 of 36) isolated between 1999 and 2002 (P < 0.001). The percentage of pneumococci resistant to penicillin (MIC, ≥2.0 μg/ml) increased from 0% (0 of 24) to 10% (8 of 40) and then 28% (10 of 36) (P = 0.008). The proportion of penicillin nonsusceptible isolates (MIC, ≥0.1 μg/ml) was 78% (28 of 36) in children younger than 15 years compared with 25% (16 of 64) in persons ≥15 years (relative risk, 3.1, 95% confidence interval, 2.0 to 4.9) (P < 0.0001).
Between December 1996 and July 2001, S. pneumoniae was isolated from the CSF of 36 adults recruited to a double-blind randomized placebo-controlled trial of dexamethasone in the treatment of bacterial meningitis. All patients in this trial were treated with ceftriaxone. A comparison of clinical features associated with the isolation of a penicillin-nonsusceptible pneumococcus among these patients is given in Table 1. The isolation of a penicillin-nonsusceptible pneumococcus was associated with a history of being referred from another hospital rather than being admitted directly from home, with having been managed for a longer period in a previous hospital, with having received antibiotic therapy before admission, and with a lower white cell count in CSF at admission. By logistic regression, referral from another hospital rather than admission from home (P = 0.005) and prior antibiotic therapy (P = 0.025) were independently predictive of the isolation of a penicillin-nonsusceptible pneumococcus. Among patients admitted to the Centre for Tropical Diseases, 7% of those admitted directly from home (mostly from Ho Chi Minh City) (1 of 15) had a penicillin-nonsusceptible pneumococcus infection compared with 52% of those referred from another hospital (11 of 21) (P = 0.01). The proportion of patients with a penicillin- nonsusceptible pneumococcus infection, was 36% (4 of 11) for those transferred from a hospital outside Ho Chi Minh City compared with 70% (7 of 10) for those transferred from a hospital in Ho Chi Minh City (P = 0.1).
TABLE 1.
Comparison of the admission clinical features of adults with bacterial meningitis due to a penicillin-susceptible or nonsusceptible S. pneumoniae
| Feature | Penicillin-susceptible S. pneumoniae (n = 24) | Penicillin-nonsusceptible S. pneumoniae (n = 12) | P |
|---|---|---|---|
| Age [med (IQR, range)]a | 37.5 (28-48, 15-86) | 33.5 (19-56, 17-69) | 0.63 |
| Sex (male) [no. (%)] | 21 (88) | 10 (83) | 1.0 |
| Duration of symptoms (days) [med (IQR, range)] | 2.5 (2-4, 1-6) | 3 (2-5, 2-11) | 0.12 |
| Admitted from another hospital [no. (%)] | 10 (42) | 11 (92) | 0.005 |
| Duration in previous hospital (days) [med (IQR, range)] | 0 (0-1, 0-3) | 1 (1-3, 0-8) | 0.004 |
| Fever [no. (%)] | 23 (96) | 12 (100) | 1.0 |
| Headache [no. (%)] | 22 (92) | 11 (92) | 1.0 |
| Vomiting [no. (%)] | 16 (67) | 9 (75) | 0.7 |
| Confused [no. (%)] | 16 (67) | 8 (67) | 1.0 |
| Unconscious [no. (%)] | 15 (63) | 4 (33) | 0.1 |
| Previous antibiotic treatment [no. (%)] | 4 (17) | 7 (58) | 0.02 |
| CSF WCCb (/mm3) [med (IQR, range)] | 4,700 (1,760-9,665, 265-24,250) | 1660 (144-4,885, 16-9,100) | 0.03 |
| CSF % neutrophils [med (IQR, range)] | 94 (90-96, 58-99) | 86 (80-96, 50-99) | 0.18 |
| CSF/blood glucose ratio [med (IQR, range)] | 0.13 (0.08-0.21, 0.03-0.44) | 0.14 (0.07-0.25, 0.02-0.48) | 0.83 |
| CSF protein (mg%) [med (IQR, range)] | 479 (405-768, 98-1,450) | 423 (231-508, 186-850) | 0.24 |
Median, interquartile range, range.
WCC, white cell count.
In vitro susceptibility.
MIC determinations were performed on 80 isolates (Table 2). Isolates used for the MIC determinations were more likely to be penicillin nonsusceptible than were those not tested (40 of 80 [50%] and 4 of 20 [20%], respectively; P = 0.03). The MIC results are given in Table 2. A total of 35 isolates (45%) were multidrug resistant (resistant to three or more antimicrobial groups). Table 3 shows the association of penicillin resistance with resistance to other antimicrobial agents.
TABLE 2.
Susceptibilities and MIC results of 80 of the isolated S. pneumoniae organisms
| Antibiotic | MIC50 (μg/ml) | MIC90 (μg/ml) | MIC range (μg/ml) | No. (%) of strains with indicated resulta:
|
Breakpoint values (μg/ml)
|
||||
|---|---|---|---|---|---|---|---|---|---|
| S | I | R | S | I | R | ||||
| Penicillin G | 0.06 | 2.00 | 0.015-2.0 | 40 (50) | 23 (29) | 17 (21) | ≤0.06 | 0.12-1.0 | ≥2.0 |
| Ceftriaxone | 0.06 | 0.5 | 0.015-2.0 | 73 (91) | 61 (8) | 1 (1) | ≤0.5 | 1.0 | ≥2.0 |
| Cefepime | 0.125 | 1.00 | 0.008-2.0 | 6 (76) | 14 (18) | 5 (6) | ≤0.5 | 1.0 | ≥2.0 |
| Imipenem | 0.03 | 0.25 | 0.003-0.50 | 61 (76) | 19 (24) | 0 | ≤0.12 | 0.25-0.5 | ≥1.0 |
| Chloramphenicol | 4.0 | 16.0 | 0.03-32.0 | 47 (59) | 33 (41) | ≤4 | ≥8 | ||
| Erythromycin | 64.0 | >256 | 0.03->256 | 31 (39) | 2 (2) | 47 (59) | ≤0.25 | 0.5 | ≥1.0 |
| Azithromycin | 128.0 | >256 | 0.06->256 | 26 (33) | 5 (6) | 49 (61) | ≤0.5 | 1.0 | ≥2.0 |
| SXTb | 2.0 | 32.0 | 0.03-128 | 27 (34) | 15 (19) | 38 (47) | ≤0.5 | 1.0-2.0 | ≥4 |
| Tetracycline | 16.0 | 64.0 | 0.06-128.0 | 27 (34) | 2 (2) | 51 (64) | ≤2 | 4 | ≥8 |
| Rifampin | 0.125 | 0.25 | 0.015-0.25 | 80 (100) | ≤1.0 | 2.0 | ≥4.0 | ||
| Vancomycin | 0.03 | 0.75 | 0.03-0.75 | 80 (100) | ≤1.0 | ||||
| Levofloxacin | 1.0 | 1.0 | 0.25-1.0 | 80 (100) | ≤2.0 | 4.0 | ≥8.0 | ||
S, susceptible; I, intermediate; R, resistant.
Trimethoprim-sulfamethoxazole. MIC refers to the trimethoprim component.
TABLE 3.
Resistance of penicillin-susceptible, -intermediate, and -resistant isolates of S. pneumoniae to other antimicrobials
| Antibiotic | No. (%) of isolates with indicated level of penicillin resistance exhibiting resistance to other agents
|
P (χ2 for trend) | ||
|---|---|---|---|---|
| Susceptible (n = 40) | Intermediate (n = 23) | Resistant (n = 17) | ||
| Ceftriaxone | 0 (0) | 0 (0) | 1 (6) | 0.11 |
| Cefepime | 0 (0) | 0 (0) | 5 (29) | 0.001 |
| Imipenem | 0 (0) | 0 (0) | 0 (0) | |
| Chloramphenicol | 6 (15) | 12 (52) | 15 (88) | <0.001 |
| Erythromycin | 7 (18) | 23 (100) | 17 (100) | <0.001 |
| Azithromycin | 9 (23) | 23 (100) | 17 (100) | <0.001 |
| SXTa | 10 (25) | 13 (57) | 15 (88) | <0.001 |
| Tetracycline | 32 (80) | 14 (61) | 5 (29) | <0.001 |
| Rifampicin | 0 (0) | 0 (0) | 0 (0) | |
| Vancomycin | 0 (0) | 0 (0) | 0 (0) | |
| Levofloxacin | 0 (0) | 0 (0) | 0 (0) | |
| Three or more antibiotic groupsb | 3 (8) | 14 (61) | 17 (100) | <0.001 |
Trimethoprim-sulfamethoxazole.
Multidrug resistant.
Serotypes.
Serotyping was performed on 60 of the isolates (Table 4). Serotyped isolates were slightly more likely to be penicillin nonsusceptible than were those not serotyped (29 of 60 [48%] and 15 of 40 (38%), respectively; P = 0.3). Serotype 23F was found in 93% of the penicillin-resistant isolates (13 of 14) 60% of the penicillin-intermediate isolates (9 of 15), and 3% of the penicillin- sensitive isolates (1 of 31) (χ2 for trend, P < 0.001). Of the 13 serotype 23F penicillin- resistant isolates, 12 (92%) were also resistant to chloramphenicol, erythromycin, and trimethoprim- sulfamethoxazole. A total of 38 (63%), 39 (65%), 40 (67%), or 45 (75%) of the 60 isolates had a serotype included in the 7-valent, 9-valent, 11-valent, or 23-valent pneumococcal vaccine, respectively. Of the 29 penicillin-nonsusceptible isolates, 28 had a serotype included in each of these vaccines.
TABLE 4.
Serotypes of the S. pneumoniae isolates
| Serotype | No. of isolates | No. showing penicillin susceptibility
|
No. in patients with age range of:
|
|||
|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistance | <15 yr | ≥15 yr | ||
| 1 | 1 | 1 | 1 | |||
| 3 | 1 | 1 | 1 | |||
| 6A | 1 | 1 | 1 | |||
| 6B | 1 | 1 | 1 | |||
| 7C | 1 | 1 | 1 | |||
| 10A | 2 | 2 | 2 | |||
| 10F | 3 | 3 | 3 | |||
| 11A | 1 | 1 | 1 | |||
| 14 | 8 | 4 | 4 | 5 | 3 | |
| 17F | 1 | 1 | 1 | |||
| 18A | 2 | 2 | 2 | |||
| 18C | 3 | 3 | 3 | |||
| 19F | 3 | 2 | 1 | 2 | 1 | |
| 20 | 3 | 3 | 3 | |||
| 22F | 1 | 1 | 1 | |||
| 23F | 23 | 1 | 9 | 13 | 16 | 7 |
| 24F | 2 | 2 | 2 | |||
| 34 | 1 | 1 | 1 | |||
| 35F | 1 | 1 | 1 | |||
| 39 | 1 | 1 | 1 | |||
| Total | 60 | 31 | 15 | 14 | 27 | 33 |
MLST.
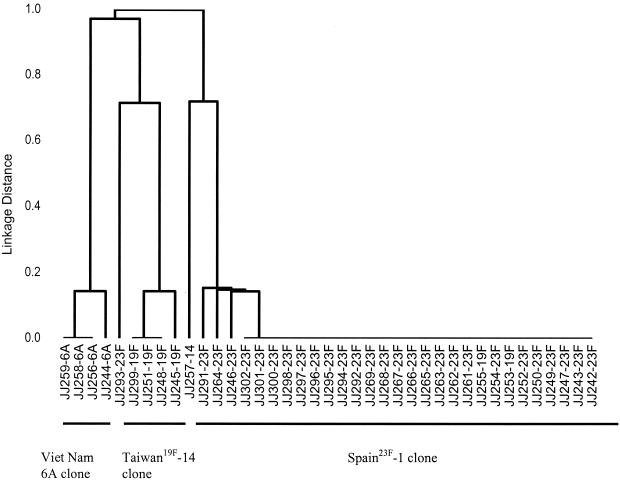
A total of 39 pneumococcal isolates that had a penicillin MIC of ≥1.0 μg/ml and one with penicillin MIC of 0.25 μg/ml were selected for MLST. A total of 15 were from CSF, 7 were from blood, and 17 were from the nasal carriage study (13 urban and 4 rural children) (24). A total of 29 isolates were serotype 23F, 6 were 19F, 3 were 6A, and 1 was 14. The results are in summarized in Fig. 1. There were three main clusters of sequence types. All but one of the invasive isolates tested and many of the carriage isolates were members of the Spain23F-1 clone. These strains were usually also resistant to chloramphenicol and erythromycin, intermediate or resistant to trimethoprim- sulfamethoxazole, but susceptible to tetracycline. Two further isolates, JJ253 and JJ255, were also Spain23F-1, although the serotype was 19F. Three of the carriage isolates and one invasive isolate were members of the previously described Taiwan19F-14 clone (27). Two other clones were seen in the carriage isolates. One cluster with serotype 6A was a clone unique to Vietnam. A single strain with serotype 14 had one match in the MLST database with an isolate from the CSF of a patient admitted to a hospital in Oxford, United Kingdom. One of the serotype 23F resistant strains from a patient with invasive disease was not a member of the Spain23F-1 clone and was not similar to any strain in the MLST database.
FIG. 1.
Dendrogram showing the relatedness of S. pneumoniae isolates from patients with invasive disease and S. pneumoniae isolates from nasal carriage in children. The dendrogram was constructed by the UPGMA method from the matrix of differences in the allelic profile of the 39 isolates. The lines show clusters of closely related sequence types that, with two exceptions (JJ255-19F and JJ253-19F), express the same serotype.
DISCUSSION
This study has shown high levels of resistance to penicillin and other antimicrobial agents in invasive S. pneumoniae strains isolated at a referral hospital in Ho Chi Minh City. There has been a significant increase in the isolation of penicillin-nonsusceptible and resistant isolates from blood and CSF in the last 9 years. In the years 1999 to 2002, more than half of the isolates were penicillin nonsusceptible and one-quarter of the isolates were fully penicillin resistant and multiresistant. These rates are comparable to those obtained in studies in other Asian countries, including South Korea (2, 13, 16, 19, 29, 30, 31, 32), Hong Kong (10, 11, 12, 18), Taiwan (7, 8, 9, 27), Singapore (15, 28, 30), Thailand (30), and Japan (30, 36), but are considerably higher than those obtained in studies in other countries in this region such as China (21, 35), Malaysia (25), and the Philippines (1). In a recent multicenter study of 996 clinical isolates from 11 Asian countries, including 176 isolates from blood or CSF, 18.3% showed intermediate susceptibility and 22.7% were fully resistant (30). In countries with a high prevalence of penicillin-resistant S. pneumoniae (MIC, ≥2.0 μg/ml) isolated from blood or CSF, the rate has varied from 10% in Hong Kong (12) and Singapore (15) to 25% in Korea (16).
The high levels of resistance observed in this study may be biased by the fact that although patients are admitted directly from home, they are also referred from other hospitals. Two-thirds of the 36 adults in the steroid trial had been referred from other hospitals in the city and surrounding provinces. The levels of resistance may be lower in patients with invasive pneumococcal infections who were admitted from home to district hospitals, where it may be that many patients are successfully treated with penicillin. In the steroid study, only 7% of adults admitted directly from home had a penicillin-nonsusceptible pneumococcus compared with 52% of those in adults referred from another hospital. This lower incidence may be more marked in rural areas. Previous work has shown that the levels of penicillin resistance in the nasal pneumococci of rural children are considerably lower than in urban children in Vietnam (24). Furthermore, in this study an adult was more likely to have a penicillin-nonsusceptible pneumococcus if referred from a hospital in Ho Chi Minh City than if referred from a hospital in the surrounding provinces.
It is clear that penicillin can no longer be relied on to cure meningitis in adults and children in Vietnam. Chloramphenicol cannot be recommended as an alternative treatment (6). A course of ceftriaxone is 100 times more expensive than penicillin, and so simple clinical predictors of the likelihood of meningitis being caused by a penicillin-nonsusceptible pneumococcus at the time of admission would be useful in guiding clinical management. A penicillin-nonsusceptible pneumococcus was isolated from more than three-quarters of the children and from one-quarter of the adults. Isolation of a penicillin- nonsusceptible pneumococcus in adults was associated with referral from another hospital, a history of prior treatment, and a lower CSF white cell count at admission. These features are consistent with a group of patients who have received initial treatment in another hospital but are then referred because of a slow response to therapy, and they would provide clues to the need to use an alternative to penicillin for therapy. If simple microbiological facilities are available, an absent zone of inhibition around a 1-μg oxacillin disc had a positive predictive value of 51% and a negative predictive value of 100% for a penicillin-resistant (MIC, ≥2.0 μg/ml) isolate.
Only one isolate, from a child admitted to hospital in 2001, was ceftriaxone resistant (MIC, 2.0 μg/ml). Vancomycin, to which all the isolates were susceptible, in combination with ceftriaxone would be a suitable, albeit extremely expensive treatment option if ceftriaxone-resistant pneumococcal meningitis became common (4, 14).
Although high-dose penicillin is likely to be still appropriate for the treatment of acute respiratory infections, the association of penicillin resistance with erythromycin and trimethoprim-sulfamethoxazole resistance suggests that neither antibiotic is a reliable alternative. All isolates were susceptible to levofloxacin and are likely to be susceptible to other broadspectrum fluoroquinolones. Although these are not yet widely used in Vietnam, there are concerns about their high cost and the possible emergence of resistance to this class of antibiotic, as has been reported from Hong Kong (10).
The majority of the penicillin-resistant and multidrug-resistant isolates were serotype 23F. The results of MLST show that these strains are members of the Spain23F-1 clone. Furthermore, all the penicillin-resistant 23F isolates from the previous nasal carriage study were also Spain23F-1. Serotype 23F has been particularly strongly associated with penicillin resistance in Korea (19, 31, 32), Taiwan (27), and Hong Kong (11) but has also been associated with resistance in Japan, Singapore, and Malaysia (30). The Vietnamese Spain23F-1 pneumococci were all erythromycin resistant but tetracycline susceptible. This is not typical of the clone in Spain (20) but is a common feature of the Spain23F-1 pneumococci in Asia (8, 9, 11, 16, 27). Two of the penicillin-resistant 19F isolates from the nasal carriage study were found to be the Spain23F-1 clone by MLST. It is probable that a serotype switch had occurred, a phenomenon previously described (3). The other 19F invasive isolates that were penicillin resistant were consistent with the Taiwan19F-14 clone previously described in Taiwan (27). The increasing business and tourist links between Vietnam, Korea, and Taiwan may have played a role in the appearance of these clones in Vietnam. Some of the nasal nonsusceptible pneumococci were serotype 6A. The allelic profiles of these strains were not similar to any other in the pneumococcal MLST database. These Vietnamese strains were isolated from children in rural schools and not from children in Ho Chi Minh City, therefore, they may have emerged locally. Finally, a serotype 14 isolate had an MLST type seen otherwise only in the CSF of a patient admitted to a hospital in Oxford. There was no epidemiological connection between the two cases.
This study has shown a significant problem with invasive S. pneumoniae strains that are nonsusceptible to penicillin and other antimicrobials in Ho Chi Minh City, Vietnam. Further surveillance is required to measure the true burden of disease caused by drug-resistant S. pneumoniae in patients admitted to hospitals throughout Vietnam. Pneumococcal vaccines currently available, or under evaluation, would cover the serotypes of up to 75% of the invasive isolates tested and more than 95% of the penicillin-nonsusceptible isolates. Consideration should be given to the role of vaccination in addressing this important issue.
Acknowledgments
We thank the hospital leaders at the Centre for Tropical Diseases for their support, the staff of the microbiology laboratory for isolating the strains, and Nguyen Minh Hoang for his expert technical assistance.
The Wellcome Trust of Great Britain funded this study.
REFERENCES
- 1.Capeding, M. R. Z., L. T. Sombrero, M. G. Lucero, and M. C. Saniel. 1994. Serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates in Filipino children. J. Infect. Dis. 169:479-480. [DOI] [PubMed] [Google Scholar]
- 2.Chong, Y., K. Lee, O. H. Kwon, and J. Henrichsen. 1995. Capsular types and antimicrobial resistance of Streptococcus pneumoniae isolated in Korea. Eur. J. Clin. Microbiol. Infect. Dis. 14:528-531. [DOI] [PubMed] [Google Scholar]
- 3.Coffey, T. J., M. C. Enright, M. Daniels, J. K. Morona, R. Morona, W. Hryniewicz, J. C. Paton, and B. G. Spratt. 1998. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 27:73-83. [DOI] [PubMed] [Google Scholar]
- 4.Doit, C. P., S. P. Bonacorsi, A. J. Fremaux, G. Sissia, R. Cohen, P. L. Geslin, and E. H. Bingen. 1994. In vitro killing activities of antibiotics at clinically achievable concentrations in cerebrospinal fluid against penicillin-resistant Streptococcus pneumoniae isolated from children with meningitis. Antimicrob. Agents Chemother. 38:2655-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 6.Friedland, I. R., and K. P. Klugman. 1992. Failure of chloramphenicol therapy in penicillin-resistant pneumococcal meningitis. Lancet 339:405-408. [DOI] [PubMed] [Google Scholar]
- 7.Fung, C. P., B. S. Hu, S. C. Lee, P. Y. Liu, T. N. Jang, H. S. Leu, B. I. Kuo, M. Y. Yen, C. Y. Liu, Y. C. Liu, Y. J. Lau, and K. W. Yu. 2000. Antimicrobial resistance of Streptococcus pneumoniae isolated in Taiwan: an island-wide surveillance study between 1996 and 1997. J Antimicrob. Chemother. 45:49-55. [DOI] [PubMed] [Google Scholar]
- 8.Hsueh, P.-R., L.-J. Teng, L.-N. Lee, P.-C. Yang, S.-W. Ho, and K.-T. Luh. 1999. Dissemination of high-level penicillin-, extended-spectrum cephalosporin-, and erythromycin-resistant Streptococcus pneumoniae clones in Taiwan. J. Clin. Microbiol. 37:221-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsueh, P.-R., L.-J. Teng, L.-N. Lee, P.-C. Yang, S.-W. Ho, and K.-T. Luh. 1999. Extremely high incidence of macrolide and trimethoprim-sulfamethoxazole resistance among clinical isolates of Streptococcus pneumoniae in Taiwan. J. Clin. Microbiol. 37:897-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho, P. L., T. L. Que, D. N. Tsang, T. K. Ng, K. H. Chow, and W. H. Seto. 1999. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob. Agents Chemother. 43:1310-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ip, M., D. J. Lyon, R. W. H. Yung, C. Chan, and A. F. B. Cheng. 1999. Evidence of clonal dissemination of multidrug-resistant Streptococcus pneumoniae in Hong Kong. J. Clin. Microbiol. 37:2834-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kam, K. M., K. Y. Luey, S. M. Fung, P. P. Yiu, T. J. Harden, and M. M. Cheung. 1995. Emergence of multiple-antibiotic-resistant Streptococcus pneumoniae in Hong Kong. Antimicrob. Agents Chemother. 39:2667-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, S. N., S. W. Kim, I. H. Choi, S. N. Pyo, and D. K. Rhee. 1996. High incidence of multidrug-resistant Streptococcus pneumoniae in South Korea. Microb. Drug Resist. 2:401-406. [DOI] [PubMed] [Google Scholar]
- 14.Klugman, K. P., I. R. Friedland, and J. S. Bradley. 1995. Bactericidal activity against cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with acute bacterial meningitis. Antimicrob. Agents Chemother. 39:1988-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koh, T. H., and R. V. Lin. 1997. Increasing antimicrobial resistance in clinical isolates of Streptococcus pneumoniae. Ann. Acad. Med. Singapore 26:604-608. [PubMed] [Google Scholar]
- 16.Lee, H. J., J. Y. Park, S. H. Jang, J. H. Kim, E. C. Kim, and K. W. Choi. 1995. High incidence of resistance to multiple antimicrobials in clinical isolates of Streptococcus pneumoniae from a university hospital in Korea. Clin. Infect. Dis. 20:826-835. [DOI] [PubMed] [Google Scholar]
- 17.Lee, N. Y., J. H. Song, S. Kim, K. R. Peck, K. M. Ahn, S. I. Lee, Y. Yang, J. Li, A. Chongthaleong, S. Tiengrim, N. Aswapokee, T. Y. Lin, J. L. Wu, C. H. Chiu, M. K. Lalitha, K. Thomas, T. Cherian, J. Perera, T. T. Yee, F. Jamal, U. C. Warsa, P. H. Van, C. C. Carlos, A. M. Shibl, M. R. Jacobs, and P. C. Appelbaum. 2001. Carriage of antibiotic-resistant pneumococci among Asian children: a multinational surveillance by the Asian Network for Surveillance of Resistant Pathogens (ANSORP). Clin. Infect. Dis. 32:1463-1469. [DOI] [PubMed] [Google Scholar]
- 18.Lyon, D. J., O. Scheel, K. S. Fung, A. F. Cheng, and J. Henrichsen. 1996. Rapid emergence of penicillin-resistant pneumococci in Hong Kong. Scand. J. Infect. Dis. 28:375-376. [DOI] [PubMed] [Google Scholar]
- 19.McGee, L., K. P. Klugman, D. Friedland, and H. J. Lee. 1997. Spread of the Spanish multi-resistant serotype 23F clone of Streptococcus pneumoniae to Seoul, Korea. Microb. Drug Resist. 3:253-257. [DOI] [PubMed] [Google Scholar]
- 20.McGee, L., L. McDougal, J. Zhou, B. G. Spratt, F. C. Tenover, R. George, R. Hakenbeck, W. Hryniewicz, J. C. Lefevre, A. Tomasz, and K. P. Klugman. 2001. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J. Clin. Microbiol. 39:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGee L., H. Wang, A. Wasas, R. Huebner, M. Chen, and K. P. Klugman. 2001. Prevalence of serotypes and molecular epidemiology of Streptococcus pneumoniae strains isolated from children in Beijing, China: identification of two novel multiply- resistant clones. Microb. Drug Resist. 7:55-63. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial sensitivity testing. Supplemental tables. M100-S10 (M2), vol 20. NCCLS, Villanova, Pa.
- 23.National Committee for Clinical Laboratory Standards. 1998. Performance standards for antimicrobial sensitivity testing. Supplemental tables. M100-S10 (M7), vol. 20. NCCLS, Villanova, Pa.
- 24.Parry, C. M., T. S. Diep, J. Wain, N. T. T. Hoa, M. Gainsborough, D. Nga, C. Davies, N. H. Phu, T. T. Hien, N. J. White, and J. J. Farrar. 2000. Nasal carriage in Vietnamese children of Streptococcus pneumoniae resistant to multiple antimicrobial agents. Antimicrob. Agents Chemother. 44:484-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohani, M. Y., A. Raudzah, A. J. Ng, P. P. Ng, A. A. Zaidatul, I. Asmah, M. Murtaza, N. Parasakthy, M. Y. Mohd Yasmin, and Y. M. Cheong. 1999. Epidemiology of Streptococcus pneumoniae infection in Malaysia. Epidemiol. Infect. 122:77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruoff, K. L. 1995. Streptococcus, p. 299-307. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. American Society for Microbiology, Washington, D.C.
- 27.Shi, Z. Y., M. C. Enright, P. Wilkinson, D. Griffiths, and B. G. Spratt. 1998. Identification of three major clones of multiple antibiotic-resistant Streptococcus pneumoniae in Taiwanese hospitals by multilocus sequence typing. J. Clin. Microbiol. 36:3514-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soh, S. W., C. L. Poh, and R. V. Lin. 2000. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates from pediatric patients in Singapore. Antimicrob. Agents Chemother. 44:2193-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song, J. H., J. W. Yang, K. R. Peck, S. Kim, N. Y. Lee, M. R. Jacobs, P. C. Applebaum, and C. H. Pai. 1997. Spread of multidrug-resistant Streptococcus pneumoniae in South Korea. Clin. Infect. Dis. 25:747-749. [DOI] [PubMed] [Google Scholar]
- 30.Song, J. H., N. Y. Lee, S. Ichiyama, R. Yoshida, Y. Hirakata, W. Fu, A. Chongthaleong, N. Aswapokee, C. H. Chiu, M. K. Lalitha, K. Thomas, J. Perera, T. T. Yee, F. Jamal, U. C. Warsa, B. X. Vinh, M. R. Jacobs, P. C. Appelbaum, and C. H. Pai. 1999. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study. Clin. Infect. Dis. 28:1206-1211. [DOI] [PubMed] [Google Scholar]
- 31.Song, J. H., J. W. Yang, J. H. Jin, S. W. Kim, C. K. Kim, H. Lee, K. R. Peck, S. Kim, N. Y. Lee, M. R. Jacobs, P. C. Applebaum, and the Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study Group. 2000. Molecular characterization of multidrug-resistant Streptococcus pneumoniae isolates in Korea. J. Clin. Microbiol. 38:1641-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarasi, A., Y. Chong, K. Lee, and A. Tomasz. 1997. Spread of the serotype 23F multidrug-resistant Streptococcus pneumoniae clone to South Korea. Microb. Drug Resist. 3:105-109. [DOI] [PubMed] [Google Scholar]
- 33.Tomasz, A. Antibiotic resistance in Streptococcus pneumoniae. Clin. Infect. Dis. 24(Suppl. 1):S85-S88. [DOI] [PubMed]
- 34.Tram, T. T., L. Q. Thinh, T. T. Nga, N. N. Tuong, F. K. Pederson, and M. Schlumberger. 1998. The etiology of bacterial pneumonia and meningitis in Vietnam. Pediatr. Infect. Dis. J. 17:S192-S194. [DOI] [PubMed] [Google Scholar]
- 35.Wang, H., R. Huebner, M. Chen, and K. Klugman. 1998. Antibiotic susceptibility patterns of Streptococcus pneumoniae in China and comparison of MICs by agar dilution and E-test methods. Antimicrob. Agents Chemother. 42:2633-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida, R., M. Kaku, S. Kohno, K. Ishida, R. Mizukane, H. Takemura, H. Tanaka, T. Usui, K. Tomono, H. Koga, and K. Hara. 1995. Trends in antimicrobial resistance of Streptococcus pneumoniae in Japan. Antimicrob. Agents Chemother. 39:1196-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou, J., M. C. Enright, and B. G. Spratt. 2000. Identification of the major Spanish clones of penicillin-resistant pneumococci via the Internet using multilocus sequence typing. J. Clin. Microbiol. 38:977-986. [DOI] [PMC free article] [PubMed] [Google Scholar]