Abstract
A gene which mediates inducible resistance to macrolides, lincosamides, and streptogramin B antibiotics, designated erm(33), was detected on the Staphylococcus sciuri plasmid pSCFS1. Analysis of the erm(33) reading frame suggested that this gene was the product of a recombination between an erm(C) gene and an erm(A) gene. Such a recombination event is a novel observation for erm genes.
Staphylococcus sciuri, a common inhabitant of the skin of rodents and other mammals, has been reported to carry a number of resistance plasmids, such as the tetracycline resistance plasmid pSTS9 (12), the chloramphenicol resistance plasmid pSCS13 (10), and also the chloramphenicol-streptomycin resistance plasmid pSCS12 (11), which differ in size and/or structure from the resistance plasmids commonly found in staphylococci. More recently, the first and, to date, only known staphylococcal chloramphenicol-florfenicol resistance plasmid was isolated from a bovine S. sciuri isolate (13). Analysis of this plasmid, designated pSCFS1, showed that it also mediated inducible resistance to macrolides, lincosamides, and streptogramin B antibiotics (MLSB antibiotics). Based on the results of PCR analysis, the MLSB resistance gene of plasmid pSCFS1 was considered to be an erm gene of class C (13). Since erm(C) genes are commonly located on small 2.3- to 4.3-kb plasmids (1-3, 16) and have very rarely been detected on larger plasmids, we decided to clone and sequence the erm gene and its adjacent regions of the ca. 17-kb plasmid pSCFS1. This approach should provide information on how the erm(C) gene has become part of plasmid pSCFS1.
To localize the erm gene in plasmid pSCFS1, hybridization studies were conducted with a gene probe that consisted of the 378-bp SacI-BclI fragment of plasmid pSES5 (3). This gene probe comprised the entire erm(C) translational attenuator and the first 219 bp of the erm(C) gene. The smallest hybridizing fragment was an EcoRI-PstI fragment of ca. 2.2 kb. This fragment was cloned into pBluescript II SK(+), and the recombinant plasmid was transformed into Escherichia coli JM107. Sequence analysis on both strands was performed by primer walking, starting with the M13 universal and reverse primers.
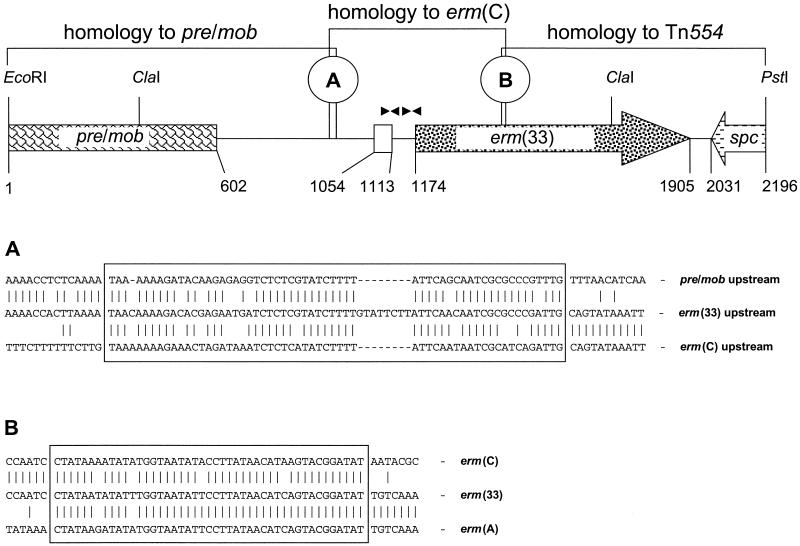
The sequence of this EcoRI-PstI fragment consisted of 2,196 bp. At the EcoRI end, the first 602 bp represented the 5′ end of a reading frame whose product showed similarity to plasmid-borne recombination-mobilization proteins of gram-positive bacteria (Fig. 1). The amino terminal 200 amino acids (aa) of this reading frame were most closely related to the corresponding parts of a recombination-mobilization protein of Listeria monocytogenes (U40997) with 82% amino acid identity, the recombination protein of the Bacillus plasmid pTB913 (X15670; 81% amino acid identity), the mobilization protein of Geobacillus stearothermophilus (M63891; 81% amino acid identity), and the recombination-mobilization protein of Staphylococcus cohnii (AF015628; 79% amino acid identity). Sequence homology to the expected erm(C) region started about 340 bp from the start codon of the aforementioned reading frame. A 66-bp region that showed similarity to the pre-mob upstream region as well as to the erm(C) upstream region indicated the junction of both sequences and might have served as a putative recombination site. This site did not exhibit similarity to the staphylococcal recombination sites RSA (on which Pre acts) or RSB (6, 7) previously found on small erm(C)-carrying plasmids (6, 7). The 8-bp sequence GTATTCTT within this region did not occur in the two partner sequences (Fig. 1). It represented an imperfect tandem duplication which might have arisen during the recombination process.
FIG. 1.
Restriction map and structural organization of the 2.2-kb EcoRI-PstI fragment of plasmid pSCFS1. The 5′ end of a pre-mob-like gene is shown as a box. The white box indicates the small reading frame for the 19-aa regulatory peptide. The black triangles represent the two pairs of inverted repeated sequences detected in the erm(33) translational attenuator. The erm(33) gene and the 3′ end of the spc reading frame are displayed as arrows, with the arrowhead showing the direction of transcription. The sequences of two potential recombination sites A and B (displayed as boxes) and their adjacent sequences are shown in detail below the map.
Further downstream, a small reading frame for a peptide of 19 aa and two pairs of inverted repeated sequences were found to precede a reading frame for a 243-aa protein (Fig. 1). This region corresponded closely to the translational attenuators seen upstream of the inducibly expressed erm(C) genes of plasmids pT48 (1), pE194 (2) or pSES5 (3). The inverted repeats appear to form different mRNA secondary structures in the presence or absence of inducing macrolides, thereby allowing or preventing translation of the erm(C) transcripts (15). The regulatory region of the erm gene of plasmid pSCFS1 differed only by four base pair exchanges and three base pair deletions from the translational attenuators of inducibly expressed erm(C) genes (1-3). All these sequence alterations were located in the noncoding parts of the regulatory region and also did not affect the inverted repeats. The 243-aa protein represented a rRNA methylase that mediates MLSB resistance. A comparison of its amino acid sequence with those of other rRNA methylase proteins revealed highest overall identity of 79% to Tn554-analogous Erm(A) proteins (A25101, AP003137, AP003363, and AB037671) (Table 1), but only 71% amino acid identity to the Erm(A) from Streptococcus pyogenes (AF002716), formerly known as Erm(TR) (14). Identity to the Erm(C) proteins found on small plasmids from Bacillus and Staphylococcus varied between 73% [Erm(C) of the Staphylococcus hominis plasmid pSES5 (Y09001)] and 76% [Erm(C) of the Staphylococcus hyicus plasmid pSES21 (Y09003)] (Table 1). Identity of the Erm protein of plasmid pSCFS1 to Erm(T) of the Lactobacillus reuteri plasmid pGT633 (M64090) was only 67% and was even lower at 65% to Erm(Y) of Staphylococcus aureus (AB014481) and Erm(G) of Bacillus sphaericus (M15332). Since this Erm protein exhibited ≤79% amino acid identity to the next most closely related Erm proteins (8), it received the designation Erm(33) from the Nomenclature Center for MLS Genes (http://faculty.washington.edu/marilynr/; M. Roberts, personal communication). Further analysis of the erm(33) gene showed that the first 284 bp at the 5′ end of the reading frame were almost identical to those of erm(C) genes (12 base pair exchanges that caused five amino acid alterations), while the 403 bp at the 3′ end of erm(33) were indistinguishable from those of erm(A) from Tn554 (Table 1). At the junction of erm(C)-homologous and erm(A)-homologous sequences, a stretch of 45 bp was seen which displayed sequence identities of 95.6% to erm(A) and 93.3% to erm(C) (Fig. 1; Table 1). Assuming that erm(33) resulted from a recombination between an erm(C) and an erm(A) gene, it is most probable that this 45-bp sequence had served as the site for the recombination. The knowledge of the entire erm(33) sequence also explains the initial misidentification of the gene as an erm(C) gene (13) since the PCR primers and the erm(C) gene probe used (3) bound exclusively in the erm(C)-homologous part of erm(33).
TABLE 1.
Comparison of different parts of the erm(33) gene and Erm(33) protein sequences with the respective parts of the most closely related erm genes and Erm proteins
| Sequence | % Identity
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
erm(33) gene
|
Erm(33) protein
|
|||||||
| 5′ end (284 bp) | Recombination area (45 bp) | 3′ end (403 bp) | Entire gene (732 bp) | N terminus (95 aa) | Recombination area (15 aa) | C terminus (133 aa) | Entire protein (243 aa) | |
| erm(A) Tn554 | 59.5 | 95.6 | 100.0 | 84.0 | 50.5 | 86.7 | 100.0 | 79.4 |
| erm(C) pSES21 | 99.3 | 88.9 | 58.9 | 76.5 | 97.9 | 86.7 | 59.4 | 76.1 |
| erm(C) pE194 | 95.4 | 91.1 | 60.4 | 76.0 | 94.7 | 86.7 | 58.3 | 74.5 |
| erm(C) pSES5 | 94.7 | 93.3 | 59.6 | 75.4 | 93.3 | 93.3 | 57.9 | 73.7 |
The 125 bp immediately downstream of erm(33) corresponded exactly to the noncoding sequence downstream of erm(A) in Tn554 (5). Furthermore, the final 166 bp of the EcoRI-PstI fragment represented the 3′ end of the spc gene of Tn554 coding for a spectinomycin adenyltransferase (5). The observation that additional Tn554-homologous sequences were detected in the sequenced part downstream of erm(33) confirmed the involvement of a Tn554-associated erm(A) gene (4) in the development of erm(33).
To the best of our knowledge, this is the first report of a natural recombination between two known erm genes resulting in the formation of a functionally active new erm gene. Both genes erm(A) and erm(C) which show ca. 62% identity in their nucleotide sequences and 58% identity in their deduced amino acid sequences are widespread among human and animal staphylococci and occasionally have been detected in the same isolates (9). Under such conditions, a recombination might have occurred by chance. Since Erm(33) did not differ in its substrate spectrum, nor in the MICs as determined for inducibly expressed Erm(A) or Erm(C) proteins (9), there is no biological need for the bacteria to develop a recombination product such as Erm(33). However, in PCR-directed studies on the occurrence of different erm genes in staphylococci, misidentification of erm(33) or failure to detect this gene by using previously described PCR assays must be taken into consideration. Therefore, a suitable primer system that specifically recognizes this gene should be used for the correct identification of erm(33).
Nucleotide sequence accession number.
The sequence of a the erm(33) gene has been deposited with the EMBL database under accession number AJ313523.
Acknowledgments
This study was supported by grants of the Deutsche Forschungsgemeinschaft (SCHW 382/6-1, SCHW 382/6-2). K. K. Ojo was financially supported by the Gesellschaft der Freunde der Kleintierforschung (GdFuF).
We thank Keith G. H. Dyke for helpful discussions.
REFERENCES
- 1.Catchpole, I., C. Thomas, A. Davies, and K. G. H. Dyke. 1988. The nucleotide sequence of Staphylococcus aureus plasmid pT48 conferring inducible macrolide-lincosamide-streptogramin B resistance and comparison with similar plasmids expressing constitutive resistance. J. Gen. Microbiol. 134:697-709. [DOI] [PubMed] [Google Scholar]
- 2.Horinouchi, S., and B. Weisblum. 1982. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide and streptogramin type B antibiotics. J. Bacteriol. 150:804-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodder, G., C. Werckenthin, S. Schwarz, and K. Dyke. 1997. Molecular analysis of naturally occurring ermC-encoding plasmids in staphylococci isolated from animals with and without previous contact with macrolide/lincosamide antibiotics. FEMS Immunol. Med. Microbiol. 18:7-15. [DOI] [PubMed] [Google Scholar]
- 4.Murphy, E. 1985. Nucleotide sequence of ermA, a macrolide-lincosamide-streptogramin B determinant in Staphylococcus aureus. J. Bacteriol. 162:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy, E., L. Huwyler, and M. C. F. Bastos. 1985. Transposon Tn554: complete nucleotide sequence and isolation of transposition-defective and antibiotic-sensitive mutants. EMBO J. 4:3357-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novick, R. P. 1989. Staphylococcal plasmids and their replication. Annu. Rev. Microbiol. 43:537-565. [DOI] [PubMed] [Google Scholar]
- 7.Novick, R. P., S. J. Projan, W. Rosenblum, and I. Edelman. 1984. Staphylococcal plasmid cointegrates are formed by host- and phage-mediated general rec systems that act on short regions of homology. Mol. Gen. Genet. 195:374-377. [DOI] [PubMed] [Google Scholar]
- 8.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz, F.-J., J. Petridou, D. Milatovic, J. Verhoef, A. C. Fluit, and S. Schwarz. 2002. In vitro activity of new ketolides against macrolide-susceptible and -resistant Staphylococcus aureus isolates with defined resistance gene status. J. Antimicrob. Chemother. 49:580-582. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz, S., and H. Blobel. 1993. Molecular analysis of chloramphenicol resistance in Staphylococcus sciuri. Mh. Vet.-Med. 48:123-127. [Google Scholar]
- 11.Schwarz, S., and S. Grölz-Krug. 1991. A chloramphenicol-streptomycin resistance plasmid from a clinical strain of Staphylococcus sciuri and its structural relationships to other staphylococcal plasmids. FEMS Microbiol. Lett. 82:319 to 322. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz, S., and W. C. Noble. 1994. Tetracycline resistance genes in staphylococci from the skin of pigs. J. Appl. Bacteriol. 76:320-326. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz, S., C. Werckenthin, and C. Kehrenberg. 2000. Identification of a plasmid-borne chloramphenicol-florfenicol resistance gene in Staphylococcus sciuri. Antimicrob. Agents Chemother. 44:2530-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seppälä, H., M. Skurnik, H. Soini, M. C. Roberts, and P. Huovinen. 1998. A novel erythromycin resistance methylase gene (ermTR) in Streptococcus pyogenes. Antimicrob. Agents Chemother. 42:257-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisblum, B. 1995. Insights into erythromycin action from studies of its activity as inducer of resistance. Antimicrob. Agents Chemother. 39:797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werckenthin, C., S. Schwarz, and H. Westh. 1999. Structural alterations in the translational attenuator of constitutively expressed ermC genes. Antimicrob. Agents Chemother. 43:1681-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]