Abstract
Most manifestations of candidiasis are associated with biofilm formation on biological or inanimate surfaces. Candida albicans biofilms are recalcitrant to treatment with conventional antifungal therapies. Here we report on the activity of caspofungin, a new semisynthetic echinocandin, against C. albicans biofilms. Caspofungin displayed potent in vitro activity against sessile C. albicans cells within biofilms, with MICs at which 50% of the sessile cells were inhibited well within the drug's therapeutic range. Scanning electron microscopy and confocal scanning laser microscopy were used to visualize the effects of caspofungin on preformed C. albicans biofilms, and the results indicated that caspofungin affected the cellular morphology and the metabolic status of cells within the biofilms. The coating of biomaterials with caspofungin had an inhibitory effect on subsequent biofilm development by C. albicans. Together these findings indicate that caspofungin displays potent activity against C. albicans biofilms in vitro and merits further investigation for the treatment of biofilm-associated infections.
Biofilms are structured microbial communities with a complex three-dimensional architecture characterized by a network of adherent cells connected by water channels and encapsulated within an extracellular matrix. Biofilms of Candida species play a growing role in human medicine. Indeed, the majority of manifestations of candidiasis at both mucosal and systemic sites are associated in one way or another with the formation of biofilms on inert or biological surfaces (11, 14, 18). More than their planktonic (free-living) counterparts, cells grown in biofilms can be very recalcitrant to antimicrobial treatment. Our group and others have demonstrated the intrinsic resistance of Candida albicans biofilms to the most commonly used antifungal agents, fluconazole and amphotericin (5, 13, 23, 27, 35-38).
Caspofungin (formerly reported as MK-0991 and L-743,872) is a fungicidal, water-soluble semisynthetic echinocandin that inhibits synthesis of 1,3-β-d-glucan, a main structural component of the fungal cell wall. Caspofungin has proven to be very effective against different clinically important fungi, especially Candida spp. and Aspergillus spp. (1-3, 9, 15, 30, 31), and it is also active against those Candida isolates displaying high levels of fluconazole resistance (4, 8, 28, 41). Caspofungin also displays favorable pharmacodynamic and pharmacokinetic characteristics and has an excellent toxicological profile (39; J. A. Stone, J. B. McCrea, P. J. Wickersham, S. D. Holland, P. J. Deutsch, S. Bi, T. Cicero, H. Greenberg, and S. A. Waldman, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 854, 2000). Because the fungal cell wall plays critical structural and adhesive roles that are deemed to be important in biofilm formation and development, we hypothesized that this new agent targeting a key step in the fungal cell wall biosynthesis could be effective against C. albicans biofilms.
MATERIALS AND METHODS
Organisms and culture conditions.
C. albicans 3153A, a well-characterized strain derived from a clinical isolate, was used throughout this study. It was stored on Sabouraud dextrose slopes (BBL, Cockeysville, Md.) at −70°C. C. albicans 3153A was propagated in yeast extract-peptone-dextrose medium (1% [wt/vol] yeast extract, 2% [wt/vol] peptone, 2% [wt/vol] dextrose; U.S. Biological, Swampscott, Mass.). Batches of medium (20 ml) were inoculated with material from yeast extract-peptone-dextrose agar plates containing freshly grown C. albicans cells and incubated overnight in an orbital shaker at 30°C. C. albicans 3153A cells grew in the budding yeast phase under these conditions. Cells were harvested and washed in sterile phosphate-buffered saline (PBS) (10 mM phosphate buffer, 2.7 mM potassium chloride, 137 mM sodium chloride, pH 7.4; Sigma, St. Louis, Mo.). Cells were resuspended in RPMI 1640 supplemented with l-glutamine and buffered with morpholinepropanesulfonic acid (MOPS; Angus Buffers and Chemicals, Niagara Falls, N.Y.), and the suspension was adjusted to the desired cellular density by using a hemacytometer to count cells (see below).
Biofilm formation and antifungal susceptibility testing of C. albicans biofilms.
Biofilms were formed in the wells of microtiter plates as previously described (36). Cells were resuspended in buffered RPMI 1640 (see above) to a cellular density equivalent to 1.0 × 106 cells/ml. All experiments were performed on commercially available presterilized, polystyrene, flat-bottomed, 96-well microtiter plates (Corning Incorporated, Corning, N.Y.). Biofilms were formed by pipetting 100 μl of the standardized cell suspensions into selected wells of the microtiter plate and incubating the plate for 24 h at 37°C. After biofilm formation, the medium was discarded and nonadherent cells were removed by thoroughly washing the biofilms three times in sterile PBS. Caspofungin (Merck Research Laboratories, Rahway, N.J.) and fluconazole (Pfizer Inc., Sandwich, United Kingdom) were then added to selected wells at serial doubling dilutions in RPMI 1640. The biofilms were then incubated in the presence of the antifungal agent for 48 h. Untreated biofilms containing RPMI 1640 were included to serve as negative controls for each isolate. Four replicate biofilms were included for each experiment. The antifungal effects were monitored by using a metabolic 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay, previously described by our group and others (22, 24, 35, 36, 40). Briefly, XTT (Sigma) was prepared as a saturated solution at 0.5 mg/ml in Ringer's lactate. This solution was filter sterilized and stored at −70°C. Prior to each assay, an aliquot of stock XTT was thawed, and menadione (10 mM prepared in acetone; Sigma) was added to obtain a final concentration of 1 μM. A 100-μl aliquot of XTT-menadione was then added to each well, and microtiter plates were incubated in the dark for 2 h at 37°C. The colorimetric change (a reflection of the metabolic activity of cells within the biofilm) was measured in a Benchmark microtiter plate reader (Bio-Rad, Hercules, Calif.) at 490 nm. The antifungal effect was measured by comparing the reduction in the mean absorbance of the antifungal-challenged biofilm to that of the unchallenged RPMI 1640 control and expressed as the MIC at which 50% of the sessile C. albicans cells were inhibited (MIC50 for sessile cells). The same microtiter plate method was used to perform additional susceptibility testing of caspofungin and fluconazole against preformed biofilms of a total of 18 C. albicans clinical isolates (4).
SEM.
For examination by scanning electron microscopy (SEM), C. albicans 3153A biofilms were formed on plastic Thermanox coverslip disks (15-mm diameter; Nalge Nunc International) within six-well cell culture plates (Corning) by dispensing standardized cell suspensions (4 ml of a suspension containing 1.0 × 106 cells/ml of RPMI 1640) onto appropriate disks and incubating the plates at 37°C for 24 h. After washings with PBS, caspofungin (0.5 μg/ml of RPMI 1640) was added to preformed biofilms and the plates were incubated for an additional 48 h at 37°C. Control biofilms were incubated in RPMI 1640 only. After incubation, the biofilms were washed with PBS and placed in fixative (4% formaldehyde [vol/vol] and 1% glutaraldehyde [vol/vol] in PBS) overnight. The samples were rinsed in 0.1 M phosphate buffer (two times for 3 min each time) and then placed in 1% Zetterquist's osmium for 30 min. The samples were subsequently dehydrated in a series of ethanol washes (70% ethanol for 10 min, 95% ethanol for 10 min, 100% ethanol for 20 min) and then treated (two times for 5 min each time) with hexamethyldisilazane (Polysciences, Inc., Warrington, Pa.) and finally air dried in a desiccator. The specimens were coated with 40% gold-60% palladium. After the samples were processed, they were observed in a scanning electron microscope (Leo 435 VP) in the high-vacuum mode at 15 kV. The images were processed for display by using Photoshop software (Adobe Systems Inc., Mountain View, Calif.).
CLSM.
C. albicans 3153A biofilms were formed as described above for SEM experiments. After treatment with caspofungin (0.5 μg/ml of RPMI 1640) or RPMI 1640 alone (control) for an additional 48 h, the biofilms were washed with PBS and stained with the FUN 1 component of the LIVE/DEAD yeast viability kit (Molecular Probes, Eugene, Oreg.) according to the manufacturer's instructions. Both live and dead cells are labeled with this dye, which results in a diffusely distributed green fluorescence; however, metabolically active cells process this dye, which results in a shift from green to red fluorescence. Stained biofilms were observed with an Olympus FV-500 confocal laser scanning microscope (CLSM) by using a rhodamine-fluorescein isothiocyanate protocol, with excitation at 488 nm (argon ion laser) and emission bands at 505 to 525 nm (for green) and >560 nm (for red). Images of sections in the xy plane were taken along the z axis and acquired by the resident software. Images were processed for display by using the Adobe PhotoShop program.
Effect of coating the wells of a microtiter plate with caspofungin on C. albicans biofilm formation.
A modified assay was used in which the wells of a microtiter plate were directly precoated with caspofungin in order to investigate the drug's ability to prevent biofilm formation. Briefly, 200-μl volumes of caspofungin at different concentrations in sterile PBS were added to selected wells of a microtiter plate and incubated overnight at 4°C. After incubation, excess caspofungin was aspirated and the plates were washed once in sterile PBS. C. albicans 3153A cells were washed in PBS and resuspended at a concentration of 106 cells per ml in RPMI 1640. The 96-well microtiter plates were then seeded with the suspension (100 μl per well) and incubated for 24 h at 37°C to allow biofilm formation. The contents of the wells were aspirated and washed three times in sterile PBS, and the extent of biofilm formation was assessed by the XTT reduction assay and by light microscopy. The inhibitory effect of caspofungin was expressed as the percentage of the optical density (OD) of caspofungin-treated wells compared to that of control (plastic) wells for the XTT assays. Statistical analysis was performed with Student's t test. P values of <0.05 were considered statistically significant. The analyses were performed by using Prism version 3.00 for Windows (GraphPad Software, San Diego, Calif.).
RESULTS
In vitro activity of caspofungin against preformed C. albicans biofilms.
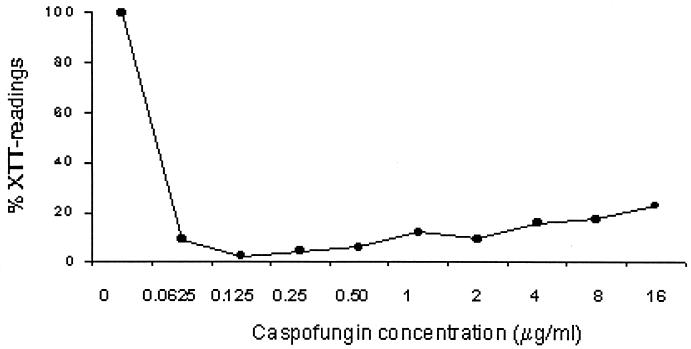
A sharp reduction of the metabolic activity of cells within the biofilm as assessed by the XTT reduction assay was demonstrated when preformed C. albicans 3153A biofilms were exposed to caspofungin (Fig. 1). By this method, the 48-h MIC50 of caspofungin for sessile C. albicans 3153A cells within biofilms was 0.0625 μg/ml. In comparison, fluconazole had little effect and displayed a MIC of ≥64 μg/ml for sessile cells. Although complete sterility of biofilms was not achieved by treatment with caspofungin, the experiments showed a >97% reduction in the metabolic activity of sessile cells with caspofungin concentrations as low as 0.125 μg/ml. Overall, these results indicated that caspofungin was highly efficacious against preformed C. albicans biofilms at concentrations similar to the MICs determined by NCCLS methodologies for planktonic cells (because caspofungin is fungicidal, the endpoint for MIC determinations is the absolute lack of growth, complete sterility). Caspofungin was also active against biofilms formed by all the C. albicans clinical isolates tested (n = 18), with MIC50s for sessile cells ranging between 0.0625 and 0.125 μg/ml, compared to fluconazole MIC50s for sessile cells of ≥64 μg/ml for all isolates. Interestingly, caspofungin showed less efficacy in killing biofilms at higher, nonphysiological concentrations of the drug (8 to 16 μg/ml).
FIG. 1.
Activities of caspofungin at different concentrations against biofilms of C. albicans strain 3153A. Values are expressed as the average percentage of OD of wells containing treated biofilms compared to that of control wells (considered to be 100%) for the XTT assays. Results are from a single experiment performed with four replicate wells.
Direct visualization of the effect of caspofungin on preformed C. albicans biofilms.
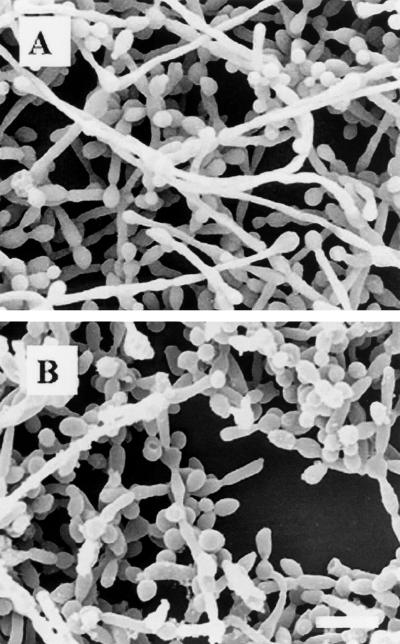
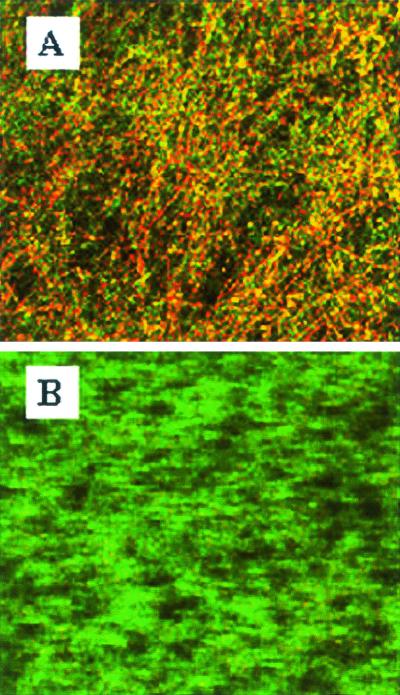
C. albicans 3153A biofilms that formed on the surface of plastic coverslips were treated with caspofungin (0.5 μg/ml) for 24 h at 37°C. SEM revealed that complete eradication of the biofilms was not achieved by treatment with caspofungin at this concentration, and fungal cells adhering to the biomaterial were still visible (Fig. 2). However, compared to control (untreated) biofilms, caspofungin-treated biofilms were less hyphal and some of the cells within the biofilms presented aberrant morphologies. The nondestructive nature of CLSM, together with vital staining with FUN 1, allowed in situ visualization of the effects of caspofungin on C. albicans biofilms (Fig. 3). The shift from green to red fluorescence seen with untreated biofilms reflected the processing of the dye by metabolically active cells within the biofilms (Fig. 3A). In contrast, in agreement with the XTT assays, only residual metabolic activity was detected in cells within the caspofungin-treated biofilms, which showed a diffuse green fluorescence pattern characteristic of dead cells (Fig. 3B). In confirmation of the SEM results, CLSM demonstrated that caspofungin treatment resulted in biofilms that were less hyphal and also showed minor distortions of the overall biofilm architecture.
FIG. 2.
Electron micrographs of preformed C. albicans 3153A biofilms on plastic coverslips after incubation for 24 h with medium alone (A) or with 0.5 μg of caspofungin per ml (B). Bar, 10 μm.
FIG. 3.
CLSM of C. albicans 3153A biofilms on plastic coverslips after incubation for 24 h with medium alone (A) or with 0.5 μg of caspofungin per ml (B). Experiments utilize the FUN 1 stain to directly visualize the effects of caspofungin on preformed biofilms. Note the shift from green to red fluorescence visible in panel A (untreated control biofilms), which reflects processing of the dye by metabolically active cells. In contrast, caspofungin-treated biofilms (B) show diffuse green fluorescence characteristic of dead cells. Images are single xy optical sections taken across the z axis. Magnification, ×200.
Ability of caspofungin to prevent C. albicans biofilm formation.
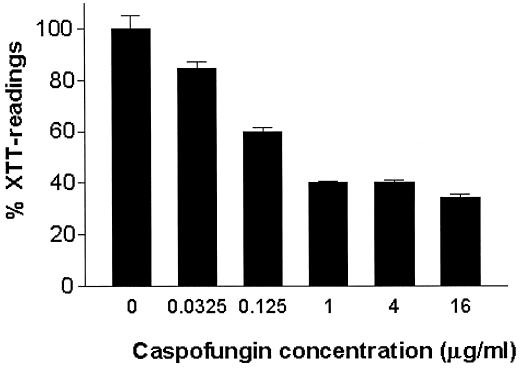
An attractive alternative to the control of biofilm formation is to prevent colonization by coating biomaterials with antifungal agents. Thus, different concentrations of caspofungin were used to directly coat the wells of a microtiter plate, and the effect on subsequent biofilm formation was assessed with the XTT reduction assay. As shown in Fig. 4, coating with caspofungin resulted in significant (up to 60%) reduction of the metabolic activity of adherent cells compared to that of cells in untreated (control) wells.
FIG. 4.
Effect of caspofungin impregnation on subsequent biofilm formation by C. albicans 3153A on the wells of microtiter plates. Values are expressed as the average percentage of OD (seven replicate wells) compared to that of control wells (considered to be 100%) for the XTT assays. Pretreatment at all caspofungin concentrations resulted in statistically significant differences in biofilm formation compared to that of control biofilms (P is 0.0379 for 0.03125 μg/ml and P is <0.001 for all other concentrations).
DISCUSSION
The epidemiology of Candida infections is dichotomous: on one side, a previously frequently encountered clinical entity, namely oropharyngeal and esophageal candidiasis affecting human immunodeficiency virus-infected patients, is becoming less common. This fact is mainly explained by the introduction of potent, immunoreconstitutional antiretroviral therapy (20, 21). In contrast, nosocomial infections with any Candida species are becoming increasingly important. In fact, surveys of the past decades list Candida spp. as the third or fourth most common bloodstream pathogens in U.S. hospitals; they surpass gram-negative rods in incidence (7, 10). One reason for the growing frequency is the increasing use of immunosuppressive therapy in cancer and transplant patients, which leads to breakdowns of the barrier between the bowel and bloodstream (29). Other sources of Candida infections include central venous catheters, voice prostheses, intrauterine devices, and prosthetic joints, to name only a few (16). Yeasts, mainly C. albicans, are the third leading cause of catheter-related infections, with the second highest colonization-to-infection rate and the highest crude mortality (14). Whereas the rate of successful treatment of oropharyngeal and esophageal candidiasis remains high, foreign-body infections with Candida spp. can be a therapeutic challenge. Fortunately, simple removal of a catheter in addition to antifungal therapy helps resolve the infection in many cases. But for some patients, insertion of a new catheter can be difficult or even relatively contraindicated. Also, the removal of an infected medical device, e.g., a prosthetic joint, is almost always associated with a remarkable morbidity, and replacement is very difficult (32).
Morbidity and mortality rates associated with candidiasis remain unacceptably high. One of the main reasons for this is the limited armamentarium of antifungal drugs. Unfortunately, there are substantially fewer antifungal than antibacterial drugs. Clinically used antifungals are basically restricted to polyenes (e.g., amphotericin B) and azoles (e.g., fluconazole). Both types of antifungal agents directly or indirectly target ergosterol, which is the major sterol in the fungal membrane. Another major reason for the low success rate in treating these infections is that a majority of them are caused by biofilms. Sessile cells within a biofilm exhibit drastic phenotypic differences compared to their planktonic counterparts. Our group and other authors have shown that C. albicans cells in biofilm conditions display dramatically increased resistance to antifungal agents (including fluconazole and amphotericin B) compared to that of cells in planktonic conditions (6, 13, 22, 27, 36-38). Again, in this study, these previous observations are confirmed. Biofilms formed by C. albicans strain 3153A and 18 clinical isolates displayed intrinsic resistance to fluconazole (the MIC was ≥64 μg/ml for sessile cells).
New antifungal agents in the development pipeline may prove superior in the treatment of biofilm-associated infections. Caspofungin was recently introduced to the market as the first compound in the class of echinocandins. Caspofungin has proven highly active against different clinically important fungi (1-3, 9, 15, 30, 31). Importantly, it is active against Candida isolates that are refractory to azole treatment (4, 8, 28, 41). Caspofungin belongs to the echinocandin family of lipopeptide antibiotics, which kill fungi by inhibiting the synthesis of β-1,3-glucan, a major component of the fungal cell wall. From a quantitative point of view, β-glucans are the main constituent of the C. albicans cell wall, accounting for approximately 60% of its weight (12, 26). Moreover, β-1,3-glucan molecules serve as a scaffold for the attachment of other macromolecules in the cell wall (mainly chitin and mannoproteins) and provide strong physical properties to the cell. The importance of the β-1,3-glucan biosynthetic pathway and the relevance of maintaining a functional cell wall to C. albicans are highlighted by two main observations: (i) FKS1, the gene encoding β-1,3-glucan synthase (the main target for caspofungin), is an essential gene (17) and (ii) a salvage pathway that results in cell wall restructuring after cell wall damage or stress is present (26). As the outermost part of the cell, the C. albicans cell wall is the structure that mediates the cell's interactions with the environment and it is where adhesive properties reside (12). Thus, the cell wall may be an attractive target for the development of strategies to combat biofilm-associated infections.
In a first set of experiments analyzing the efficacy of caspofungin against preformed C. albicans biofilms by using our recently described microtiter plate model of biofilm formation (36), this agent displayed potent activity against adherent C. albicans populations. Caspofungin MIC50s for sessile cells were consistently low, between 0.0625 and 0.125 μg/ml, compared to fluconazole MIC50s for sessile cells, which were always ≥64 μg/ml (caspofungin MICs for planktonic cells ranged from 0.25 to 1 μg/ml, and fluconazole MICs for planktonic cells ranged from 0.25 to >64 μg/ml). Although adherent populations were not completely eradicated by treatment with caspofungin, a >97% reduction in the metabolic activity of adherent cells was detected at caspofungin concentrations as low as 0.125 μg/ml (Fig. 1), well within the drug's therapeutic range (39). These results indicated caspofungin's potent activity against preformed C. albicans biofilms at physiological concentrations similar to the MICs reported for most Candida strains (4, 8, 28), with the caveat that, since caspofungin is fungicidal, MICs determined by NCCLS methodology are considered the lowest concentrations at which complete growth inhibition is observed.
Most techniques evaluating the efficacy of antimicrobial agents, including the XTT reduction assay used in our laboratory, use cell viability measurements or correlates. However, direct visualization of biofilms after exposure to the agents can provide valuable information on the effect of antimicrobials. The efficacy of caspofungin on C. albicans biofilms was confirmed by SEM and CLSM. As depicted in Fig. 2 and 3, caspofungin-treated biofilms were less hyphal and showed minor defects in the overall biofilm architecture. Caspofungin treatment did not eradicate preformed biofilms (Fig. 2), but vital staining observed with CLSM demonstrated that the majority of cells within caspofungin-treated biofilms were dead (Fig. 3B), in accordance with XTT colorimetric readings.
Because of the intrinsic recalcitrance of microbial biofilms and the difficulty in eradicating cells once the biofilm has been established, coating of biomaterials with antimicrobials constitutes an alternative therapeutic strategy to prevent biofilm-associated infections (33, 34). This surface modification can inhibit adhesion of fungal cells and also cause direct inhibitory activity through the release of the drug from the biomaterial. This approach may be particularly important in the prophylaxis of biofilm-related infections and has been used with certain success in the management of catheter-related bacteremias (33, 34). Caspofungin and other lipopeptides have been previously described to bind strongly to wells of polystyrene plates without preactivation (25). In our experiments, pretreatment with caspofungin at a range of concentrations had an inhibitory effect on subsequent biofilm formation on the bottoms of wells of microtiter plates (Fig. 4). Although a maximum reduction in metabolic activity to about 40% of that displayed by control biofilms was observed in our experiments using this strategy, direct microscopic examination indicated that only scattered adherent cells, mostly showing enlarged, aberrant morphologies consistent with growth inhibition (19), were present in those wells coated with caspofungin but no typical biofilms with three-dimensional structure were formed. Current experiments in our laboratory are aimed at developing methods for the sustained release of antifungals, which may improve their inhibitory effects on biofilm formation.
In conclusion, our results suggest that therapeutic concentrations of caspofungin display potent in vitro activity against C. albicans biofilms, which have been shown to be otherwise recalcitrant to treatment with fluconazole and amphotericin B, the two most commonly used antifungal agents. These results are in agreement with those presented in a recent report by Kuhn and colleagues, who also demonstrated potent in vitro activity by echinocandins against preformed biofilms of C. albicans (27). The efficacy of caspofungin, together with its pharmacokinetic and toxicological properties, merits further investigation of this new antifungal agent for the therapy of biofilm-associated infections.
Acknowledgments
This work was supported by a grant from Merck & Co., Inc. Additional support was provided by grant ATP 3659-0080 from the Texas Higher Education Coordinating Board (Advance Technology Program, Biomedicine) (to J.L.L.-R. and B.L.W.) and Public Health Service grant 5 R01 DE11381-04A2 from the National Institute of Dental and Craniofacial Research (to T.F.P). J.L.L.-R. is the recipient of a New Investigator Award in molecular pathogenic mycology from the Burroughs Wellcome Fund.
We thank Peggy Miller and Victoria Frohlich for assistance with SEM and CLSM experiments.
REFERENCES
- 1.Abruzzo, G. K., C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, and H. Rosen. 2000. Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamide-induced immunosuppressed mice. Antimicrob. Agents Chemother. 44:2310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2002. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob. Agents Chemother. 46:245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann, S. P., T. F. Patterson, and J. L. López-Ribot. 2002. In vitro activity of caspofungin (MK-0991) against Candida albicans clinical isolates displaying different mechanisms of azole resistance. J. Clin. Microbiol. 40:2228-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baillie, G. S., and L. J. Douglas. 1999. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 310:644-656. [DOI] [PubMed] [Google Scholar]
- 6.Baillie, G. S., and L. J. Douglas. 1998. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 42:1900-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee, S. N., T. G. Emori, D. H. Culver, R. P. Gaynes, W. R. Jarvis, T. Horan, J. R. Edwards, J. Tolson, T. Henderson, W. J. Martone, et al. 1991. Secular trends in nosocomial primary bloodstream infections in the United States, 1980-1989. Am. J. Med. 91:86S-89S. [DOI] [PubMed] [Google Scholar]
- 8.Barchiesi, F., A. M. Schimizzi, A. W. Fothergill, G. Scalise, and M. G. Rinaldi. 1999. In vitro activity of the new echinocandin antifungal, MK-0991, against common and uncommon clinical isolates of Candida species. Eur. J. Clin. Microbiol. Infect. Dis. 18:302-304. [DOI] [PubMed] [Google Scholar]
- 9.Bartizal, K., C. J. Gill, G. K. Abruzzo, A. M. Flattery, L. Kong, P. M. Scott, J. G. Smith, C. E. Leighton, A. Bouffard, J. F. Dropinski, and J. Balkovec. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2326-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck-Sague, C., W. R. Jarvis, et al. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 11.Cannon, R. D., and W. L. Chaffin. 1999. Oral colonization by Candida albicans. Crit. Rev. Oral Biol. Med. 10:359-383. [DOI] [PubMed] [Google Scholar]
- 12.Chaffin, W. L., J. L. Lopez-Ribot, M. Casanova, D. Gozalbo, and J. P. Martinez. 1998. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol. Mol. Biol. Rev. 62:130-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandra, J., P. K. Mukherjee, S. D. Leidich, F. F. Faddoul, L. L. Hoyer, L. J. Douglas, and M. A. Ghannoum. 2001. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 80:903-908. [DOI] [PubMed] [Google Scholar]
- 14.Crump, J. A., and P. J. Collignon. 2000. Intravascular catheter-associated infections. Eur. J. Clin. Microbiol. Infect. Dis. 19:1-8. [DOI] [PubMed] [Google Scholar]
- 15.Del Poeta, M., W. A. Schell, and J. R. Perfect. 1997. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob. Agents Chemother. 41:1835-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donlan, R. M. 2001. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 33:1387-1392. [DOI] [PubMed] [Google Scholar]
- 17.Douglas, C. M., J. A. D'Ippolito, G. J. Shei, M. Meinz, J. Onishi, J. A. Marrinan, W. Li, G. K. Abruzzo, A. Flattery, K. Bartizal, A. Mitchell, and M. B. Kurtz. 1997. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41:2471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ell, S. R. 1996. Candida ‘the cancer of silastic'. J. Laryngol. Otol. 110:240-242. [PubMed] [Google Scholar]
- 19.Ernst, E. J., M. E. Klepser, M. E. Ernst, S. A. Messer, and M. A. Pfaller. 1999. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn. Microbiol. Infect. Dis. 33:75-80. [DOI] [PubMed] [Google Scholar]
- 20.Greenspan, D., A. J. Canchola, L. A. MacPhail, B. Cheikh, and J. S. Greenspan. 2001. Effect of highly active antiretroviral therapy on frequency of oral warts. Lancet 357:1411-1412. [DOI] [PubMed] [Google Scholar]
- 21.Haddad, N. E., and W. G. Powderly. 2001. The changing face of mycoses in patients with HIV/AIDS. AIDS Read. 11:365-368, 375-378. [PubMed] [Google Scholar]
- 22.Hawser, S. 1996. Comparisons of the susceptibilities of planktonic and adherent Candida albicans to antifungal agents: a modified XTT tetrazolium assay using synchronised C. albicans cells. J. Med. Vet. Mycol. 34:149-152. [PubMed] [Google Scholar]
- 23.Hawser, S. P., and L. J. Douglas. 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 39:2128-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawser, S. P., H. Norris, C. J. Jessup, and M. A. Ghannoum. 1998. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J. Clin. Microbiol. 36:1450-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karkhanis, Y. D., and D. M. Schmatz. 1998. Novel enzyme-linked immunoassay to determine nanogram levels of pneumocandins in human plasma. J. Clin. Microbiol. 36:1414-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klis, F. M., P. de Groot, and K. Hellingwerf. 2001. Molecular organization of the cell wall of Candida albicans. Med. Mycol. 39(Suppl. 1):1-8. [PubMed] [Google Scholar]
- 27.Kuhn, D. M., T. George, J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marco, F., M. A. Pfaller, S. A. Messer, and R. N. Jones. 1998. Activity of MK-0991 (L-743,872), a new echinocandin, compared with those of LY303366 and four other antifungal agents tested against blood stream isolates of Candida spp. Diagn. Microbiol. Infect. Dis. 32:33-37. [DOI] [PubMed] [Google Scholar]
- 29.Nucci, M., and E. Anaissie. 2001. Revisiting the source of candidemia: skin or gut? Clin. Infect. Dis. 33:1959-1967. [DOI] [PubMed] [Google Scholar]
- 30.Petraitiene, R., V. Petraitis, A. H. Groll, T. Sein, R. L. Schaufele, A. Francesconi, J. Bacher, N. A. Avila, and T. J. Walsh. 2002. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrob. Agents Chemother. 46:12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., F. Marco, S. A. Messer, and R. N. Jones. 1998. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn. Microbiol. Infect. Dis. 30:251-255. [DOI] [PubMed] [Google Scholar]
- 32.Phelan, D. M., D. R. Osmon, M. R. Keating, and A. D. Hanssen. 2002. Delayed reimplantation arthroplasty for candidal prosthetic joint infection: a report of 4 cases and review of the literature. Clin. Infect. Dis. 34:930-938. [DOI] [PubMed] [Google Scholar]
- 33.Raad, I. 2000. Management of intravascular catheter-related infections. J. Antimicrob. Chemother. 45:267-270. [DOI] [PubMed] [Google Scholar]
- 34.Raad, I., and H. Hanna. 1999. Intravascular catheters impregnated with antimicrobial agents: a milestone in the prevention of bloodstream infections. Support. Care Cancer 7:386-390. [DOI] [PubMed] [Google Scholar]
- 35.Ramage, G., S. Bachmann, T. F. Patterson, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 49:973-980. [DOI] [PubMed] [Google Scholar]
- 36.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. López-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramage, G., K. VandeWalle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Characteristics of biofilm formation by Candida albicans. Rev. Iberoam. Micol. 18:163-170. [PubMed] [Google Scholar]
- 38.Ramage, G., B. L. Wickes, and J. L. Lopez-Ribot. 2001. Biofilms of Candida albicans and their associated resistance to antifungal agents. Am. Clin. Lab. 20:42-44. [PubMed] [Google Scholar]
- 39.Stone, J. A., S. D. Holland, P. J. Wickersham, A. Sterrett, M. Schwartz, C. Bonfiglio, M. Hesney, G. A. Winchell, P. J. Deutsch, H. Greenberg, T. L. Hunt, and S. A. Waldman. 2002. Single- and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob. Agents Chemother. 46:739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tellier, R., M. Krajden, G. A. Grigoriew, and I. Campbell. 1992. Innovative endpoint determination system for antifungal susceptibility testing of yeasts. Antimicrob. Agents Chemother. 36:1619-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vazquez, J. A., M. Lynch, D. Boikov, and J. D. Sobel. 1997. In vitro activity of a new pneumocandin antifungal, L-743,872, against azole-susceptible and -resistant Candida species. Antimicrob. Agents Chemother. 41:1612-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]