Abstract
The increased resistance of the protozoan parasite Trypanosoma cruzi to nitro derivatives is one of the major problems for the successful treatment of Chagas' disease. In the present study, we have tested the effects of 1-O-hexadecylphosphocholine (miltefosine) against strains of T. cruzi that are partially resistant (strain Y) and highly resistant (strain Colombiana) to the drugs in clinical use. As expected, epimastigotes of strain Colombiana showed higher levels of resistance to benznidazole than those of strain Y. However, the level of resistance to miltefosine was the same for both strains. This alkylphospholipid was also extremely toxic against intracellular amastigotes of both strains. This ether-lipid analogue induced in a dose-dependent manner the production of tumor necrosis factor alpha and nitric oxide (NO) radicals by infected and noninfected macrophages, suggesting that miltefosine may activate macrophages in vitro. Nevertheless, the cytotoxic effect of miltefosine against intracellular amastigotes was independent of the amount of NO produced by the infected macrophages since the same dose-response curves for miltefosine were observed when the NO production was blocked by the NO synthase inhibitor NG-monomethyl-l-arginine monoacetate. Preliminary in vivo studies with BALB/c mice infected with strain Y indicated that oral miltefosine promoted survival and reduced the parasitemia to levels comparable to those observed when benznidazole was used. Four months after treatment, no parasites were detected in the blood or spleen tissue sections maintained in culture. Together, these results support the hypothesis that miltefosine may be used for the treatment of Chagas' disease, including cases caused by resistant strains of T. cruzi.
Chagas' disease is an inflammatory condition caused by the intracellular protozoan parasite Trypanosoma cruzi that affects 18 million people in Latin America. It is a major cause of heart disease and represents an important cause of economic loss due to infectious disease (27). Despite research efforts toward the development of novel drugs for the treatment of Chagas' disease, only two substances, the nitro derivatives nifurtimox and benznidazole, are in clinical use. Unfortunately, narrow therapeutic windows, serious side effect profiles, and various drug susceptibilities among T. cruzi strains result in low clinical efficacies for these nitro derivatives (10, 42). The recognized impact on public health should lead to the development of novel trypanocidal agents with favorable side effect profiles and sufficient clinical efficacies.
Miltefosine (1-O-hexadecylphosphocholine) is a membrane-active synthetic ether-lipid analogue originally used for the treatment of cutaneous metastasis from mammary carcinomas (15, 20). This compound is in clinical trials for the treatment of visceral and cutaneous leishmaniasis, with high overall cure rates achieved (2, 11, 17, 30, 31, 40-41). Previous studies have also demonstrated that miltefosine is toxic in vitro to other protozoan parasites including Entamoeba histolytica (38), Acanthamoeba spp. (43), and T. cruzi (5, 21, 36); however, it has a low level of toxicity against Trypanosoma brucei subspecies (18). Little is known about the mechanisms of the antitumor (35) and antiparasite actions of ether-lipid analogues. In the case of parasites, hypotheses about the mechanisms of action include interference with signal transduction pathways and glycosylphosphatidylinositol anchor biosynthesis (22), metabolism of alkylglycero-phosphocholine (23), and de novo synthesis of phosphatidylcholine through the Greenberg pathway (21).
In this study, we report novel data concerning the in vitro and in vivo cytotoxic effects of miltefosine against a partially resistant strain (strain Y) and a naturally resistant strain (strain Colombiana) of T. cruzi (32), expanding the potential antiprotozoal spectrum of this ether-lipid analogue.
MATERIALS AND METHODS
Materials.
Lipopolysaccharide (LPS) from Escherichia coli O111:B4 was purchased from Sigma (St. Louis, Mo.). Recombinant murine gamma interferon (IFN-γ), recombinant murine tumor necrosis factor alpha (TNF-α), and anti-TNF-α monoclonal antibodies were purchased from PharMingen (San Diego, Calif.). NG-Monomethyl-l-arginine monoacetate (l-NMMA) was purchased from Calbiochem-Novabiochem (La Jolla, Calif.). Benznidazole was purchased from Roche (Rio de Janeiro, Brazil). Miltefosine was provided by Zentaris/ASTA Medica AG (Frankfurt am Main, Germany).
Parasites.
T. cruzi strains Y and Colombiana were obtained from the Fundação Oswaldo Cruz culture collection. Epimastigotes were axenically cultured in brain heart infusion (BHI) supplemented with 10 mg of hemin liter−1 and 5% heat-inactivated fetal calf serum (FCS) (BHI-FCS medium) at 28°C with shaking (∼80 rpm), as described previously (34). In vitro differentiation of epimastigotes into metacyclic trypomastigote (MCT) forms was achieved with a chemically defined triatomine artificial urine medium (3). Tissue culture-derived trypomastigotes (TCTs) were obtained after infection of confluent monolayers of Vero cells with MCTs to establish the intracellular cycle for 6 days and maintained in RPMI 1640 medium containing 10% FCS under an atmosphere of 5% CO2 at 37°C (1). MCTs and TCTs were used to infect murine peritoneal macrophages or primary heart muscle cells (HMCs) in vitro, and TCTs were used for inoculation into mice (see below).
Treatment of T. cruzi epimastigote forms in vitro.
Miltefosine and benznidazole were stored as 10- and 20-mg ml−1 stock solutions in methanol, respectively, and were serially diluted (1:2) in BHI-FCS medium before use. Drug-free control medium contained comparable final concentrations of methanol. Epimastigotes (2 × 106 ml−1) were incubated in BHI-FCS medium with or without drugs in a final volume of 200 μl in 96-well flat-bottom plates (Corning, Corning, N.Y.), as described previously (23). After 72 h at 28°C, the number of parasites was determined by direct counting with a Neubauer chamber. The 50% inhibitory concentrations (IC50s) were determined by linear regression analysis (23).
Mφ, HMCs, and infection.
Exudate cells removed from the peritoneal cavities of BALB/c mice were cultured in complete RPMI 1640 medium containing 2 mM l-glutamine, 1 mM sodium pyruvate, 10 μg of gentamicin ml−1, minimal essential medium with nonessential amino acids, 10 mM HEPES, 50 μM 2-mercaptoethanol, and 5% FCS with 3 × 105 cells ml−1 on 24-well plates (Corning). Macrophages (Mφ) were infected with 1.5 × 106 MCT forms of the Y strain per well (12) or 1.5 × 106 TCT forms of the Y and Colombiana strains at a ratio of five parasites per Mφ. After 24 h (MCT) or 2 h (TCT), noninternalized parasites were removed and infected Mφ were cultured in complete medium (1 ml) alone, medium containing LPS (10 ng ml−1) plus IFN-γ (40 U ml−1), or medium containing different doses of methanol or miltefosine at 37°C under 5% CO2 for up to 10 days. Extracellular motile trypomastigotes were counted in the supernatants of cultures after 5, 7, and 10 days of infection. In addition, some cultures received LPS plus IFN-γ or different doses of miltefosine together with 1 mM l-NMMA. To assess the number of intracellular amastigote forms, Mφ were plated onto 13-mm2 coverslips (Thomas Scientific, Swedesboro, N.J.) in 24-well plates and infected as described above. After 3 days of culture the monolayers of infected Mφ (treated or not treated with miltefosine) were washed with phosphate-buffered saline (PBS) at 37°C, fixed in methanol, and stained with Giemsa. The number of amastigotes was determined by counting at least 400 Mφ in duplicate cultures, and the results were expressed as the percentage of infected Mφ and the average number of amastigotes per infected Mφ. Murine embryo primary HMC cultures were obtained as described previously (25), and infections and treatments were performed with MCT forms as described above for Mφ.
NO and TNF-α production assays.
Nitric oxide (NO) levels produced by primary Mφ cultures (assayed in quadruplicate) were estimated by reducing the nitrate accumulated over 48 h to nitrite with nitrate reductase (37) and measuring the nitrite concentration by the method of Green et al. (14). The NO−2 concentrations were quantified by using a double three-point standard curve of NaNO2 concentrations (in a linear range between 1 and 80 μM). The levels of TNF-α in the supernatants of Mφ cultures were quantified with enzyme-linked immunosorbent assay kits from PharMingen. Briefly, each well of microtiter plates (Immuno PlateMaxiSorp Surface; Nunc) was coated overnight at 4°C with 2 μg of purified capture monoclonal antibody to mouse TNF-α (PharMingen) in 100 mM carbonate buffer (pH 9.5). The plates were washed five times with PBS-Tween (PBS-T). Nonspecific binding sites were saturated with 10% FCS in PBS for 1 h and washed with PBS-T. Thereafter, the supernatants and cytokine standards diluted in PBS-FCS were added, and the mixtures were incubated at room temperature for 40 min with biotinylated detection antibody (PharMingen). After the plates were washed, avidin-conjugated horseradish peroxidase was added and the mixture was incubated for 30 min. 3,3′,5,5′-Tetramethylbenzidine-1.2 mM H2O2 in citrate buffer (pH 5.0) was used as a substrate for color development. The reaction was terminated by addition of 1 N HCl. The absorbance was measured at 450 nm (Anthos 2010; Anthos Labtec Instruments, Salzburg, Austria) and cytokine concentrations (in a linear range between 50 and 1,500 pg ml−1) were quantified by using a double four-point standard curve.
Mouse infection and treatments.
Fifteen male BALB/c mice (age, 6 weeks; weight, ∼20 to 22 g) were inoculated intraperitoneally with 105 MCT forms of T. cruzi Y strain, and groups of five mice were either left untreated or immediately treated orally for 20 consecutive days with 100 mg of benznidazole kg of body weight−1 or 25 mg of miltefosine kg−1. The compounds were suspended in PBS, with each mouse receiving 0.1 ml of drug suspension daily by gavage. Untreated controls received only PBS. Blood collected from the tail was examined microscopically for living parasites as described previously (10). After 120 days of treatment, the presence of parasites was assessed by direct blood examination and by culture of spleen slices in RPMI 1640 medium for 15 days at 37°C under a 5% CO2 atmosphere and in BHI-FCS medium for 10 days at 28°C (26). All experiments were conducted according to protocols approved by the Committee on Ethics and Regulations of Animal Use of the Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro.
RESULTS
Toxicities of miltefosine and benznidazole against epimastigotes in culture.
Various sensitivities to nitro derivatives have been observed among several T. cruzi strains isolated from human patients, domestic and sylvatic reservoirs, or vectors in vitro (10). In addition, marked molecular differences have been observed within the most widely studied strains, strains CL, Y, and Colombiana (32). To test the profile of benznidazole sensitivity of the Y strain versus that of the Colombian strain, axenically grown epimastigotes were incubated in the presence of increasing amounts of the drug (Fig. 1). A clear difference in the amount of drug necessary to reduce the viabilities of the parasites of each strain in axenic culture was observed. The IC50 of benznidazole for epimastigotes of the Y strain was determined to be 15 ± 4 μg ml−1 or 3 ± 1 μM and is about four to five times lower than that observed for parasites of the Colombiana strain (Fig. 1), as reported previously (24, 33). Miltefosine had a higher level of cytotoxic activity against Y-strain epimastigotes than benznidazole, with the miltefosine IC50 of 3.5 ± 1 μg ml−1 (8 ± 1 μM) being similar to data reported previously (21, 36). In contrast to benznidazole, there were no differences in susceptibilities to miltefosine between epimastigotes of the Y and Colombiana strains (Fig. 1). The results suggest that the cytotoxicity of miltefosine against T. cruzi may be as effective as that originally observed against different species of Leishmania (4-5, 7, 19, 23).
FIG. 1.
Dose-dependent inhibition of epimastigote viability by miltefosine and benznidazole in axenic culture. Parasites from strains Y and Colombiana were cultivated in 96-well plates in BHI-FCS medium with different concentrations of miltefosine or benznidazole. The viability of each culture was determined after 72 h at 28°C by direct counting of the number of parasites per milliliter, and each experimental point corresponds to the mean ± standard deviation for duplicates from three independent experiments. ▪, controls; •, strain Y cultivated with miltefosine; ▴, strain Colombiana cultivated with miltefosine; ○, strain Y cultivated with benznidazole; ▵, strain Colombiana cultivated with benznidazole.
Effects of miltefosine on the development of amastigotes from strains Y and Colombiana inside BALB/c-derived macrophages.
Four different lyso-alkylphospholipids (including miltefosine) have been reported to exhibit variable activities against intracellular T. cruzi Y-strain amastigotes in mouse peritoneal Mφ (5). To study the effects of miltefosine on amastigotes from strains Y and Colombiana, the IC50s were determined for BALB/c-infected Mφ (Fig. 2). As reported previously (5), dose-dependent decreases in the percentage of Mφ infected with Y-strain amastigotes (IC50 after 3 days, 0.3 ± 0.1 μg ml−1 or 0.7 ± 0.2 μM) and in the number of amastigotes per Mφ were observed (Fig. 2A). Similar to epimastigotes of strain Colombiana, amastigotes demonstrated the same dose-dependent sensitivity to miltefosine as amastigotes of strain Y (Fig. 2B). Recent studies report that lyso-alkylphospholipids are also able to reduce the proliferation of intracellular amastigotes of the Y strain in murine HMC (36) and Vero cells (21), with IC50s of 3 and 0.3 μg ml−1, respectively. However, despite the 10-fold difference in susceptibility observed by others (36), experiments performed by us following the conditions described in the legend to Fig. 2 for Mφ but with murine-infected HMC cultures (25) and miltefosine resulted in a much lower IC50 of 0.6 ± 0.3 μg ml−1 or 1.4 ± 0.7 μM (data not shown).
FIG. 2.
Miltefosine inhibits the percentage of infected Mφ and the number of amastigotes per Mφ. Murine peritoneal Mφ plated onto coverslips were infected with strain Y (A) or Colombiana (B) TCT forms (ratio of five parasites per Mφ) for 2 h, washed, and incubated with medium alone or medium containing increasing amounts of miltefosine, as indicated. After 3 days, the coverslips were fixed and stained with Giemsa, and the percentage of infected Mφ as well as the number of amastigotes per Mφ were determined by direct counting, as described in Materials and Methods. The graphs represent the means of duplicates from four independent experiments.
Effects of miltefosine on release of trypomastigotes and production of NO and TNF-α by infected macrophages.
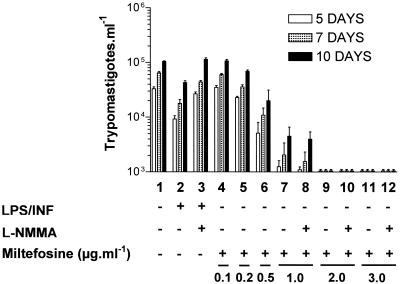
It has previously been shown that hexadecylphosphocholine is able to induce the release of TNF-α and NO from mouse peritoneal Mφ (44) and the human histiocytic cell line U937 (8). To study the effect of miltefosine on the release of trypomastigotes from infected cells and Mφ activation, infected and noninfected mouse peritoneal Mφ were incubated in the absence and presence of LPS plus INF-γ or increasing amounts of miltefosine (Fig. 3). After 2 days of infection, the amounts of NO (Fig. 3B) and TNF-α (Fig. 3C) in the supernatants of each culture were determined. After 5, 7, and 10 days of infection, the numbers of trypomastigotes (Fig. 3A) in the supernatants of infected cells were counted. In comparison to the control (Fig. 3A, lane 3), there was a dose-dependent decrease in the number of trypomastigotes in the culture supernatant (Fig. 3A, lanes 6, 8, 10, and 12). Parasites were not detected in the culture supernatants when miltefosine concentrations greater than 2 μg ml−1 were used (data not shown). Expression of NO (Fig. 3B) and TNF-α (Fig. 3C) by infected (Fig. 3, lanes 6, 8, 10, and 12) and noninfected (Fig. 3, lanes 5, 7, 9, and 11) Mφ was stimulated by miltefosine in a dose-dependent manner compared to the results for the controls (Fig. 3A and B, lanes 1 and 3). These findings suggest that miltefosine treatment promotes Mφ activation that may result in toxicity against the parasite (8, 44). The level of stimulation of NO and TNF-α production by miltefosine (Fig. 3B and C, lanes 5 to 12) was equivalent to that observed with the classical Mφ activator LPS plus IFN-γ. Despite a high level of induction of NO and TNF-α secretion, LPS and IFN-γ treatment of Mφ resulted in a small decrease in the amount of trypomastigotes found in the culture supernatant (Fig. 3A, lane 4). In contrast, despite the induction of only a small increase in the levels of NO and TNF-α production, miltefosine treatment resulted in a pronounced decrease in the number of trypomastigotes in the supernatants of infected Mφ (Fig. 3A; compare lane 6 with lanes 3 and 4). These results suggest that the observed effect of miltefosine on T. cruzi-infected Mφ is independent of cellular activation and NO and TNF-α production.
FIG. 3.
Miltefosine stimulates the synthesis of TNF-α and NO by infected and noninfected Mφ. Plated murine peritoneal Mφ were infected (+) or not infected (−) with Y-strain MCT forms for 24 h, washed, and then incubated with fresh medium containing (+) or not containing (−) LPS plus INF-γ or increasing amounts of miltefosine, as indicated at the bottom. After 2 days of culture, aliquots of the supernatants were taken for measurement of the levels of production of NO (B) and TNF-α (C); and after 5, 7, and 10 days of culture, the number of trypomastigotes found in the supernatants was determined (A). The graphs represent the mean of duplicates from two independent experiments.
Effects of l-NMMA on synthesis of NO by T. cruzi-infected macrophages and cytotoxicity of miltefosine against the parasites.
To further investigate the involvement of the NO produced by Mφ in the cytotoxicity against T. cruzi parasites induced by LPS plus IFN-γ or miltefosine, infected Mφ were treated with l-NMMA, a specific inhibitor of NO production (9, 13). As observed previously, when infected Mφ were activated with LPS plus IFN-γ, fewer trypomastigotes accumulated in the culture supernatants compared to the number that accumulated in supernatants of nonactivated cell cultures (Fig. 4; compare lanes 1 and 2). The decrease was in part because of the toxic action of the NO produced by the activated Mφ since the number of trypomastigotes released was restored to the control levels when the production of NO was inhibited (down to 3.1 ± 0.1 μM; data not shown) by l-NMMA (Fig. 4; compare lanes 1, 2, and 3). However, the cytotoxicity of miltefosine against intracellular amastigotes and/or trypomastigotes was shown to be independent of NO production since the same IC50 (0.6 ± 0.1 μM) was observed if NO production was inhibited by l-NMMA even with the highest concentrations of miltefosine (Fig. 4, lanes 7 to 12).
FIG. 4.
l-NMMA inhibits the synthesis of NO by T. cruzi-infected macrophages but does not change the cytotoxicity of miltefosine against the parasites. Plated Mφ were infected with T. cruzi as described in the legend to Fig. 3 and incubated in the absence (−) or the presence (+) of LPS plus INF-γ, l-NMMA, or miltefosine, as indicated at the bottom. After 2 days of infection the levels of NO were similar to those observed in Fig. 3B, except that in the presence of l-NMMA there was 85 to 90% inhibition in the level of NO production (data not shown). The graph shows the number of trypomastigotes released to the culture supernatants after 5, 7, and 10 days after infection and represents the mean of duplicates from two independent experiments.
Effects of miltefosine on parasitemia and survival of BALB/c mice infected with T. cruzi strain Y.
The in vivo activity of miltefosine against the Y strain of T. cruzi has been studied in the BALB/c mouse model following oral administration of miltefosine (25 mg kg−1 for 20 days) and compared to treatment with benznidazole (100 mg kg−1 for 20 days) (10). Preliminary results in terms of levels of parasitemia and rates of survival obtained with groups of five animals are presented in Fig. 5. Both benznidazole and miltefosine had suppressive activities during the acute phase of infection, bringing about considerable reductions in the levels of parasitemia (Fig. 5A), and promoted 100% survival (Fig. 5B). The most impressive difference observed during the treatments was the complete absence of fur bristling, weight loss, and diarrhea in the miltefosine-treated mice. Four months after treatment, parasites have not been detected in tail blood by microscopy or in spleen tissue sections cultured in BHI-FCS culture medium (data not shown).
FIG. 5.
Miltefosine promotes a dramatic decrease in the level of parasitemia and parasitological cure rate in BALB/c mice infected with T. cruzi strain Y. Results are from a single experiment in which a total of 15 BALB/c mice were infected intraperitoneally with 105 Y-strain MCTs and groups of 5 mice each were left untreated (○) or were immediately treated orally for 20 days with 100 mg of benznidazole kg−1 (▵) or 25 mg of miltefosine kg−1 (▪). As indicated, the number of parasites in the blood was determined at days 6, 7, 8, 13, 14, and 15 after infection (A); and the rate of survival was monitored for 30 days (B).
DISCUSSION
Studies with human and murine models indicate that the efficacy of treatment of Chagas' disease varies according to the strain of T. cruzi. On the basis of susceptibility or resistance to benznidazole, Filardi and Brener (10) investigated 47 T. cruzi strains and separated them into three groups: resistant, partially resistant, and susceptible to treatment. The cytotoxic effects of miltefosine against the different life-cycle stages of the resistant Colombiana strain have been demonstrated for the first time in the present study. Unlike benznidazole, the in vitro effectiveness of miltefosine was also comparable to that observed against parasites from the partially resistant Y strain of T. cruzi (36; this study) and from other species involving Leishmania spp. (5, 7, 23), E. histolytica (38), and Acanthamoeba spp. (43). While the mechanisms of action remain unknown, the results strongly suggest that miltefosine has a broad antiparasitic spectrum.
Several immunomodulatory effects of hexadecylphosphocholine have been described, including enhanced levels of IFN-γ secretion, enhanced levels of granulocyte-macrophage colony-stimulating factor mRNA expression by human mononuclear cells (16), and enhanced levels of induction of NO production in the human histiocyte cell line U937 (8) and murine peritoneal Mφ after treatment with LPS (44). However, despite these observations suggesting that miltefosine has an effect on the development of the immune response, recent studies demonstrate that miltefosine has equal leishmanicidal effects in mouse models deficient in T cells, endogenous IFN-γ, Mφ production of reactive nitrogen and oxygen radicals, TNF-α, and functionally deficient B and T cells (6, 28-30). In a similar manner, our study has confirmed and extended observations that at least NO production is not necessary for the activity of miltefosine against T. cruzi, as the doses-responses were similar in both the absence and the presence of NO produced after Mφ activation.
On the basis of the results presented in this report, as far as NO and TNF-α production is considered, the mechanism of Mφ activation caused by mitefosine differs from that caused by LPS plus IFN-γ. As previously observed by Silva et al. (39) and confirmed in our study, in vitro infection of mouse peritoneal Mφ with T. cruzi resulted in production of almost no TNF-α, which alone had no significant effect on the induction of NO production or intracellular killing. It has recently been shown that liposomal hexadecylphosphocholine induces human mammary carcinoma cytotoxicity mediated by Mφ (9). This effect is dependent on interleukin-6 (IL-6) and TNF-α and is independent of IL-1α and NO. Therefore, the possible participation of other cytokines in the mediation of the cytotoxic effects induced by miltefosine on T. cruzi-infected Mφ cannot be ruled out and requires further analysis. In addition, although it was shown in the present work that miltefosine presented a high level of toxicity against T. cruzi parasites inside nonphagocytic HMCs, the participation of reactive oxygen intermediates should also be investigated in order to correlate the possible activation of Mφ to toxicity triggered by the ether-lipid analogue.
Although the results presented here are preliminary, they show that miltefosine is able to promote a reduction in the level of parasitemia in an experimental mouse model of acute Chagas' disease comparable to that observed with benznidazole, one of the drugs of choice for clinical use in the treatment of Chagas' disease (10). More extensive studies on parasitemia and histopathology are under way with murine models of both acute and chronic Chagas' disease in order to establish if miltefosine could be considered for use in future clinical trials with humans with Chagas' disease.
Acknowledgments
This work was supported by grants from the Third World Academy of Sciences (TWAS), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Universitária José Bonifácio, and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro. V.B.S. is the recipient of a CNPq master-student fellowship, and L.M.-P. is a Howard Hughes Medical Institute International Research Scholar.
We thank Vincent Aguirre for critical reading of the manuscript.
REFERENCES
- 1.Andrews, N. W., M. J. M. Alves, R. I. Schumacher, and W. Colli. 1985. Trypanosoma cruzi: protection in mice immunized with 8-methoxypsolaren-inactivated trypomastigotes. Exp. Parasitol. 60:255-262. [DOI] [PubMed] [Google Scholar]
- 2.Arana, B., N. Rizzo, and A. Diaz. 2001. Chemotherapy of cutaneous leishmaniasis: a review. Med. Microbiol. Immunol. 190:93-95. [DOI] [PubMed] [Google Scholar]
- 3.Contreras, V. T., J. M. Salles, N. Thomas, C. M. Morel, and S. Goldenberg. 1985. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol. Biochem. Parasitol. 16:315-327. [DOI] [PubMed] [Google Scholar]
- 4.Croft, S. L., R. A. Neal, W. Pendergast, and J. H. Chan. 1987. The activity of alkyl phosphorylcholines and related derivatives against Leishmania donovani. Biochem. Pharmacol. 36:2633-2636. [DOI] [PubMed] [Google Scholar]
- 5.Croft, S. L., D. Snowdon, and V. Yardley. 1996. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J. Antimicrob. Chemother. 38:1041-1047. [DOI] [PubMed] [Google Scholar]
- 6.Escobar, P., V. Yardley, and S. L. Croft. 2001. Activities of hexadecylphosphocholine (miltefosine), Ambisome, and sodium stibogluconate (Pentostam) against Leishmania donovani in immunodeficient scid mice. Antimicrob. Agents Chemother. 45:1872-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escobar, P., S. Matu, C. Marques, and S. L. Croft. 2002. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop. 81:151-157. [DOI] [PubMed] [Google Scholar]
- 8.Eue, I., R. Zeisig, and D. Arndt. 1995. Alkylphosphocholine-induced production of nitric oxide and tumor necrosis factor alpha by U937 cells. J. Cancer Res. Clin. Oncol. 121:350-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eue, I. 2001. Growth inhibition of human mammary carcinoma by liposomal hexadecylphosphocholine: participation of activated macrophages in the antitumor mechanism. Int. J. Cancer 92:426-433. [DOI] [PubMed] [Google Scholar]
- 10.Filardi, L. S., and Z. Brener. 1987. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used in Chagas’ disease. Trans. R. Soc. Trop. Med. Hyg. 81:755-759. [DOI] [PubMed] [Google Scholar]
- 11.Fischer, C., A. Voss, and J. Engel. 2001. Development status of miltefosine as first oral drug in visceral and cutaneous leishmaniasis. Med. Microbiol. Immunol. 190:85-87. [DOI] [PubMed] [Google Scholar]
- 12.Freire-de-Lima, C. G., D. O. Nascimento, M. B. P. Soares, P. T. Bozza, H. C. Castro-Faria-Neto, F. G. de Mello, G. A. DosReis, and M. F. Lopes. 2000. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature 403:199-203. [DOI] [PubMed] [Google Scholar]
- 13.Gantt, K. R., T. L. Goldman, M. L. McCormick, M. A. Miller, S. M. B. Jeronimo, E. T. Nascimento, B. E. Britigan, and M. E. Wilson. 2001. Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J. Immunol. 167:893-901. [DOI] [PubMed] [Google Scholar]
- 14.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 15.Hilgard, P., T. Klenner, J. Stekar, and C. Unger. 1993. Alkylphosphocholines: a new class of membrane active anticancer agents. Cancer Chemother. Pharmacol. 32:90-95. [DOI] [PubMed] [Google Scholar]
- 16.Hochhuth, C. H., K. Vehmeyer, H. Eibl, and C. Unger. 1992. Hexadecylphosphocholine induces INF-γ secretion and expression of GM-CSF mRNA in human mononuclear cells. Cell. Immunol. 141:161-168. [DOI] [PubMed] [Google Scholar]
- 17.Jha, T. K., S. Sundar, C. P. Thakur, P. Bachmann, J. Karbwang, C. Fischer, A. Voss, and J. Berman. 1999. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniais. N. Engl. J. Med. 341:1795-1800. [DOI] [PubMed] [Google Scholar]
- 18.Konstantinov, S. M., R. Kaminsky, R. Brun, M. R. Berger, and U. Zillmann. 1997. Efficacy of anticancer alkylphosphocholines in Trypanosoma brucei subspecies. Acta Trop. 64:145-154. [DOI] [PubMed] [Google Scholar]
- 19.Kuhlencord, A., T. Maniera, H. Eibl, and C. Unger. 1992. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrob. Agents Chemother. 36:1630-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonard, R., J. Hardy, G. van Tienhoven, S. Houston, P. Simmonds, M. David, and J. Mansi. 2001. Randomized, double-blind, placebo-controlled, multicenter trial of 6% miltefosine solution, a topical chemotherapy in cutaneous metastases from breast cancer. J. Clin. Oncol. 19:4150-4159. [DOI] [PubMed] [Google Scholar]
- 21.Lira, R., L. M. Contreras, R. Santa-Rita, and J. A. Urbina. 2001. Mechanism of action of anti-proliferative lyso-phospholipid analogues against the protozoan parasite Trypanosoma cruzi: potentiation of in vitro activity by sterol biosynthesis inhibitor ketoconazole. J. Antimicrob. Chemother. 47:537-546. [DOI] [PubMed] [Google Scholar]
- 22.Lux, H., D. T. Hart, P. J. Parker, and T. Klenner. 1996. Ether-lipid metabolism, GPI anchor biosynthesis, and signal transduction are putative targets for anti-leishmania alkyl-phospholipid analogues. Adv. Exp. Med. Biol. 406:201-211. [DOI] [PubMed] [Google Scholar]
- 23.Lux, H., N. Heise, T. Klenner, D. T. Hart, and F. R. Opperdoes. 2000. Ether-lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether-lipid analogues in Leishmania. Mol. Biochem. Parasitol. 111:1-14. [DOI] [PubMed] [Google Scholar]
- 24.Martínez-Díaz, R. A., J. A. Escario, J. J. Nogal-Ruiz, and A. Gómez-Barrio. 2001. Biological characterization of Trypanosoma cruzi strains. Mem. Inst. Oswaldo Cruz 96:53-59. [DOI] [PubMed] [Google Scholar]
- 25.Meirelles, M. N. L., T. C. Araújo-Jorge, C. F. Miranda, and H. S. Barbosa. 1986. Interaction of Trypanosoma cruzi with heart muscle cells: ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur. J. Cell Biol. 41:198-205. [PubMed] [Google Scholar]
- 26.Michailowsky, V., S. M. F. Murta, L. Carvalho-Oliveira, M. E. S. Pereira, L. R. P. Ferreira, Z. Brener, A. J. Romanha, and R. T. Gazzinelli. 1998. Interleukin-12 enhances in vivo parasiticidal effect of benznidazole during acute experimental infection with a naturally drug-resistant strain of Trypanosoma cruzi. Antimicrob. Agents Chemother. 42:2549-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moncayo, A. 1999. Progress towards interruption of transmission of Chagas disease. Mem. Inst. Oswaldo Cruz 94:401-404. [DOI] [PubMed] [Google Scholar]
- 28.Murray, H. W. 2000. Suppression of posttreatment recurrence of experimental visceral leishmaniasis in T-cell-deficient mice by oral miltefosine. Antimicrob. Agents Chemother. 44:3235-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray, H. W., and S. Delph-Etiene. 2000. Visceral leishmanicidal activity of hexadecylphosphocholine (miltefosine) in mice deficient in T cells and activated macrophage microbicidal mechanisms. J. Infect. Dis. 181:795-799. [DOI] [PubMed] [Google Scholar]
- 30.Murray, H. W., A. Jungbluth, E. Ritter, C. Montelibano, and M. W. Marino. 2000. Visceral leishmaniasis in mice devoid of tumor necrosis factor and response to treatment. Infect. Immun. 68:6289-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray, H. W. 2001. Clinical and experimental advances in treatment of visceral leishmaniasis. Antimicrob. Agents Chemother. 45:2185-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murta, S. M., R. T. Gazzinelli, Z. Brener, and A. J. Romanha. 1998. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol. Biochem. Parasitol. 93:203-214. [DOI] [PubMed] [Google Scholar]
- 33.Neal, R. A., and J. van Bueren. 1988. Comparative studies of drug susceptibility of five strains of Trypanosoma cruzi in vivo and in vitro. Trans. R. Soc. Trop. Med. Hyg. 82:709-714. [DOI] [PubMed] [Google Scholar]
- 34.Previato, J. O., C. Jones, L. P. B. Gonçalves, R. Wait, L. R. Travassos, and L. Mendonça-Previato. 1994. O-Glycosidically linked N-acetylglucosamine-bound oligosaccharides from glycoproteins of Trypanosoma cruzi. Biochem. J. 301:151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rybczynska, M., M. Spitaler, N. G. Knebel, G. Boeck, H. Grunicke, and J. Hofmann. 2001. Effects of miltefosine on various biochemical parameters in a panel of tumor cell lines with different sensitivities. Biochem. Pharmacol. 62:765-772. [DOI] [PubMed] [Google Scholar]
- 36.Santa-Rita, R. M., H. S. Barbosa, M. N. S. L. Meirelles, and S. L. de Castro. 2000. Effect of the alkyl-lysophospholipids on the proliferation and differentiation of Trypanosoma cruzi. Acta Trop. 75:219-228. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt, H. H. W., P. Wilke, B. Evers, and E. Bohme. 1989. Enzymatic formation of nitrogen oxides from l-arginine in bovine brain cytosol. Biochem. Biophys. Res. Commun. 165:284-291. [DOI] [PubMed] [Google Scholar]
- 38.Seifert, K., M. Duchene, W. H. Wernsdorfer, H. Kollaritsch, O. Scheiner, G. Wiedermann, T. Hottkowitz, and H. Eibl. 2001. Effects of miltefosine and other alkylphosphocholines on human intestinal parasite Entamoeba histolytica. Antimicrob. Agents Chemother. 45:1505-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva, J. S., G. N. R. Vespa, M. A. G. Cardoso, J. C. S. Aliberti, J. C. S., and F. Q. Cunha. 1995. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect. Immun. 63:4862-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundar, S., F. Rosenkaimer, M. K. Makharia, A. K. Goyal, A. K. Mandal, A. Voss, P. Hilgard, and H. W. Murray. 1998. Trial of oral miltefosine for visceral leishmaniasis. Lancet 352:1821-1823. [DOI] [PubMed] [Google Scholar]
- 41.Sundar, S., A. Makharia, D. K. More, G. Agrawal, A. Voss, C. Fischer, P. Bachmann, and H. W. Murray. 2000. Short-course of oral miltefosine for treatment of visceral leishmaniasis. Clin. Infect. Dis. 31:1110-1113. [DOI] [PubMed] [Google Scholar]
- 42.Urbina, J. A. 1999. Parasitological cure of Chagas’ disease: is it possible? Is it relevant? Mem. Inst. Oswaldo Cruz 94:349-355. [DOI] [PubMed] [Google Scholar]
- 43.Walochnik, J., M. Duchene, K. Seifert, A. Obwaller, T. Hottkowitz, G. Wiedermann, H. Eibl, and H. Aspock. 2002. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob. Agents Chemother. 46:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeisig, R., M. Rudolf, I. Eue, and D. Arndt. 1995. Influence of hexadecylphosphorylcholine on the release of tumor necrosis factor and nitroxide from peritoneal macrophages in vitro. J. Cancer Res. Clin. Oncol. 121:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]