Abstract
We have examined the in vitro activities of fluconazole, amphotericin B, and caspofungin against Candida albicans biofilms by time-kill methodology. Fluconazole was ineffective against biofilms. Killing of biofilm cells was suboptimal at therapeutic concentrations of amphotericin B. Caspofungin displayed the most effective pharmacokinetic properties, with ≥99% killing at physiological concentrations.
Yeasts (mainly Candida albicans) are the third leading cause of catheter-related infections, with the overall highest crude mortality (4, 5). Biofilms are a well-described phenomenon in the microbial world, which have gained notoriety from their ability to resist antimicrobials (10). Determining the effectiveness of different antifungal agents in this setting has important clinical implications in that it may guide therapeutic decisions that may affect the outcome for patients with these difficult-to-treat infections.
Antifungal treatment strategies for C. albicans are limited to a small armamentarium of compounds, mainly azoles, polyenes, and echinocandins (8). Azoles such as fluconazole act on ergosterol biosynthesis and are fungistatic. Emergence of resistance to azoles is an increasing problem. Polyenes, such as amphotericin B, bind to membrane sterols, leading to membrane permeability, leakage, and cell death. These drugs have clinical drawbacks based on their toxicity. Echinocandins are a new class of antifungal agents that inhibit the synthesis of 1,3-β-d-glucan, a key component of the cell wall (11). Caspofungin, a member of this class, exhibits excellent pharmacokinetic characteristics, is fungicidal (against yeasts), and displays good safety profiles (18).
The pharmacodynamic properties of all three antifungals have been described in studies using free-floating (planktonic) cells (2, 6, 7). These studies may be useful in selecting dosing regimens for a range of Candida infections, mainly for free-floating cells encountered in bloodstream infections. However, the pharmacodynamic profiles of sessile cells that are encountered in many biofilm-associated infections are not adequately addressed in these types of studies, especially since sessile C. albicans cells display dramatically altered resistance phenotypes in comparison to planktonic cells (1, 3, 15, 17).
Biofilms of C. albicans strains SC5314 and 3153A were formed as previously described by our group by using microtiter plates (15, 16). Fluconazole (Pfizer, Inc., New York, N.Y.), amphotericin B (Bristol-Myers Squibb, Princeton, N.J.), and caspofungin (Merck Research Laboratories, Rahway, N.J.) were then added to the preformed biofilms at various concentrations, and plates were incubated for selected time intervals (2, 4, 8, 12, 24, and 48 h). Antifungal effects were monitored by a metabolic assay based on the reduction of2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT), a tetrazolium salt (15).
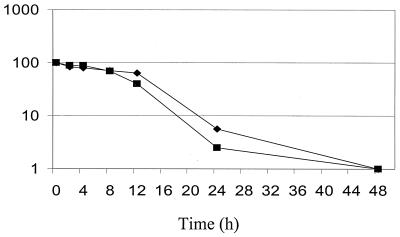
An initial set of experiments was performed to validate the XTT-colorimetric readings for determining cell viability compared to total viable cell counts in caspofungin-treated biofilms. For plate counts, triplicate biofilms were removed from microtiter wells by scraping the biofilms and vortexed vigorously to disperse the cells. Cell counts were estimated by plating serial dilutions of these suspensions. Figure 1 shows a parallel examination of caspofungin-treated biofilms with both XTT measurements and total viable cell counts. The Pearson correlation coefficient was r = 0.9667. The excellent correlation supports the use of the more rapid and simpler XTT-based method to allow high-throughput analysis of the efficacy of the antifungal treatments.
FIG. 1.
Relationship between viable counts determined by plating (▪) and XTT-colorimetric readings (⧫) to measure the survival of biofilm populations formed in microtiter plates following exposure to caspofungin (0.125 μg/ml). Results are expressed as percentages of reduction in viability. Results were analyzed statistically to determine the Pearson correlation coefficient (r = 0.9667).
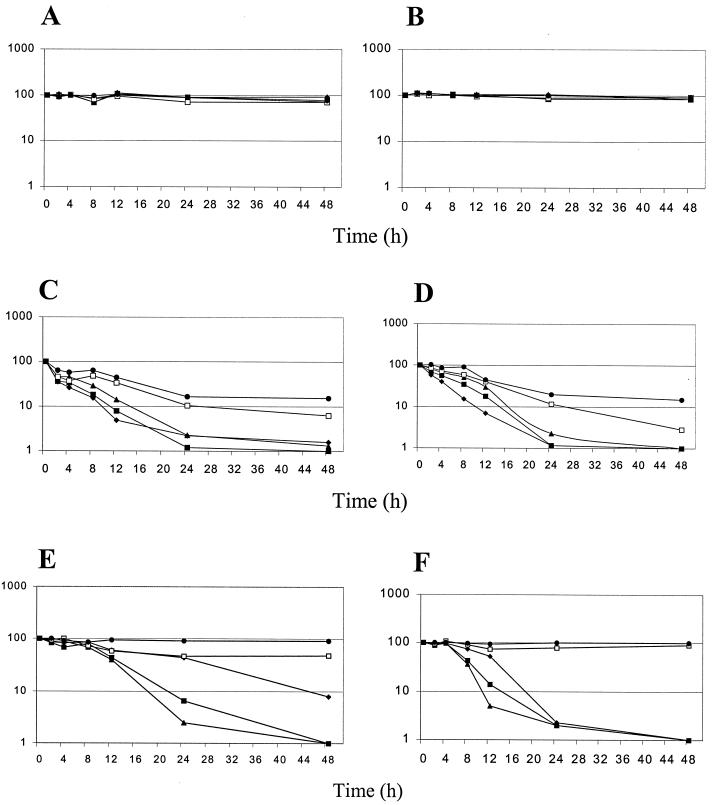
Log plots of decreased viability (percent) in biofilms treated with fluconazole versus time for each isolate are presented in Fig. 2A and B. Fluconazole challenge of preformed C. albicans biofilms showed minimal activity at all concentrations tested over the 48-h period, with a maximal 30% decrease in biofilm activity exhibited after 48 h. Fluconazole is used routinely in the treatment of C. albicans infections, including those exhibiting a biofilm etiology. Indeed, the lack of correlation between susceptibility testing results determined by NCCLS methods (14) and clinical outcome in patients with deep-seated candidiasis (9, 12) could be explained by the increased resistance of biofilms that is not adequately addressed by NCCLS techniques.
FIG. 2.
Log plots of killing kinetics of fluconazole (A and B) (0.5 [•], 2 [□], 16 [▴], 64 [▪], and 1,024 [⧫] μg/ml), amphotericin B (C and D) (0.125 [•], 0.5 [□], 2 [▴], 8 [▪], and 64 [⧫] μg/ml), and caspofungin (E and F) (0.00195 [•], 0.0156 [□], 0.125 [▴], 1 [▪], and 8 [⧫] μg/ml) against preformed biofilms of C. albicans type strains 3153A (A, C, and E) and SC5314 (B, D, and F). Results are averages of eight replicate biofilms for each condition tested and are expressed as percentages of reduction in absorbance by the XTT assay. By this method, the limit of quantification was a 99% killing.
The effects of amphotericin B against sessile cells in biofilms were rapid in comparison to those of fluconazole. As shown in Fig. 2C and D, after 2 h of challenge a 36% and a 64% reduction at the lowest and highest concentrations, respectively, of amphotericin B (0.125 and 64 μg/ml, respectively) were observed for C. albicans 3153A. Thereafter, the antifungal killing effects of the biofilms were generally linear in a concentration-dependent manner with >95% killing at the three highest concentrations but not at therapeutic concentrations (0.125 and 0.5 μg/ml). Thus, the toxicity of this antifungal agent is its major drawback; therefore, if this was overcome then this drug would help reduce morbidity and mortality rates associated with biofilm-associated infections. Interestingly, a recent report indicates the efficacy of lipid formulations of amphotericin B against Candida biofilms, probably due to the higher doses of drug present in these formulations (13), which is consistent with our results.
Caspofungin challenge of preformed C. albicans biofilms showed efficacious antifungal properties over the 48-h period (Fig. 2E and F). C. albicans SC5314 and 3153A exhibited 95 and 60% killing after 12 h at 0.125 μg/ml, respectively. Furthermore, after 24 and 48 h of caspofungin challenge (0.125 and 1 μg/ml, respectively), >99% killing was observed for both C. albicans strains at concentrations achievable in humans (18). Interestingly, the highest concentration of caspofungin (8 μg/ml) was less efficacious than were lower concentrations (0.125 and 1 μg/ml).
Overall our results indicate that caspofungin exhibits the most effective pharmacodynamic properties against C. albicans biofilms in comparison to both amphotericin B and fluconazole (Fig. 2). Whereas amphotericin B kills sessile cells within the biofilm rapidly in a concentration-dependent manner, the concentrations required to initiate these effects are high above its therapeutic margin. Fluconazole, a fungistatic drug, shows little or no efficacy against sessile cells. Caspofungin kills >99% of sessile cells within the biofilm at therapeutically attainable concentrations. These observations, together with the toxicological and pharmacological profiles displayed by caspofungin, support its further investigation and use in difficult-to-treat biofilm-associated infections.
Acknowledgments
This work was supported by grant ATP 3659-0080 from the Texas Higher Education Coordinating Board (Advanced Technology Program, Biomedicine). J.L.L.-R. is the recipient of a New Investigator Award in Molecular Pathogenic Mycology from the Burroughs Wellcome Fund.
We thank Merck & Co., Inc., for providing caspofungin for this study.
REFERENCES
- 1.Baillie, G. S., and L. J. Douglas. 1998. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 42:1900-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgess, D. S., R. W. Hastings, K. K. Summers, T. C. Hardin, and M. G. Rinaldi. 2000. Pharmacodynamics of fluconazole, itraconazole, and amphotericin B against Candida albicans. Diagn. Microbiol. Infect. Dis. 36:13-18. [DOI] [PubMed] [Google Scholar]
- 3.Chandra, J., P. K. Mukherjee, S. D. Leidich, F. F. Faddoul, L. L. Hoyer, L. J. Douglas, and M. A. Ghannoum. 2001. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 80:903-908. [DOI] [PubMed] [Google Scholar]
- 4.Crump, J. A., and P. J. Collignon. 2000. Intravascular catheter-associated infections. Eur. J. Clin. Microbiol. Infect. Dis. 19:1-8. [DOI] [PubMed] [Google Scholar]
- 5.Donlan, R. M. 2001. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 33:1387-1392. [DOI] [PubMed] [Google Scholar]
- 6.Ernst, E. J., M. E. Klepser, M. E. Ernst, S. A. Messer, and M. A. Pfaller. 1999. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn. Microbiol. Infect. Dis. 33:75-80. [DOI] [PubMed] [Google Scholar]
- 7.Ernst, E. J., K. Yodoi, E. E. Roling, and M. E. Klepser. 2002. Rates and extents of antifungal activities of amphotericin B, flucytosine, fluconazole, and voriconazole against Candida lusitaniae determined by microdilution, Etest, and time-kill methods. Antimicrob. Agents Chemother. 46:578-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgopapadakou, N. H., and T. J. Walsh. 1994. Human mycoses: drugs and targets for emerging pathogens. Science 264:371-373. [DOI] [PubMed] [Google Scholar]
- 9.Ghannoum, M. A. 1997. Susceptibility testing of fungi and correlation with clinical outcome. J. Chemother. 9(Suppl. 1):19-24. [PubMed] [Google Scholar]
- 10.Gilbert, P., J. Das, and I. Foley. 1997. Biofilm susceptibility to antimicrobials. Adv. Dent. Res. 11:160-167. [DOI] [PubMed] [Google Scholar]
- 11.Graybill, J. R. 2001. The echinocandins, first novel class of antifungals in two decades: will they live up to their promise? Int. J. Clin. Pract. 55:633-638. [PubMed] [Google Scholar]
- 12.Krcmery, V., Jr. 2000. Is there in vivo-in vitro correlation between antifungal susceptibility, species of Candida spp. and clinical outcome? Int. J. Antimicrob. Agents 16:537-539. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn, D. M., T. George, J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. National Committee for Clinical Laboratory Standards document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramage, G., K. VandeWalle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Characteristics of biofilm formation by Candida albicans. Rev. Iberoam. Micol. 18:163-170. [PubMed] [Google Scholar]
- 17.Ramage, G., B. L. Wickes, and J. L. Lopez-Ribot. 2001. Biofilms of Candida albicans and their associated resistance to antifungal agents. Am. Clin. Lab. 20:42-44. [PubMed] [Google Scholar]
- 18.Stone, J. A., S. D. Holland, P. J. Wickersham, A. Sterrett, M. Schwartz, C. Bonfiglio, M. Hesney, G. A. Winchell, P. J. Deutsch, H. Greenberg, T. L. Hunt, and S. A. Waldman. 2002. Single- and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob. Agents Chemother. 46:739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]