Abstract
The ongoing selection of multidrug-resistant strains of Mycobacterium tuberculosis has markedly reduced the effectiveness of the standard treatment regimens. Thus, there is an urgent need for new drugs that are potent inhibitors of M. tuberculosis, that exhibit favorable resistance profiles, and that are well tolerated by patients. One promising drug target for treatment of mycobacterial infections is dihydrofolate reductase (DHFR; EC 1.5.1.3), a key enzyme in folate utilization. DHFR is an important drug target in many pathogens, but it has not been exploited in the search for drugs effective against M. tuberculosis. The triazine DHFR inhibitor WR99210 has been shown to be effective against other mycobacteria. We show here that WR99210 is also a potent inhibitor of M. tuberculosis and Mycobacterium bovis BCG growth in vitro and that resistance to WR99210 occurred less frequently than resistance to either rifampin or isoniazid. Screening of drugs with M. tuberculosis cultures is slow and requires biosafety level 3 facilities and procedures. We have developed an alternative strategy: initial screening in an engineered strain of the budding yeast Saccharomyces cerevisiae that is dependent on the M. tuberculosis DHFR for its growth. Using this system, we have screened 19 compounds related to WR99210 and found that 7 of these related compounds are also potent inhibitors of the M. tuberculosis DHFR. These studies suggest that compounds of this class are excellent potential leads for further development of drugs effective against M. tuberculosis.
The ongoing selection of multidrug-resistant strains of Mycobacterium tuberculosis has markedly reduced the effectiveness of the standard treatment regimens (27). Thus, there is an urgent need for new drugs that are potent inhibitors of M. tuberculosis, that exhibit favorable resistance profiles, and that are well tolerated by patients. One promising drug target for treatment of mycobacterial infections is dihydrofolate reductase (DHFR; EC 1.5.1.3), a key enzyme in folate utilization that catalyzes the reduction of dihydrofolate to tetrahydrofolate. Tetrahydrofolate is necessary for the one-carbon transfer reactions used in the biosynthesis of nucleic and amino acids including thymidylate, adenine, methionine, glycine, and histidine. DHFR inhibitors act by halting the synthesis of these DNA, RNA, and protein subunits, thereby arresting cell growth. Specific competitive inhibitors of DHFR have been used for more than 50 years in combination with sulfa and sulfone compounds as inexpensive effective inhibitors of bacterial and parasitic infections (13, 32). DHFR has distinct advantages as a potential drug target. First, the biochemistry of the folate pathway and the DHFR enzyme are well characterized, and the crystal structures of 10 DHFR enzymes have been solved. Second, this long history means that the safety and selectivity of these inhibitors have been intensively studied (38) and compounds with a higher degree of selectivity for the microbial enzymes than for the human enzyme have been identified (3, 21, 32, 40).
As a class, inhibitors of folate metabolism show promise as antimycobacterial agents. For example, the dihydropteroate synthase inhibitor dapsone is a cornerstone of leprosy therapy (44), and a number of DHFR inhibitors have reasonable efficacy against mycobacteria (23, 33-35). In particular, the experimental inhibitor WR99210 (4, 17) has been shown to inhibit Mycobacterium avium growth in vitro and in animal models (23, 33-35).
WR99210 has been most intensively studied as an inhibitor of the DHFR enzyme in the malaria parasite Plasmodium falciparum. In the early 1980s, WR99210 was shown to selectively inhibit parasite growth in vitro, with a 50% inhibitory concentration (IC50) in the nanomolar range (24). Despite better selectivity for the malaria parasite enzyme than for the enzyme in human cells in culture (42), trials of WR99210 with humans showed serious problems of bioavailability, and further development was abandoned. However, Canfield and colleagues (4) demonstrated that in vivo, the biguanide precursor of WR99210, PS-15, is converted to the active triazine form and that this formulation improved the bioavailability substantially. This approach revived interest in the series, and related molecules are now at the late stages of preclinical development for antimalaria therapy (17). In addition to indications that WR99210 is effective against several mycobacteria, the crystal structure of the M. tuberculosis DHFR was recently solved, with the brominated analogue of WR99210 bound in the active site (20).
Taken together, these advances suggested that a detailed study of the inhibition of M. tuberculosis by WR99210 and related compounds would be productive. Because screening of drugs with M. tuberculosis cultures is slow, we have developed an alternative strategy: initial screening in an engineered strain of the budding yeast Saccharomyces cerevisiae that is dependent on the M. tuberculosis DHFR for its growth. S. cerevisiae has several advantages that we have exploited to develop similar systems for screening of inhibitors of the DHFR enzymes from P. falciparum, Cryptosporidium parvum, and Pneumocystis carinii (2, 19, 36, 43). We have now constructed a yeast-based system for comparison of compounds that inhibit the DHFR enzyme from M. tuberculosis and used this approach to identify three compounds that are even stronger inhibitors of the M. tuberculosis enzyme than WR99210.
MATERIALS AND METHODS
Mycobacterial culture.
Mycobacterium bovis BCG Montreal and M. tuberculosis H37Rv were grown in 7H9 medium (4.7 g of Difco powder/liter, 0.5% glycerol, 0.05% Tween 80, 10% oleic acid-albumin-dextrose-catalase [OADC] enrichment) at 37°C on rollers. Cell density was determined by measuring the optical density at 600 nm (OD600). To assay the growth on plates, standard 7H10 medium was prepared, autoclaved, and supplemented with OADC enrichment to a final concentration of 10%. To assay either the BCG or the H37Rv cell concentration in liquid culture, a luciferase expression system based on the plasmid pMH109 was used (12). Cells were grown in 0 to 70 μM (0 to 30.3 μg/ml) WR99210 at 37°C on a roller. Growth was assayed by adding 100 μl of nonlysed cells to 100 μl of luciferin (Promega, Madison, Wis.) The samples were incubated at room temperature for 7 min, and the light emitted was measured in a Turner Designs Luminometer TD-20/20.
Determination of frequency of resistant colonies.
The calculated MIC of rifampin ranges from 0.07 to 0.3 μM (0.06 to 0.25 μg/ml) (6). We chose 0.12 μM (0.1 μg/ml), at the middle of the range of estimates, and prepared plates that contained four times the MIC, 0.5 μM (0.4 μg/ml). The calculated MIC of isoniazid ranges from 0.22 to 0.44 μM (0.03 to 0.06 μg/ml) (45). We chose 0.44 μM (0.06 μg/ml) and prepared plates that contained four times the MIC, 1.75 μM (0.24 μg/ml). Prior to this work, the range of MICs of WR99210 had not been determined, but we estimated the range to be 1.6 to 6 μM (0.7 to 2.6 μg/ml) (Fig. 1) and used 24 μM (10.3 μg/ml) as four times the MIC of WR99210. Stock solutions of drugs were dissolved in dimethyl sulfoxide (DMSO) and added directly to cooled medium. The final DMSO concentration in the plates was <1%. A total of 109 cells were plated on each of 4 plates with rifampin or isoniazid and 107 to 109 cells/plate and 10 plates with WR99210 and 109 cells/plate. The plates were sealed in plastic bags and incubated at 37°C. Colonies that grew on drug-containing plates were picked and retested in liquid culture to verify the drug-resistant phenotype.
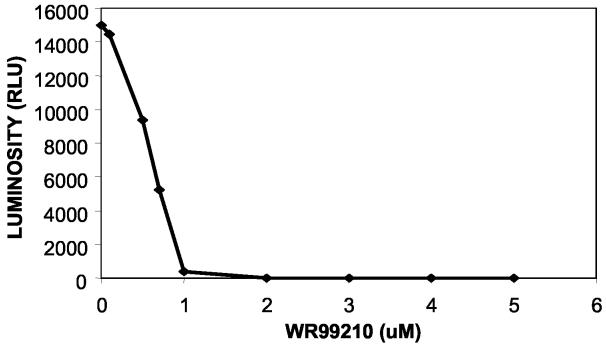
FIG. 1.
Inhibition of BCG growth by WR99210. BCG cells transformed with the pMH109 luciferase plasmid were grown in the presence of WR99210 for 3 days at the indicated drug concentrations. Growth was monitored by measuring the light emitted by the cells during a 6-min period.
Yeast strains.
DHFR-deficient S. cerevisiae strain TH5 (MATaleu2-3,112 trp1 ura3-52 dfr1::URA3 tup1) was generously provided by Tun Huang (14); TH5 requires supplementation with adenine, histidine, methionine, and dTMP for growth. Strain TH5 was transformed with a pRS314-derived shuttle vector (37) containing 600 bp of the S. cerevisiae dfr1 promoter, the complete coding region of the dfr1 gene, and 400 bp of its terminator (43). Yeasts were cultured by standard techniques in rich (yeast extract-peptone-dextrose) or tryptophan-deficient medium at 30°C. Both medium types contained adenine, histidine, and methionine. In addition, yeast growth was supplemented with 100 μg of dTMP/ml unless the strain was transformed with a functional DHFR gene or when otherwise necessary. Transformations were carried out by standard lithium acetate methods (16), and transformation reaction mixtures were plated onto plates with tryptophan-deficient medium plus dTMP.
Construction of strain TH5 transformed with dfrA gene from M. tuberculosis.
Previous amplification and sequencing in our laboratory had shown that the sequences of the dfrA genes from M. tuberculosis and BCG are identical, so primers to M. tuberculosis dfrA were used to amplify the DHFR gene from M. bovis BCG (Montreal). The dfrA gene was amplified by the PCR and cloned by homologous recombination into the p414CYC1 yeast shuttle vector, which contains a TRP marker for selection in yeast, a centromere sequence to maintain the plasmid at the single-copy level, and the yeast cyc1 promoter (25). The primers were designed to match the external sequence of the vector, which allowed us to clone the PCR product directly into the yeast shuttle vector by homologous recombination. Cell lysates were diluted 1:10, and amplification was carried out with Herculase polymerase (Stratagene, La Jolla, Calif.) and 250 μM deoxynucleoside triphosphates, 500 nM primers (upstream primer, 5′-CGCAAACACAAATACACACACTAAATTAATACTGCAGATGGTGGGGCTGATTTGGGCT-3′; downstream primer, 5′-GAATGTAAGCGTGACATAACTAATTACATGAGAATTCTGAGCGGTGGTAGCTGTACAACCGG-3′), and 0.9% DMSO on a thermo- cycler (PTC 200; MJ Research, Boston, Mass.). Cycling parameters were as follows: 95°C for 3 min, 34 cycles of 95°C for 15 s, 60°C for 20 s, and 72°C for 45 s, followed by 10 min at 72°C. To clone the gene, the plasmid was linearized in an XbaI restriction digest and was transformed into the yeasts along with the PCR product. The presence and sequence of dfrA in the vector were verified by sequencing.
Complementation.
DHFR-deficient yeast strain TH5 was transformed with the p414CYC1 vector with no DHFR insert, the dhf1 gene from yeast, or the dfrA gene from M. tuberculosis (TB-yeast). Transformants were patched onto rich medium with 100 μg of dTMP/ml, grown for 3 days, and then sequentially replica plated onto rich medium lacking dTMP. After 3 days the replica plate was checked for growth. Growth of a yeast patch in the absence of dTMP indicated complementation of the TH5 dfr1 disruption.
Drugs.
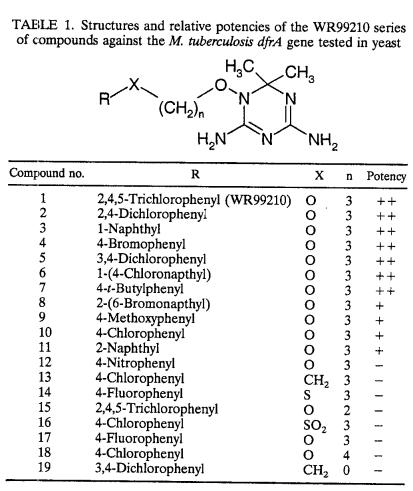
The WR99210 analogs, chlorcycloguanil, and epiroprim were synthesized by Jacek Terpinski (Jacobus Pharmaceutical Company, Princeton, N.J.). Sulfanilamide and pyrimethamine were obtained from Sigma (St. Louis, Mo.). Trimetrexate was provided by Mohamed Nasr at the National Institute of Allergy and Infectious Diseases. The structures of the WR99210 analogs used in this study are listed in Table 1, and the method of their synthesis is described by Jensen (17). All drugs were dissolved in DMSO (Sigma) and stored at −20°C until use.
TABLE 1.
Structures and relative potencies of the WR99210 series of compounds against the M. tuberculosis dfrA gene tested in yeast
Radial spoke assay.
The spoke assay-based screening of the activities of the test compounds against TB-yeast was performed as described in detail by Sibley et al. (36). Addition of 1 mM sulfanilamide to the growth medium improved the sensitivities of the TB-yeast to the test compounds, so it was included in the growth assays. For the radial assay, sulfanilamide was spread onto fresh plates and allowed to absorb into the medium overnight. A template streaked with TB-yeast that had grown for 3 days was sequentially replica plated onto these plates, and a 10-μl aliquot of a 10 mM test compound was placed in the center of the plate. The final concentration of DMSO in the assay plates was <0.6%. Assay plates were incubated for 3 days to allow growth of the yeast before the spoke length was measured. Spokes that grew to a length at least 0.4 cm shorter than the spoke from the control plate with DMSO alone were scored as inhibited by the drug on the plate.
Quantitative liquid assay.
The liquid assay was also conducted as described previously (36). Briefly, 8 × 104 cells from a log-phase culture were aliquoted into the wells of a 96-well plate to which sulfanilamide and a range of concentrations of the test compound were added. The final concentrations of sulfanilamide (1 mM) and DMSO (1%) were constant in each well. For a single assay, tests with each drug concentration were repeated in three or four wells except for tests with the control lacking a test compound, for which tests were repeated 3 to 12 times. Cultures were grown until the control cultures reached the mid-log phase, approximately 40 h later. Growth was measured by reading the OD650s of the cells. The average OD650 for the control wells was scored as 100% growth, and the average readings for the cultures at each drug concentration were divided by this value to obtain relative growth values. The IC50 was calculated by using the two relative growth values that flanked the 50% mark and the formula y = mx + b, where m is the slope, b is the y intercept, and the solution for x at y equal to 50% yielded the IC50.
RESULTS
WR99210 inhibits growth of both BCG and M. tuberculosis (H37Rv) in vitro.
Although there was good evidence that WR99210 could inhibit other species of mycobacteria (23, 33-35), we first confirmed that WR99210 could inhibit M. tuberculosis growth in vitro. M. bovis BCG or M. tuberculosis strains that carry the luc expression plasmid pMH109 (12, 30) were incubated in the presence of 0 to 70 μM WR99210, and the growth of each culture was monitored between 4 and 6 days by measurement of the light emitted. Figure 1 shows the results of a representative experiment performed with BCG. The range of MICs measured in several experiments was 1.6 to 6 μM. The MIC for M. tuberculosis was within this range, and subsequent experiments used BCG alone.
Frequency of colonies resistant to WR99210 is low.
In a number of pathogens, resistance to inhibitors of DHFR evolves simply by the accumulation of point mutations in the dhfr gene (15, 32), and point mutations in the target genes are known to be the principal mechanism of drug resistance in M. tuberculosis (6, 26). To assess the frequency at which resistance to WR99210 resistance might occur, we spread BCG onto plates containing either WR99210 or the standard antituberculosis drugs rifampin and isoniazid and compared the rate at which resistant colonies appeared. We tested each drug at four times its MIC (rifampin, 0.5 μM; isoniazid, 1.75 μM; WR99210, 24 μM). These data are summarized in Table 2. The frequency of colonies resistant to rifampin was about 6.7 × 10−8, and the frequency of colonies resistant to isoniazid was about 1.7 × 10−6; both of these values are within the range predicted by previously published work (6, 8, 31).Numerous microcolonies were observed on all plates containing WR99210 that were originally inoculated with 109 BCG cells. Several colonies were picked and again grown in liquid medium with or without WR99210. The cultures without WR99210 grew within a week at 37°C, whereas none of the cultures grew after 3 weeks in the presence of 24 μM WR99210. We concluded that WR99210 was exerting a bacteriostatic effect rather than a bactericidal effect on BCG. No colonies resistant to WR99210 were observed on any of the plates. These data suggest that the frequency of mutations for resistance to WR99210 is less than 1.2 × 10−10 and that further development of drugs of this class will not be rapidly undermined by selection of resistant strains of M. tuberculosis.
TABLE 2.
Frequency of mutant colonies arising on plates with rifampin, isoniazid, or WR99210a
| Drug | Concn (μg/ml) | No. of cells/plate | No. of plates | Time incubated (wk) | Total no. of colonies | Frequency of resistance |
|---|---|---|---|---|---|---|
| None | NA | 107 | 2 | 2 | Lawn | |
| Isoniazid | 0.24 | 107 | 2 | 2 | 5-7 | 1.7 × 10−6 |
| Rifampin | 0.4 | 107 | 4 | 6 | 0 | |
| Rifampin | 0.4 | 108 | 6 | 6 | 0 | |
| Rifampin | 0.4 | 109 | 4 | 6 | 6 | 6.7 × 10−8 |
| WR99210 | 10.3 | 109 | 14 | 6 | Microcoloniesb | <1.2 × 10−10 |
The indicated number of BCG cells were plated with each drug at four times the estimated MIC and grown for the indicated times at 37°C, and the colonies were counted.
Microcolonies were observed at the indicated frequency; however, several colonies were picked and failed to grow in liquid medium with an appropriate concentration of WR99210.
M. tuberculosis dfrA complements a DHFR-deficient yeast strain.
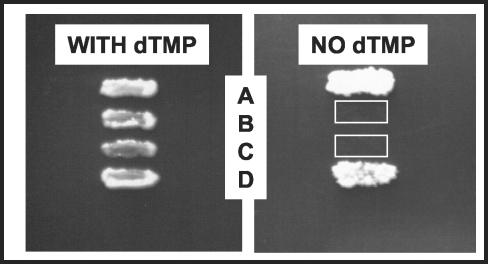
The slow growth and difficulty of working with the mycobacteria directly led us to develop a system that could more quickly and safely screen potential inhibitors of the M. tuberculosis DHFR enzyme. As a first step, we constructed a yeast strain dependent on the M. tuberculosis DHFR enzyme for growth. The TH5 strain of yeast (14) lacks endogenous expression of DHFR and is maintained by supplementing the medium with dTMP and a full complement of amino acids, uracil, and adenine. To construct this strain, the M. tuberculosis DHFR dfrA gene was cloned into a series of yeast shuttle vectors with a range of promoters that drove expression of the linked M. tuberculosis gene at several levels: p414GPD, p414TEF, and p414CYC1 (25), as well as GR7, a pRS314-based vector with a truncated promoter from the yeast dhfr gene (7). The vectors were transformed into TH5 and tested for their ability to grow without dTMP and for the level of inhibition by WR99210. The optimal strain for the screening of DHFR inhibitors must express the enzyme at a level high enough that the yeast can grow without supplementation but low enough that a proportional decrease in growth is achieved when an inhibitor of DHFR function is added. The plasmids that carried the p414GPD and p414TEF promoters drove such a high level of DHFR expression that WR99210 did not inhibit yeast growth, even at the highest levels that can be attained on plates (10−4 M), and the GR7 plasmid drove expression to a level that was insufficient for yeast growth without supplementation with dTMP (data not shown). The strain that carried dfrA on the p414CYC1 vector drove an intermediate level of DHFR expression, and so this plasmid was chosen for use in all subsequent experiments and was designated TB-yeast. Figure 2 shows that TH5 was unable to grow on rich medium without supplementation of dTMP but that TH5 transformed with a single copy of a plasmid bearing the M. tuberculosis or yeast DHFR gene grew without supplementation. Transformation of the vector alone lacking a DHFR gene was insufficient for complementation, as expected.
FIG. 2.
Complementation of the TH5 dfr1 growth defect by TB-yeast. TH5 cells were transformed with the yeast shuttle plasmid and patched onto rich medium with 100 μg of dTMP/ml. After 3 days, the patches were sequentially replica plated to rich medium lacking dTMP. Rows: A, plasmid with the yeast dfr1 gene; B, no plasmid; C, plasmid alone; D, plasmid with the M. tuberculosis dfrA gene.
Several analogs of WR99210 inhibited TB-yeast.
Although WR99210 is an excellent lead compound, there were a number of barriers to its development (4). For that reason, a series of related compounds has been synthesized (17), and our goal was to use the yeast expression system to identify the compounds with the most promise for further development. Our panel of test compounds included 19 WR99210 analogs (Table 1), trimetrexate, pyrimethamine, chlorcycloguanil, and epiroprim. Trimetrexate was selected as a positive control because it inhibits all DHFR enzymes (18), and pyrimethamine was selected because it had shown some in vitro activity against the M. avium complex (34). Epiroprim was a negative control, as it is ineffective against M. tuberculosis (9).
The first step was to determine the relative potencies of these compounds compared with that of WR99210 by a simple radial assay with the TB-yeast (19). Briefly, TB-yeast was streaked from the center to the edge of a plate to make a template. The template was replica plated onto plates containing 1 mM sulfanilamide, and 10 μl of 10 mM test compound dissolved in DMSO was spotted into the center of the replica. All drug assays with the TB-yeast contained 1 mM sulfanilamide in the growth medium to improve sensitivity to the DHFR inhibitors. DMSO was used as a negative control to ensure that the solvent and 1 mM sulfanilamide were not inhibiting the yeast growth nonspecifically.
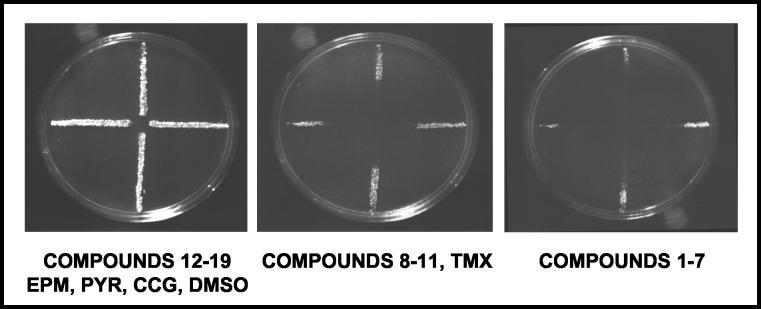
The assay results grouped into three distinct categories; Fig. 3 shows representative data. The yeast grew to the center of the plate in the assays with the DMSO negative control, compounds 12 to 19, epiroprim, pyrimethamine, and chlorcycloguanil, indicating little or no inhibition of M. tuberculosis DHFR by these compounds. Dramatically shorter spokes were observed for compounds 1 to 7, suggesting that these drugs are much stronger inhibitors. An intermediate level of potency was observed for compounds 8 to 11 and trimetrexate. The spokes in the assays with these test compounds did not grow to the center of the plate but were noticeably longer than the spokes seen on plates with compounds 1 to 7.
FIG. 3.
Radial assay of the WR99210 series of compounds. The TB-yeast cells were streaked onto rich medium in a radial pattern, grown for 3 days at 30°C, and then replica plated onto a plate onto which a 10-μl volume of each drug at a 10 mM concentration in DMSO was spotted at the center. The plates were photographed after 3 days of growth at 30°C. EPM, epiroprim; PYR, pyrimethamine; CCG, cycloguanil; TMX, trimetrexate.
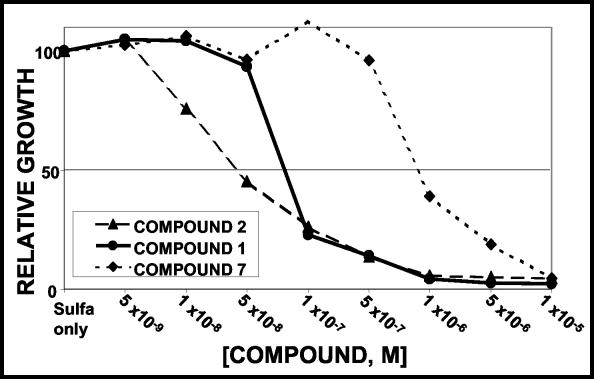
The radial assays are only semiquantitative. To measure the relative effectiveness of the seven compounds that demonstrated high levels of inhibition in the spoke assays more precisely, TB-yeast was grown in liquid culture with 0 to 10−5 M concentrations of each test compound and 1 mM sulfanilamide. The relative growth of the cells in the presence and absence of the drug was calculated, and Fig. 4 shows representative data. For all seven compounds tested, increasing concentrations of drug resulted in decreased growth of the yeast.
FIG. 4.
Quantitative assay of sensitivity to the WR99210 series compounds. A total of 8 × 10 4 actively growing TB-yeast cells were plated in triplicate or quadruplicate into wells of a 96-well plate containing 1 mM sulfanilamide and the indicated concentrations of the compound to be tested. Growth was assessed after 40 h by reading the OD650. Growth is expressed relative to that in the control wells with DMSO alone.
The relative drug efficacies were calculated by using the mean IC50 of each compound, and the data are compiled in Table 3. The IC50s of the seven compounds ranged from 0.05 to 0.97 μM; the values for each compound were reproducible within a twofold range, and with the exception of compounds 3 and 6, the values overlapped little. The IC50 of WR99210 was 0.10 μM; compounds 2, 3, and 6 had lower IC50s, indicating that they are more effective than WR99210; and compounds 4, 5, and 7 were less effective. The 2,4-dichlorophenyl compound was the most potent of the drugs tested, with an IC50 of 0.05 μM.
TABLE 3.
IC50s of the eight compounds with apparent high degrees of efficacy against TB-yeasta
| Test compound | IC50 (μM)
|
|
|---|---|---|
| Mean | SD | |
| 2 | 0.05 | 0.01 |
| 3 | 0.08 | 0.02 |
| 6 | 0.08 | 0.01 |
| WR99210 | 0.10 | 0.03 |
| 5 | 0.61 | 0.16 |
| 4 | 0.78 | 0.05 |
| 7 | 0.92 | 0.04 |
Cells were grown in liquid culture as described in the legend to Fig. 4. The IC50s were calculated from three or four different experiments. SD, standard deviation.
DISCUSSION
The effectiveness of WR99210 against both BCG and M. tuberculosis suggests that this class of drugs has real potential for development for treatment of mycobacterial infections. In addition, the apparent low frequency of colonies resistant to WR99210 indicates that the frequency of selection of M. tuberculosis strains resistant to drugs of this class may be less than that observed with rifampin or isoniazid. In all likelihood, any clinical use would combine the DHFR inhibitor with a sulfa drug or sulfones like dapsone, along with other currently used therapies, so the selection of multidrug-resistant isolates would be minimized.
The radial assays allowed us to identify modifications of lead compound WR99210 that alter the level of inhibition of the M. tuberculosis enzyme. The WR99210 analogs tested in this screen differ in three ways: the identity of the R group, the length of the carbon linker chain, and the substitution of a sulfonyl group for an oxygen atom in the carbon chain (Table 1). First, all four compounds that contained a naphthyl group as the R group moderately to strongly inhibited the TB-yeast. Second, in pairs of analogs that differed only in the length of the carbon linker, the analogs with three carbons caused the strongest inhibition. WR99210 showed strong activity with three carbons in the linker, but the analog with two carbons (compound 15) showed little or no activity. Furthermore, compound 10 showed moderate activity with three carbons, but the four-carbon variant (compound 18) caused no inhibition. These results suggest that the three-carbon linker is an important determinant of effective inhibition. Third, in a pair of analogs in which the compounds differed by an oxygen or a sulfonyl group between the linker chain and the R group, the compound with the oxygen (compound 10) caused moderate inhibition and the one with the sulfonyl group (compound 16) caused little or no inhibition. Thus, replacement of the oxygen with a sulfonyl group resulted in reduced inhibition. Observations about changes in drug structure that alter activity will support computer-aided modeling to predict drugs with improved activity and binding to the enzyme.
The quantitative liquid assay yielded more precise data about the relative efficacies of the test compounds. Compounds 1 and 2 (WR99210 and the 2,4-dichloro analog of WR99210, respectively) were two of the four most potent analogs in the quantitative liquid assay and are the most promising lead compounds identified in the screen. Compounds 3 and 6 were equally potent, and the presence of the naphthyl group in both compounds suggests that the presence of a bulky hydrophobic group at that position might increase the level of binding to the enzyme as well. The 3,4-dichloro compound (compound 5) was about sixfold less effective against the M. tuberculosis enzyme than WR99210, but it was still among the strong inhibitors. This compound is of special interest because it is in trials with primates as a potential treatment for falciparum malaria.
The simple yeast system described here offers the possibility to screen rapidly for other inhibitors of the M. tuberculosis DHFR enzyme. Inhibitors of DHFR have been synthesized for more than 50 years, and many drugs of this class are available for screening. The heterologous system circumvents the slow growth of M. tuberculosis and the health risks inherent in working with mycobacteria. One might have chosen to screen these drugs in Mycobacterium smegmatis, taking advantage of its rapid growth (the growth rate is about equal to that of S. cerevisiae) and ease of genetic manipulation relative to those for M. tuberculosis (11). However, drug screening in M. smegmatis has not always been an accurate predictor of activity (28, 29) or the mechanism of action (1, 22) in M. tuberculosis. In addition, S. cerevisiae has several advantages that we have exploited to develop similar systems for the screening of inhibitors of the DHFR enzymes from P. falciparum, C. parvum, and P. carinii DHFRs (2, 19, 36, 43). First, plasmids that carry a centromere are maintained at one copy per cell in yeasts (37), so the level of expression of an introduced gene can be controlled even on a plasmid. In bacterial systems, control of plasmid number is far less precise, and integration of genes into the chromosome would be required for equivalent studies to be carried out in M. smegmatis. Second, a series of yeast centromere plasmids that carry promoters that drive different levels of expression of linked genes had already been created (25). These plasmids were used to rapidly identify yeast clones that express the heterologous enzyme at a level convenient for drug screening. Last, homologous recombination is highly active in S. cerevisiae and foreign genes can be integrated into a plasmid simply by cotransformation with a gapped plasmid, a significant advantage when a large number of different alleles is to be tested (36).
The IC50 that one measures for the yeast is a complex function of the level of the M. tuberculosis DHFR enzyme, the level of penetration of the yeast by the drug tested, and the actual level of inhibition of the enzyme within the cell. For that reason, the absolute IC50 cannot predict the MIC for M. tuberculosis. However, the relative inhibition of yeast growth by this related series of inhibitors is valuable information for drug testing. Further work to develop these compounds will require working with mycobacterial cultures, purified enzyme, and animal models of tuberculosis infection; but we have reduced the amount of initial work necessary to identify drug candidates by working in this yeast system. In addition, further drug screening can be completed with BCG, significantly simplifying the process. Screening of more analogs may be necessary to identify molecules with antimycobacterial activities and other favorable properties in vivo (39).
To increase the sensitivity of this yeast system, we included 1 mM sulfanilamide in the growth medium for all yeast assays. Sulfa drugs inhibit dihydropteroate synthase, an enzyme upstream of DHFR in the folate pathway (5, 10, 41). The sulfa drugs are always used in combination with DHFR inhibitors because the two drugs work in synergy in many pathogens. In this case, they increase the effectiveness of the DHFR inhibitors in the heterologous yeast system and facilitate the comparative screening. The addition of sulfanilamide to these assays does not compromise the results, since we are measuring the relative inhibition of a set of closely related compounds. In any case, it is likely that a sulfa drug would be part of a combination therapy that included a DHFR inhibitor for the treatment of tuberculosis.
This system offers both a qualitative spoke assay and a quantitative liquid assay for determination of the efficacies of DHFR inhibitors. The spoke assays were useful for rapid classification of test compounds into categories of approximate potency. One surprising result was that trimetrexate showed only moderate activity against the TB-yeast. We included trimetrexate as our positive control, as it is a strong, nonspecific inhibitor of most DHFR enzymes. Furthermore, it has shown strong inhibition of isogenic yeast models that were used to screen the activities of drugs against the DHFR enzymes from other pathogens (2). The observation that trimetrexate shows only moderate activity against the TB-yeast strain suggests that there may be some fundamental differences between the M. tuberculosis enzyme and the DHFR enzymes that have been characterized previously.
For our in vitro assay, we used the listed triazine compounds, but the poor bioavailabilities and pharmacokinetic profiles of the triazines in vivo will be circumvented by the synthesis of biguanide prodrugs (4, 17). Although the development of prodrugs is at an early stage, several of these analogues are in the preclinical stage of development as antimalaria drugs (17). Thus, further study of their efficacies against the mycobacteria is likely to be a fruitful avenue of research.
Acknowledgments
We thank Reiling Liao, Kristi Guinn, and Maribel Harrell for excellent technical assistance; Jacek Terpinski for synthesis of the compounds; the laboratory of C. H. Sibley; and an anonymous reviewer for valuable comments and corrections in the manuscript.
The work was supported by Public Health Service grants AI/CI-46065 to D.P.J. and C.H.S. and AI47744 to D.R.S.
REFERENCES
- 1.Boshoff, H. I., V. Mizrahi, and C. E. Barry III. 2002. Effects of pyrazinamide on fatty acid synthesis by whole mycobacterial cells and purified fatty acid synthase I. J. Bacteriol. 184:2167-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brophy, V. H., J. Vasquez, R. G. Nelson, J. R. Forney, A. Rosowsky, and C. H. Sibley. 2000. Identification of Cryptosporidium parvum dihydrofolate reductase inhibitors by complementation in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 44:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brumfitt, W., J. M. Hamilton-Miller, and A. Gooding. 1980. Resistance to trimethoprim. Lancet i:1409-1410. [PubMed]
- 4.Canfield, C. J., W. K. Milhous, A. L. Ager, R. N. Rossan, T. R. Sweeney, N. J. Lewis, and D. P. Jacobus. 1993. PS-15: a potent, orally active antimalarial from a new class of folic acid antagonists. Am. J. Trop. Med. Hyg. 49:121-126. [DOI] [PubMed] [Google Scholar]
- 5.Chio, L. C., L. A. Bolyard, M. Nasr, and S. F. Queener. 1996. Identification of a class of sulfonamides highly active against dihydropteroate synthase from Toxoplasma gondii, Pneumocystis carinii, and Mycobacterium avium. Antimicrob. Agents Chemother. 40:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole, S. T., and A. Telenti. 1995. Drug resistance in Mycobacterium tuberculosis. Eur. Respir. J. Suppl. 20:701s-713s. [PubMed] [Google Scholar]
- 7.Cortese, J. F., and C. V. Plowe. 1998. Antifolate resistance due to new and known Plasmodium falciparum dihydrofolate reductase mutations expressed in yeast. Mol. Biochem. Parasitol. 94:205-214. [DOI] [PubMed] [Google Scholar]
- 8.David, H. L., and C. M. Newman. 1971. Some observations on the genetics of isoniazid resistance in the tubercle bacilli. Am. Rev. Respir. Dis. 104:508-515. [DOI] [PubMed] [Google Scholar]
- 9.Dosso, M., L. Ouattara, A. M. Cherif, S. A. Bouzid, L. Haller, and M. Fernex. 2001. Experimental in vitro efficacy study on the interaction of epiroprim plus isoniazid against Mycobacterium tuberculosis. Chemotherapy (Basel) 47:123-127. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez, A. H., O. G. Berlin, and D. A. Bruckner. 1989. In-vitro activity of dapsone and two potentiators against Mycobacterium avium complex. J. Antimicrob. Chemother. 24:19-22. [DOI] [PubMed] [Google Scholar]
- 11.Hatfull, G. F., and W. R. Jacobs, Jr. 2000. Molecular genetics of mycobacteria. American Society for Microbiology, Washington, D.C.
- 12.Hickey, M. J., T. M. Arain, R. M. Shawar, D. J. Humble, M. H. Langhorne, J. N. Morgenroth, and C. K. Stover. 1996. Luciferase in vivo expression technology: use of recombinant mycobacterial reporter strains to evaluate antimycobacterial activity in mice. Antimicrob. Agents Chemother. 40:400-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hitchings, G. H., Jr. 1989. Nobel lecture in physiology or medicine—1988. Selective inhibitors of dihydrofolate reductase. In Vitro Cell Dev. Biol. 25:303-310. [DOI] [PubMed] [Google Scholar]
- 14.Huang, T., B. J. Barclay, T. I. Kalman, R. C. VonBorstel, and P. J. Hastings. 1992. The phenotype of a dihydrofolate reductase mutant in Saccharomyces cerevisiae. Gene 121:167-171. [DOI] [PubMed] [Google Scholar]
- 15.Hyde, J. E. 1990. The dihydrofolate reductase-thymidylate synthase gene in the drug resistance of malaria parasites. Pharmacol. Ther. 48:45-59. [DOI] [PubMed] [Google Scholar]
- 16.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen, N. P., A. L. Ager, R. A. Bliss, C. J. Canfield, B. M. Kotecka, K. H. Rieckmann, J. Terpinski, and D. P. Jacobus. 2001. Phenoxypropoxybiguanides, prodrugs of DHFR-inhibiting diaminotriazine antimalarials. J. Med. Chem. 44:3925-3931. [DOI] [PubMed] [Google Scholar]
- 18.Kuyper, L. F., D. P. Baccanari, M. L. Jones, R. N. Hunter, R. L. Tansik, S. S. Joyner, C. M. Boytos, S. K. Rudolph, V. Knick, H. R. Wilson, J. M. Caddell, H. S. Friedman, J. C. Comley, and J. N. Stables. 1996. High-affinity inhibitors of dihydrofolate reductase: antimicrobial and anticancer activities of 7,8-dialkyl-1,3-diaminopyrrolo[3,2-f]quinazolines with small molecular size. J. Med. Chem. 39:892-903. [DOI] [PubMed] [Google Scholar]
- 19.Lau, H., J. T. Ferlan, V. H. Brophy, A. Rosowsky, and C. H. Sibley. 2001. Efficacies of lipophilic inhibitors of dihydrofolate reductase against parasitic protozoa. Antimicrob. Agents Chemother. 45:187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, R., R. Sirawaraporn, P. Chitnumsub, W. Sirawaraporn, J. Wooden, F. Athappilly, S. Turley, and W. G. Hol. 2000. Three-dimensional structure of M. tuberculosis dihydrofolate reductase reveals opportunities for the design of novel tuberculosis drugs. J. Mol. Biol. 295:307-323. [DOI] [PubMed] [Google Scholar]
- 21.Locher, H. H., H. Schlunegger, P. G. Hartman, P. Angehrn, and R. L. Then. 1996. Antibacterial activities of epiroprim, a new dihydrofolate reductase inhibitor, alone and in combination with dapsone. Antimicrob. Agents Chemother. 40:1376-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mdluli, K., D. R. Sherman, M. J. Hickey, B. N. Kreiswirth, S. Morris, C. K. Stover, and C. E. Barry III. 1996. Biochemical and genetic data suggest that InhA is not the primary target for activated isoniazid in Mycobacterium tuberculosis. J. Infect. Dis. 174:1085-1090. [DOI] [PubMed] [Google Scholar]
- 23.Meyer, S. C., S. K. Majumder, and M. H. Cynamon. 1995. In vitro activities of PS-15, a new dihydrofolate reductase inhibitor, and its cyclic metabolite against Mycobacterium avium complex. Antimicrob. Agents Chemother. 39:1862-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milhous, W. K., N. F. Weatherly, J. H. Bowdre, and R. E. Desjardins. 1985. In vitro activities of and mechanisms of resistance to antifol antimalarial drugs. Antimicrob. Agents Chemother. 27:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mumberg, D., R. Muller, and M. Funk. 1995. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156:119-122. [DOI] [PubMed] [Google Scholar]
- 26.Musser, J. M. 1995. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin. Microbiol. Rev. 8:496-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pablos-Mendez, A., M. C. Raviglione, A. Laszlo, N. Binkin, H. L. Rieder, F. Bustreo, D. L. Cohn, C. S. Lambregts-van Weezenbeek, S. J. Kim, P. Chaulet, P. Nunn, et al. 1998. Global surveillance for antituberculosis-drug resistance, 1994-1997. N. Engl. J. Med. 338:1641-1649. (Erratum, 339:139.) [DOI] [PubMed] [Google Scholar]
- 28.Parrish, N. M., T. Houston, P. B. Jones, C. Townsend, and J. D. Dick. 2001. In vitro activity of a novel antimycobacterial compound, N- octanesulfonylacetamide, and its effects on lipid and mycolic acid synthesis. Antimicrob. Agents Chemother. 45:1143-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quan, S., H. Venter, and E. R. Dabbs. 1997. Ribosylative inactivation of rifampin by Mycobacterium smegmatis is a principal contributor to its low susceptibility to this antibiotic. Antimicrob. Agents Chemother. 41:2456-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riska, P. F., and W. R. Jacobs, Jr. 1998. The use of luciferase-reporter phage for antibiotic-susceptibility testing of mycobacteria. Methods Mol. Biol. 101:431-455. [DOI] [PubMed] [Google Scholar]
- 31.Sander, P., and E. C. Bottger. 1999. Mycobacteria: genetics of resistance and implications for treatment. Chemotherapy (Basel) 45:95-108. [DOI] [PubMed] [Google Scholar]
- 32.Schweitzer, B. I., A. P. Dicker, and J. R. Bertino. 1990. Dihydrofolate reductase as a therapeutic target. FASEB J. 4:2441-2452. [DOI] [PubMed] [Google Scholar]
- 33.Shah, L. M., M. S. DeStefano, and M. H. Cynamon. 1996. Enhanced in vitro activity of WR99210 in combination with dapsone against Mycobacterium avium complex. Antimicrob. Agents Chemother. 40:2644-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah, L. M., S. C. Meyer, and M. H. Cynamon. 1996. Enhanced in vitro activity of pyrimethamine in combination with dapsone against Mycobacterium avium complex. Antimicrob. Agents Chemother. 40:2426-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoen, C. M., O. Choromanska, R. C. Reynolds, J. R. Piper, C. A. Johnson, and M. H. Cynamon. 1998. In vitro activities of several diaminomethylpyridopyrimidines against Mycobacterium avium complex. Antimicrob. Agents Chemother. 42:3315-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sibley, C. H., V. H. Brophy, S. Cheesman, K. L. Hamilton, E. G. Hankins, J. M. Wooden, and B. Kilbey. 1997. Yeast as a model system to study drugs effective against apicomplexan proteins. Methods 13:190-207. [DOI] [PubMed] [Google Scholar]
- 37.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sirotnak, F. M., J. J. Burchall, W. B. Ensminger, and J. A. Montgomery (ed.). 1984. Folate antagonists as therapeutic agents, vol. 2. Academic Press, Inc., Orlando, Fla.
- 39.Stover, C. K., P. Warrener, D. R. VanDevanter, D. R. Sherman, T. M. Arain, M. H. Langhorne, S. W. Anderson, J. A. Towell, Y. Yuan, D. N. McMurray, B. N. Kreiswirth, C. E. Barry, and W. R. Baker. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962-966. [DOI] [PubMed] [Google Scholar]
- 40.Then, R. L. 1993. History and future of antimicrobial diaminopyrimidines. J. Chemother. 5:361-368. [PubMed] [Google Scholar]
- 41.Wallace, R. J., Jr., K. Wiss, M. B. Bushby, and D. C. Hollowell. 1982. In vitro activity of trimethoprim and sulfamethoxazole against the nontuberculous mycobacteria. Rev. Infect. Dis. 4:326-331. [DOI] [PubMed] [Google Scholar]
- 42.Winstanley, P. A., E. K. Mberu, I. S. Szwandt, A. M. Breckenridge, and W. M. Watkins. 1995. In vitro activities of novel antifolate drug combinations against Plasmodium falciparum and human granulocyte CFUs. Antimicrob. Agents Chemother. 39:948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wooden, J. M., L. H. Hartwell, B. Vasquez, and C. H. Sibley. 1997. Analysis in yeast of antimalaria drugs that target the dihydrofolate reductase of Plasmodium falciparum. Mol. Biochem. Parasitol. 85:25-40. [DOI] [PubMed] [Google Scholar]
- 44. World Health Organization. 1994. Chemotherapy of leprosy. Report of a World Health Organization study group. World Health Organization, Geneva, Switzerland. [PubMed]
- 45.Zhang, Y., and D. B. Young. 1993. Molecular mechanisms of isoniazid: a drug at the front line of tuberculosis control. Trends Microbiol. 1:109-113. [DOI] [PubMed] [Google Scholar]