Male Infertility
The desire to have children is a natural instinct, yet infertility is an age-old problem that has plagued humankind since Abraham and Sarah. Infertility in humans is an issue that affects 10%–15% of couples, and approximately half of these cases of infertility are attributable to men. The categories of treatment for infertile men are separated into three groups. The first group, men with an untreatable sterility condition, comprises 12% of infertile men (Baker et al. 1986). Obvious causes are present in approximately one-third of these patients and include Klinefelter syndrome, undescended testes, testicular torsion, trauma, and exposure to cytotoxic agents or radiotherapy producing testicular atrophy (Baker 1994). Additional causes include idiopathic primary seminiferous tubule failure with appearances, on testicular biopsy specimens, of Sertoli cell only (no germ cells) or germ-cell arrest (Baker 1994). The second group, men with a potentially treatable condition, comprises 13% of infertile men (Baker et al. 1986). Potentially treatable conditions include genital tract obstructions, sperm autoimmunity, gonadotropin deficiency, coital disorders, and reversible impairment of semen quality (Baker 1994). The third group, men with an untreatable subfertility, comprises 75% of infertile men (Baker et al. 1986). These men are either oligospermic, in that they produce less than the normal number of sperm (<20 million sperm/ml; World Health Organization 1992), or asthenozoospermic, in that a high percentage of sperm is immotile. In a study presented in this issue of the Journal, Ruiz-Pesini et al. (2000) describe their finding of a likely cause of asthenozoospermia—namely, mutations in mtDNA—in some men.
The Genetics of Infertility
In recent years, three candidate genes have been implicated in genetic infertility in men. The DAZ (deleted in azoospermia) gene, which is located in a cluster on the AZFc (azoospermia factor) region of the Y chromosome, has been shown to be deleted in 10%–15% of oligospermic and azoospermic men (Reijo et al. 1995, 1996; Pryor et al. 1997). The RBM (RNA-binding motif) gene homologues, which are located throughout the Y-chromosome, are actively transcribed in the AZFb region (Ma et al. 1993; Elliott and Cooke 1997). Deletions in the AZFb region have also been found in azoospermic and oligospermic men (Ma et al. 1993; Elliott and Cooke 1997). Additional deletions encompassing DFFRY (Drosophila fat facets related Y), a gene located in the AZFa region of the Y chromosome, have also been observed in a small percentage of infertile men (Brown et al. 1998). These genes have different expression patterns: DAZ and RBM are testis specific, with transcription restricted to germ cells (Ma et al. 1993; Reijo et al. 1995), whereas DFFRY is expressed in a wide range of tissues (Brown et al. 1998). The functions of these genes remain to be determined, but evidence strongly suggests a role for both DAZ and RBM in germ-cell development in men.
Evidence that supports a role for genetics in human infertility is rare. The most concrete method to examine the genetics of infertility is to screen for a mutation(s) or deletions(s) in candidate gene(s). This method has been used successfully by Ruiz-Pesini et al. (2000 [in this issue]). They have identified mutations in mtDNA that are implicated in an asthenozoospermic phenotype.
Mitochondrial Genetics
Mutations in the mitochondria have been implicated in a variety of human diseases ranging from degenerative diseases and aging to infertility (Følgero et al. 1993; Wallace 1995). This may be because of the increase in the number of mutations in mitochondria, which is the result of several properties that make mtDNA distinct from cellular DNA. First, replication of mtDNA is rapid and lacks proofreading (Clayton et al. 1974; Fukunaga et al. 1979). This results in a mitochondrial mutation rate that is 10–100 times higher than that of nuclear DNA (Richter et al. 1988; Nagley et al. 1993). In addition, mitochondria lack an adequate DNA-repair mechanism, which can further increase the mutation rate (Clayton et al. 1974). Finally, the mode of inheritance of mitochondria is unique because the genetic information is inherited maternally. This creates an asymmetry in the natural selection process. mtDNA variations may be more prevalent because the mtDNA mutation rate is higher and because selection occurs only in females. For example, if a germline mitochondrial mutation arises that has serious effects in the male only, it could be passed with high frequency from generation to generation, since natural selection occurs only in females.
Current understanding of the role of mitochondrial mutations in human disease is expanding. Tissues that require high levels of respiratory energy, such as the brain, heart, skeletal muscle, kidney, liver, endocrine system, and other somatic tissues, can malfunction if they possess defects in their mitochondria (Johns 1995). Sperm are also included in this category. It has been proposed that the ATP produced by mitochondria is essential for sperm motility (Mitchell et al. 1976). Other evidence supports an essential role for mitochondrial formation and function in spermatogenesis (Følgero et al. 1993; Kao et al. 1995; Frank and Hurst 1996; St. John et al. 1997; Zeviani and Antozzi 1997; Ruiz-Pesini et al. 1998).
Mitochondrial Structure in Spermatozoa
The maturation of spermatogonia to a spermatozoon capable of fertilization involves rearrangement of mitochondria and development of a functional tail. Asthenozoospermia could potentially be caused by defects in tail formation in spermatozoa or by defects in the energy-producing machinery required to drive motility. In sperm, mitochondria are located around the midpiece and are arranged in a helix of 11–13 gyri (or individual spirals around the core), with two mitochondria per gyrus (Zamboni et al. 1991). In the absence of glycolytic support, ATP generated from the mitochondria is delivered to the axoneme and is used for flagellar propulsion. Reductions in motility could potentially arise from defects in any of the 200–300 separate genes that are necessary for proper assembly and function of the sperm axoneme and tail. “Primary ciliary dyskinesia” (PCD) and “immotile cilia syndrome” are terms used to designate a diverse constellation of syndromes in which ciliated structures within the body are dysfunctional (Oates 1998). Defects in spermatozoa tail formation can include a lack of inner or outer dynein arms, the absence of radial spokes, or the deletion of the central or outer doublets (arrays of microtubules composed of a complete and partial microtubule that share a common wall). Previous research has also suggested a correlation between mitochondrial volume and sperm length and flagellar beat frequency (Cardullo and Baltz 1991). Moreover, the sperm midpiece length has been shown to be significantly shorter in asthenozoospermic subjects than in normal subjects (Mundy et al. 1995). In brief, mutations in mtDNA could contribute to a large percentage of asthenozoospermia. Kao et al. (1995) identified an accumulation of a 4,977-bp deletion in the mtDNA of spermatozoa of men with infertility or subfertility. The deletion was observed in normal individuals, but the highest frequency of occurrence was in men with the lowest sperm motility. The results revealed a negative correlation between sperm motility and the proportion of 4,977 bp of deleted mtDNA.
Mitochondria Enzymatic Complexes Needed for Sperm Motility
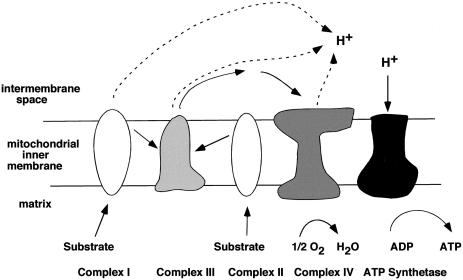
The primary goal of the study by Ruiz-Pesini et al. (2000) was to investigate whether fixed mtDNA variants have phenotypic consequences in human sperm quality. The authors first established that the motility of human spermatozoa is fully dependent on the functionality of the oxidative phosphorylation pathway. The electron-transfer chain, which produces the energy generated by mitochondria, is composed of four multimeric enzymatic complexes (complexes I–IV; fig. 1). Three of the four multimeric complexes (complexes I, III, and IV) are partially encoded by mtDNA. Ruiz-Pesini et al. used drugs that specifically inhibit the activity of these complexes, to demonstrate the progressive impairment of sperm motility and to ultimately block flagellar movement. Their observation underscores the importance of mitochondrial activity in sperm motility. It is supported by the results of studies in which inhibitors of mitochondrial energy metabolism (Ford and Harrison 1981; Halangk et al. 1985; Weinberg et al. 1995; Pascual et al. 1996) were used and the enzymatic activities of spermatozoa with normal and impaired motility were measured (Ruiz-Pesini et al. 1998).
Figure 1.
Substrates enter the respiratory chain at complex I and II and deliver electrons to complex III. Complex III delivers electrons to complex IV and reduces oxygen atoms inside the matrix to H2O. A proton-motive force is generated in the intermembrane space by coupling electron transport to proton translocation at complexes I, III, and IV. The enzyme ATP synthetase generates ATP when protons are transported down their concentration gradient into the matrix. Complexes I, III, and IV, which are crucial for generating the proton-motive force, are partially encoded by mtDNA.
mtDNA Haplogroup Associated with Asthenozoospermia
On the basis of their observation that inhibitors of mitochondrial activity reduced sperm motility, Ruiz-Pesini et al. decided to investigate the potential genetic association between mtDNA variance and sperm motility. The authors used sperm samples from 545 donors (predominantly whites) and characterized the mtDNA into known haplogroups on the basis of polymorphic sites in the mtDNA (Richard et al. 1996; Torroni et al. 1996, 1997; Brown et al. 1997; Hofmann et al. 1997a, 1997b). They then grouped the sperm samples, on the basis of sperm motility, into three groups: nonasthenozoopermic (non-AP; ⩾50% of progressive spermatozoa), moderately asthenozoospermic phenotype (MAP; ⩾25% and <50% of progressive spermatozoa), and severely asthenozoospermic phenotype (SAP; <25% of progressive spermatozoa). They analyzed the percentage of the different haplogroups in each motility group. A significant overrepresentation of haplogroup H was found in the non-AP individuals (normal sperm motility), and a significant overrepresentation of haplogroup T was found in the MAP individuals (reduced sperm motility), even though both haplogroups belong to a population of white males. The authors also observed a significant difference between the maximum swimming distance for sperm from haplogroup H and that for sperm from haplogroup T, with the distance for sperm from haplogroup H being longer. These results established a correlation between mtDNA variation and sperm motility.
Identification of Possible Cause for Type T–Associated Asthenozoospermia
Ruiz-Pesini et al. (2000) next investigated the molecular mechanism that might underlie the observed association of haplogroup-T mtDNA and reduced spermatozoa motility. This was accomplished through analysis of the enzymatic activities of the different mitochondrial complexes within each haplogroup. Of interest, two multimeric complexes (complexes I and IV) that are partially encoded by mtDNA showed a significantly lower activity when assayed from haplogroup T, compared with haplogroup H. In contrast, the mitochondrial enzymatic complex II, which is encoded only by nuclear DNA, did not show any difference in activity when compared between haplogroups H and T. Finally, to determine a possible cause for the lower mitochondrial performance seen in haplogroup T, compared with the mitochondrial performance seen in other haplogroups, the authors looked for mutations in genes encoded by the mtDNA. Several polymorphic markers in haplogroup T introduce at least two amino acid changes in the primary sequence for the enzymatic complex I gene. These mutations could explain the lower performance of complex I in the mtDNA of individuals carrying haplogroup T. In addition, Ruiz-Pesini et al. (2000) identified two mutations in the arginine and threonine tRNAs in the mtDNA of individuals carrying haplogroup T. Both tRNA mutations were previously identified (MITOMAP: A Human Mitochondrial Genome Database) and described as T-specific polymorphic sites (Macaulay et al. 1999). Further molecular analysis will be needed to determine whether any of these tRNA variants—alone or synergistically—has an influence on mitochondrial protein synthesis that could explain the lower performance of complex IV in organelles carrying haplogroup-T mtDNA (Ruiz-Pesini et al. 2000).
Impact of the Finding on Infertility
The way that we identify and characterize the cause of untreatable subfertility will be greatly impacted by the finding of Ruiz-Pesini et al. that mutations in mtDNA are associated with low sperm motility. Asthenozoospermia is not rare. It may either contribute to or be the primary cause of infertility in ⩽30% of infertile men. Mutations in mtDNA could be either the primary cause of or a secondary contributing factor to asthenozoospermia in a significant portion of these men. Because there exist 200–300 proteins (all of which are encoded by nuclear genes) that are needed for proper tail formation (Oates 1998), heterozygous mutations in these genes on a weak mitochondrial background (haplogroup T) could result in varying degrees of severity of asthenozoospermia. Thus, the results of analysis, at the morphological level, of the sperm from asthenozoospermic patients may indicate an increased percentage of nonmotile sperm, but other hidden factors could be affecting the quality of sperm from such patients.
The lack of effective treatments for defects in sperm quality and production prompts the use of assisted reproductive techniques such as IVF (in vitro fertilization) or ICSI (intracytoplasmic single-sperm injection) to treat infertile couples. There are a number of increased risks associated with the use of IVF or, more recently, ICSI. The use of sperm from oligospermic or azoospermic men for ICSI is one risk, because of the possible defects in nuclear genes required for cellular function. For example, Nudell at el. (2000) showed that the mutation rate is higher in the germline DNA, compared with the somatic (blood) DNA, of men with meiotic arrest or incomplete meiotic arrest (oligospermic). This finding suggests that oligospermic or azoospermic men may have defects in their meiosis-specific DNA-repair enzymes. The use of their sperm, which have passed the meiotic checkpoint for ICSI, may have a serious effect on the health of a child, as a result of defects in the nuclear genes required to repair DNA. If we carefully consider the implications of the use of sperm from men in the untreatable subfertility group (oligospermic and asthenozoospermic men), then possible defects in the nuclear DNA and even the mtDNA could have a combined effect on the health of a child.
The finding by Ruiz-Pesini et al. that variations in mtDNA between different haplogroups play a role in asthenozoospermia also suggests that variations in mtDNA may underlie other human characteristics. Since mutations in mtDNA accumulate sequentially along radiating female lineages, individual mtDNA lineages have diverged as women have migrated from Africa and into the various continents during the ∼150,000 years of human evolution (Wallace 1995). Each ethnic group contains population-specific polymorphisms. Haplotyping the mtDNA of different lineages may help us to understand more about human diseases or even the characteristics that might be associated with mutations in mtDNA.
It is of interest that this is the first instance of a mitochondrial mutation that has an affect on the health of men but not on that of women. Additional mitochondrial mutations that lead to male-specific diseases could be predicted on the basis of the fact that mitochondrial selection occurs only in females. This new finding by Ruiz-Pesini et al. raises new questions and issues that need to be considered in the field of infertility. Does the lower performance of the mitochondria of these men who are carrying haplogroup T predispose them to complex disorders (heart conditions, etc.) resulting from some environmental exposure later in life? Do women who harbor these same mutations have the same risk? Can we retrace, in certain ethnic groups, the origin of mtDNA mutations that cause prevalent disease? The impact of this finding gives the scientific community a new tool with which to analyze the choices used to treat human disease and to safely treat infertility in males.
Acknowledgments
We are grateful to Dr. Paul Turek, Dr. Eugene Xu, and Joyce Tung for their helpful comments and support. F.A.M. is supported by a Ford Foundation predoctoral fellowship. R.A.R.-P. is supported by the Searle Scholarship Foundation and grants from the National Institutes of Health.
Footnotes
This article represents the opinion of the authors and has not been peer reviewed.
Electronic-Database Information
The URL for data in this article is as follows:
- MITOMAP: A Human Mitochondrial Genome Database, http://www.gen.emory.edu/mitomap.html
References
- Baker H, Burger H, de Kretser D, Hudson B (1986) Relative incidence of etiologic disorders in male infertility. In: Santen RJ, Swerdloff RS (eds) Male reproductive dysfunction: diagnosis and management of hypogonadism, infertility and impotence. Marcel Dekker, New York, pp 341–372 [Google Scholar]
- Baker HW (1994) Clinical male infertility. II. Critical evaluation of the prospects for therapy. Reprod Fertil Dev 6:9–12 [DOI] [PubMed] [Google Scholar]
- Brown GM, Furlong RA, Sargent CA, Erickson RP, Longepied G, Mitchell M, Jones MH, Hargreave T, Cooke H, Affara N (1998) Characterization of the coding sequence and fine mapping of the human DFFRY gene and comparative expression analysis and mapping to the Sxrb interval of the mouse Y chromosome of the DFFRY gene. Hum Mol Genet 7:97–107 [DOI] [PubMed] [Google Scholar]
- Brown MD, Sun F, Wallace DC (1997) Clustering of Caucasian Leber hereditary optic neuropathy patients containing the 11778 or 144 mutations on an mtDNA lineage. Am J Hum Genet 60:381–387 [PMC free article] [PubMed] [Google Scholar]
- Cardullo RA, Baltz JM (1991) Metabolic regulation in mammalian sperm: mitochondrial volume determines sperm length and flagellar beat frequency. Cell Motil Cytoskeleton 19:180–188 [DOI] [PubMed] [Google Scholar]
- Clayton DA, Doda JN, Friedberg EC (1974) The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci USA 71:2777–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D, Cooke H (1997) The molecular genetics of male infertility. Bioessays 19:801–809 [DOI] [PubMed] [Google Scholar]
- Folgero T, Bertheussen K, Lindal S, Tobergsen T, Oian P (1993) Mitochondrial disease and reduced sperm motility. Hum Reprod 8:1863–1868 [DOI] [PubMed] [Google Scholar]
- Ford WC, Harrison A (1981) The role of oxidative phosphorylation in the generation of ATP in human spermatozoa. J Reprod Fertil 63:271–278 [DOI] [PubMed] [Google Scholar]
- Frank SA, Hurst LD (1996) Mitochondria and male disease. Nature 383:224 [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Yielding K (1979) Fate during cell growth of yeast mitochondrial and nuclear DNA after photolytic attachment of the monoazide analog of ethidium bromide. Biochem Biophys Res Commun 90:582–586 [DOI] [PubMed] [Google Scholar]
- Halangk W, Bohnensack R, Kunz W (1985) Interdependence of mitochondrial ATP production and extramitochondrial ATP utilization in intact spermatozoa. Biochim Biophys Acta 808:316–322 [DOI] [PubMed] [Google Scholar]
- Hofmann S, Bezold R, Jaksch M, Obermaier-Kusser B, Mertens S, Kaufhold P, Rabl W, Hecker W, Gerbitz K (1997a) Wolfram (DIDMOAD) syndrome and Leber hereditary optic neuropathy (LHON) are associated with distinct mitochondrial DNA haplotypes. Genomics 39:8–18 [DOI] [PubMed] [Google Scholar]
- Hofmann S, Jaksch M, Bezold R, Mertens S, Aholt S, Paprotta A, Gerbitz KD (1997b) Population genetics and disease susceptibility: characterization of central European haplogroups by mtDNA gene mutations, correlation with D loop variants and association with disease. Hum Mol Genet 6:1835–1846 [DOI] [PubMed] [Google Scholar]
- Johns DR (1995) Mitochondrial DNA and disease. N Engl J Med 333:638–644 [DOI] [PubMed] [Google Scholar]
- Kao SH, Chao HT, Wei YH (1995) Mitochondrial deoxyribonucleic acid 4,977-bp deletion is associated with diminished fertility and motility of human sperm. Biol Reprod 52:729–736 [DOI] [PubMed] [Google Scholar]
- Ma K, Inglis J, Sharkey A, Bickmore W, Hill R, Prosser E, Speed R, Thomson E, Jobling M, Taylor K, Wolfe J, Cooke H, Hargreave T, Chandley A (1993) A Y-chromosome gene family with RNA-binding protein homology: candidates for the azoospermia factor AZF controlling human spermatogenesis. Cell 75:1287–1295 [DOI] [PubMed] [Google Scholar]
- Macaulay V, Richards M, Hickey E, Vega E, Cruciani F, Guida V, Scozzari R, Bonne-Tamir B, Sykes B, Torroni A (1999) The emerging tree of West Eurasian mtDNAs: a synthesis of control-region sequences and RFLPs. Am J Hum Genet 64:232–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JA, Nelson L, Hafez E (1976) Motility of spermatozoa. In: Hafez ESE (ed) Human semen and fertility regulation in men. C. V. Mosby, St. Louis, pp 83–106 [Google Scholar]
- Mundy AJ, Ryder TA, Edmonds DK (1995) Asthenozoospermia and the human sperm mid-piece. Hum Reprod 10:116–119 [DOI] [PubMed] [Google Scholar]
- Nagley P, Zhang C, Martinus RD, Vaillant F, Linnane AW (1993) Mitochondrial DNA mutation and human aging: molecular biology, bioenergetics, and redox therapy. In: DiMauro S, Wallace DC (eds) Mitochondrial DNA in human pathology. Raven Press, New York, pp 137–157 [Google Scholar]
- Nudell D, Castillo M, Turek P, Reijo-Pera R (2000) Increased frequency of mutations in DNA from infertile men with meiotic arrest. Hum Reprod 15:1289–1294 [DOI] [PubMed] [Google Scholar]
- Oates R (1998) Immotile cilia syndromes. Paper presented at a conference of the American Society of Reproductive Medicine: Male Reproduction—Basic, Genetic, and Clinical Information for the Next Millennium, San Francisco, October 3–4 [Google Scholar]
- Pascual ML, Cebrian-Perez JA, Lopez-Perez MJ, Muino-Blanco T (1996) Short-term inhibition of the energy metabolism affects motility but not surface properties of sperm cells. Biosci Rep 16:35–40 [DOI] [PubMed] [Google Scholar]
- Pryor JL, Kent-First M, Muallem A, Van Bergen AH, Nolten WE, Meisner L, Roberts KP (1997) Microdeletions in the Y chromosome of infertile men [see comments]. N Engl J Med 336:534–549 [DOI] [PubMed] [Google Scholar]
- Reijo R, Alagappan RK, Patrizio P, Page DC (1996) Severe oligozoospermia resulting from deletions of azoospermia factor gene on Y chromosome [see comments]. Lancet 347:1290–1293 [DOI] [PubMed] [Google Scholar]
- Reijo R, Lee T, Salo P, Alagappan R, Brown L, Rosenberg M, Rozen S, Jaffe T, Straus D, Hovatta O, Page D (1995) Diverse spermatogenic defects in humans caused by Y-chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet 10:383–393 [DOI] [PubMed] [Google Scholar]
- Richard M, Corte-Real H, Forster P, Macaulay V, Wilkinson-Herbots H, Demaine A, Papiha S, Hedges R, Bandelt H, Sykes B (1996) Paleolithic and neolithic lineages in the European mitochondrial gene pool. Am J Hum Genet 59:185–203 [PMC free article] [PubMed] [Google Scholar]
- Richter C, Park JW, Ames BN (1988) Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci USA 85:6465–6467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Diez C, Lapeña AC, Pérez-Martos A, Montoya J, Alvarez E, Arenas J, Lopez-Perez M (1998) Correlation of sperm motility with mitochondrial enzymatic activities. Clin Chem 44:1616–1620 [PubMed] [Google Scholar]
- Ruiz-Pesini E, Lapena A, Diez-Sanchez C, Perez-Martos A, Enriquez J, Diaz M, Urries A, Montoro L, Lopez-Perez M, Enriquez J (2000) Human mitochondrial DNA haplogroups associated with high or reduced spermatozoa motility. Am J Hum Genet 67:682–696 (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John J, Cooke I, Barratt C (1997) Mitochondrial mutations and male infertility. Nat Med 3:124–125 [DOI] [PubMed] [Google Scholar]
- Torroni A, Huoponen K, Francalacci P, Petrozzi M, Morelli L, Scozzari R, Obinu D, Savontaus M, Wallace D (1996) Classification of European mtDNAs from an analysis of three European populations. Genetics 144:1835–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroni A, Petrozzi M, D'Urbano L, Sellitto D, Zeviani M, Carrara F, Carducci C, Leuzzi V, Carelli V, Barboni P, De Negri A, Scozzari R (1997) Haplotype and phylogenetic analyses suggest that one European-specific mtDNA background plays a role in the expression of Leber hereditary optic neuropathy by increasing the penetrance of the primary mutations 11778 and 14484. Am J Hum Genet 60:1107–1121 [PMC free article] [PubMed] [Google Scholar]
- Wallace DC (1995) 1994 William Allan Award Address: mitochondrial DNA variation in human evolution, degenerative disease, and aging. Am J Hum Genet 57:201–223 [PMC free article] [PubMed] [Google Scholar]
- Weinberg JB, Doty E, Bonaventura J, Haney AF (1995) Nitric oxide inhibition of human sperm motility. Fertil Steril 64:408–413 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1992) World Health Organization laboratory manual for the examination of human semen and sperm-cervical mucus interactions. Cambridge University Press, Cambridge [Google Scholar]
- Zamboni L (1991) Physiology and pathophysiology of the human spermatozoon: the role of electron microscopy. J Electron Microscopy Technique 17:412–436 [DOI] [PubMed] [Google Scholar]
- Zeviani M, Antozzi C (1997) Mitochondrial disorders. Mol Hum Reprod 3:133-148 [DOI] [PubMed] [Google Scholar]