Abstract
Germline mutations of the adenomatous polyposis coli (APC) tumor-suppressor gene result in familial adenomatous polyposis (FAP). Patients with FAP typically develop hundreds to thousands of benign colorectal tumors and early-onset colorectal cancer. A subset of germline APC mutations results in an attenuated FAP (AFAP) phenotype, in which patients develop fewer tumors and develop them at an older age. Although a genotype-phenotype correlation between the locations of APC germline mutations and the development of AFAP has been well documented, the mechanism for AFAP has not been well defined. We investigated the mechanism for AFAP in patients carrying a mutant APC allele (APCAS9) that has a mutation in the alternatively spliced region of exon 9. APCAS9 was found to down-regulate β-catenin–regulated transcription, the major tumor-suppressor function of APC, as did the wild-type APC. Mutation analysis showed that both APCAS9 and the wild-type APC alleles were somatically mutated in most colorectal tumors from these patients. Functional analysis showed that 4666insA, a common somatic mutation in APCAS9 in these tumors, did not inactivate the wild-type APC. Our results indicate that carriers of APCAS9 develop fewer colorectal tumors than do typical patients with FAP because somatic inactivation of both APC alleles is necessary for colorectal tumorigenesis. However, these patients develop colorectal tumors more frequently than does the general population because APCAS9 is inactivated by mutations that do not inactivate the wild-type APC.
Introduction
Familial adenomatous polyposis (FAP [MIM 175100]) is an autosomal dominant inherited disease that predisposes patients to colorectal cancer. Patients with FAP typically develop hundreds to thousands of colorectal adenomas in their 2d or 3d decades of life. If not surgically removed, some of these benign adenomas will progress to malignant carcinoma. FAP is caused by germline mutation of the adenomatous polyposis coli (APC) tumor-suppressor gene (Groden et al. 1991; Joslyn et al. 1991; Kinzler et al. 1991; Nishisho et al. 1991). Somatic mutations in APC also occur in most sporadic colorectal tumors (Miyoshi et al. 1992; Powell et al. 1992; Miyaki et al. 1994). Consistent with Knudson’s two-hit hypothesis that both alleles of a tumor-suppressor gene are mutated in a tumor (Knudson 1985), mutation of both APC alleles has been demonstrated in FAP-related and sporadic colorectal tumors (Miyoshi et al. 1992; Powell et al. 1992; Miyaki et al. 1994). The formation of most colorectal tumors is believed to be initiated by the mutation of both APC alleles (Kinzler and Vogelstein 1996).
APC expresses a major mRNA of ∼10.5 kb that encodes a protein of 2,843 amino acids (Groden et al. 1991; Joslyn et al. 1991; Kinzler et al. 1991). APC exon 15 contains 6.5 kb of the 3′ coding region and includes a mutation-cluster region in which most somatic mutations occur (Groden et al. 1991; Joslyn et al. 1991; Miyoshi et al. 1992; Miyaki et al. 1994). The tumor-suppressor function of APC is believed to involve the regulation of β-catenin–regulated transcription (CRT [Korinek et al. 1997; Morin et al. 1997; Polakis 1999; Peifer and Polakis 2000]). β-Catenin can associate with the Tcf/Lef family of DNA-binding proteins and can form DNA sequence–specific transcription complexes (Behrens et al. 1996; Molenaar et al. 1996; Korinek et al. 1997). APC regulates CRT by associating with β-catenin, together with glycogen synthase kinase-3β (GSK3β) and axin or conductin, and promoting its degradation (Munemitsu et al. 1995; Rubinfeld et al. 1996; Behrens et al. 1998; Hart et al. 1998; Polakis 1999; Peifer and Polakis 2000). The identification of human and rat colorectal tumors that do not have inactivating mutations in APC but instead express β-catenin mutants resistant to APC-mediated regulation strongly supports the hypothesis that APC exerts its tumor-suppressor function by regulation of CRT (Morin et al. 1997; Dashwood et al. 1998; Sparks et al. 1998; Takahashi et al. 1998).
In addition to the major mRNA, APC also expresses several minor alternatively spliced mRNA (Groden et al. 1991; Samowitz et al. 1995). One alternatively spliced APC mRNA, APCdA9, lacks 303 nucleotides of the alternatively spliced region of exon 9 (Groden et al. 1991). The APCdA9 protein encoded by APCdA9 contains all domains known to be necessary for regulation of CRT (Groden et al. 1991; Munemitsu et al. 1995), although this function could be impaired by altered three-dimensional structure.
Patients with a form of FAP called “attenuated FAP” (AFAP) develop fewer colorectal tumors, and at older ages, than do patients with typical FAP (Lynch et al. 1992, 1995). Mounting evidence indicates that individuals carrying an APCAFAP allele usually develop AFAP (Spirio et al. 1993; van der Luijt et al. 1995, 1996; Friedl et al. 1996; Soravia et al. 1998), although there could be significant variation in the phenotype of individuals carrying the same germline mutation (Brensinger et al. 1998; Soravia et al. 1998). APCAFAP includes APC alleles with a germline mutation in the first four coding exons (APC1–4), in the alternatively spliced region of exon 9 (APCAS9), or in the 3′ half of the coding region (APC3H; for descriptions of alleles, see the Appendix).
There are two alternative genetic explanations for how patients carrying APCAFAP develop AFAP, according to Knudson’s two-hit model. One is that APCAFAP does not have tumor-suppressor activity and that a single somatic mutation inactivating the wild-type APC allele will lead to colorectal tumorigenesis. This is similar to what happens in patients with FAP and could explain why patients with AFAP are susceptible to development of colorectal tumors. However, for patients with AFAP to have the attenuated phenotype, inactivation of the wild-type APC allele must either occur less frequently than in typical FAP or not be sufficient for tumorigenesis. Alternatively, the APCAFAP alleles still have tumor-suppressor activity, and somatic inactivation of both APC alleles is necessary for tumor formation. This resembles the development of sporadic colorectal tumors in people without a germline APC mutation and could explain why patients with AFAP have the attenuated phenotype. However, APC must be inactivated more readily in patients with AFAP than in the general population, because patients with AFAP are still predisposed to colorectal tumorigenesis.
A study of colorectal tumors from patients carrying APC1–4 demonstrated that some of these tumors had two somatic mutations (Spirio et al. 1998). A specific APC mutation, 4666insA, was found frequently in these tumors and was found to occur at the APC1–4 allele. It was proposed that, for tumors to form in these patients, the wild-type APC allele must be somatically inactivated first and that then the germline mutant allele must be somatically inactivated by either small mutations or loss of heterozygosity (LOH). However, it remained unclear why these patients were predisposed to colorectal tumorigenesis. The present report describes our investigation of the underlying mechanism of AFAP in patients carrying APCAS9.
Patients, Material, and Methods
Patients and Tumor Samples
The two patients were from a family carrying a germline nonsense mutation at APC codon 332 (CGA→TGA; data not shown). Patient 1 (a 47-year-old male) had 30 colorectal adenomas <2 mm and a history of 2 1-cm adenomas that had been previously excised. Patient 2 (a 15-year-old male) had three colorectal adenomas <1 mm. Neither patient had a history of cancer or surgery. Informed consent for collection of tumor samples was obtained from patients, and the research was approved by the M.D. Anderson Cancer Center's institutional review board.
The adenomas were separated from adjacent normal tissues by microdissection of fresh biopsy samples. Single crypts were isolated from fresh adenoma biopsies by a modification of a method described elsewhere (Cheng et al. 1984). In brief, fresh mucosal biopsy samples were incubated at 37°C for 10 min in Ca2+- and Mg2+-free Hanks' balanced salt solution containing 30 mM EDTA, then were vibrated gently to separate the epithelium from the stroma. The isolated crypts were then incubated in a buffer containing CaCl2 and MgCl2, to restore normal chromatin structure and crypt architecture. Finally, the crypts were fixed first in 10% buffered formalin for 2 h, then in 70% ethanol overnight. Confocal microscopy of propidium iodide–stained single crypts confirmed the complete isolation of the epithelial crypt cells from the surrounding mucosa.
Mutation Detection
Genomic DNA was isolated from individual crypts by proteinase K digestion, followed by phenol-chloroform extraction and ethanol precipitation. Mutations of APC were detected using the in vitro synthesized-protein assay (IVSP), followed by DNA sequencing, as described elsewhere (Powell et al. 1993; Su et al. 2000). All tumors were analyzed for codons 686–1686, and tumors with only one somatic mutation in this region were then analyzed for codons 1554–2843. Templates for IVSP were generated by nested PCR. Primers used for the first-step PCR were 5′-CAAATCCTAAGAGAGAACAAC-3′ and 5′-GACGCAGATGCTTGCTGG-3′ for codons 686–1686 and 5′-GATTTTCTTGTTCATCCAGCC-3′ and 5′-GAGTGGATCCCAAAATAAGACC-3′ for codons 1554–2843. PCR was performed using the Expand Long Template PCR System (Boehringer-Mannheim). One tenth of the genomic DNA obtained from a single crypt was used in the first-step PCR. The reaction consisted of 1 cycle of 2 min at 92°C; 20 cycles each of 15 s at 92°C, 1 min at 55°C, and 2.5 min at 68°C; and 1 cycle of 5 min at 68°C. One microliter of the first-step amplification product was used in the second-step PCR. The PCR and the coupled in vitro transcription and translation reactions were performed as described elsewhere (Su et al. 2000).
Plasmids
The reporter plasmids for assessment of CRT, pTOPFLASH and pFOPFLASH were gifts from Dr. H. Clevers (University Hospital, the Netherlands) and carry the luciferase gene regulated by wild-type and mutant T cell factor–binding motifs, respectively (Korinek et al. 1997). The control reporter pcDNA3Luc was generated by insertiing the coding region of luciferase downstream of the cytomegaloviral early promoter in the plasmid pcDNA3 and was a gift from Drs. M.-C. Hung and D.-H. Yan (M.D. Anderson Cancer Center). The plasmid pBI-MCS-EGFP (Yu et al. 1999) and plasmids containing the wild-type APC and APC1309 and were gifts from Drs. K.W. Kinzler and B. Vogelstein (Johns Hopkins University). The plasmid pUHD15-1 (Gossen and Bujard 1992) was provided by Dr. H. Bujard (Universität Heidelberg), and pRL-TK was purchased from Promega.
APCdA9 was generated by replacement of a fragment of the full-length APC cDNA, containing the entire exon 9, by a corresponding fragment that did not have the alternatively spliced region of exon 9 obtained by reverse transcription–PCR. APC1556 was generated by replacement of a fragment of wild-type APC containing codon 1556 by a corresponding PCR fragment containing an A inserted at codon 1556. Fragments that replaced the wild-type APC were completely sequenced, to ensure that there were no unintended sequence alterations. APCdA,1556 was generated by replacement of the 5′ 2.2-kb BamHI-StuI fragment of APC1556 by the corresponding fragment from APCdA9.
Three different expression vectors, pCMV-NEO-BAM (Baker et al. 1990), pCIN, and pTBI, were used to express APC. To generate pCIN, the SalI-BamHI fragment containing rabbit β-globin intron 2 and part of its flanking exons was isolated from pCMV-NEO-BAM, the SalI site was filled in, and the fragment was inserted between the BamHI and the filled-in HindIII site of pcDNA3 (Invitrogen). To generate pTBI, the HindIII-XhoI fragment containing β-globin intron was isolated from pCIN, the HindIII site was filled in, and the fragment was inserted between the PvuII and XhoI sites of pBI-MCS-EGFP. APC cDNAs were inserted at the BamHI site of pCIN and pCMV-NEO-BAM and at the BglII site of pTBI.
Cell Lines
All cell lines were obtained from American Type Culture Collection. MCF7 is a human breast cancer cell line. SW480, DLD-1, and HCT116 are human colon cancer cell lines. SW480 and DLD-1 express the wild-type β-catenin that can be regulated by APC, whereas HCT116 expresses a mutant β-catenin that is resistant to APC regulation (Morin et al. 1997).
Results
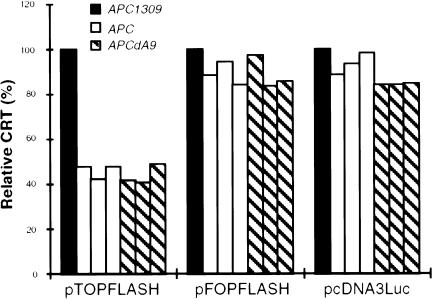
We first determined whether APCdA9 had tumor-suppressor activity. The alternatively spliced APCdA9 transcript expressed from APCAS9 does not contain the germline mutation and is expected to express the same APCdA9 protein that is expressed from the wild-type APC. Thus, APCAS9 would have tumor-suppressor activity if APCdA9 has tumor-suppressor activity. We compared the ability of APCdA9 to regulate CRT with that of the wild-type APC and of the mutant APC1309, a common APC mutant deficient in regulation of CRT (Nagase and Nakamura 1993; Miyaki et al. 1994; Morin et al. 1997). As it did in previous reports of the wild-type APC (Korinek et al. 1997; Morin et al. 1997), APCdA9 reduced the expression of a reporter controlled by CRT (pTOPFLASH) but did not significantly affect the expression of reporters that were not controlled by CRT (pcDNA3Luc and pFOPFLASH; fig. 1). These results suggested that APCAS9 had tumor-suppressor activity. However, because of the normally low expression level of APCdA9 (Groden et al. 1991), it was not clear whether APCAS9 needed to be somatically mutated for colorectal tumors to form in APCAS9 carriers.
Figure 1 .
Regulation of CRT by APCdA9. SW480 cells were transfected with the combination of pCMVβgal, a reporter plasmid (pTOPFLASH, pFOPFLASH, or pcDNA3Luc), and an APC expression vector, as indicated. Results of three separate experiments using pCIN to express APC are shown. The activities of the wild-type APC and APCdA9 in the regulation of CRT are compared with that of APC1309. Similar results were obtained using pCMV-NEO-BAM to express APC (data not shown).
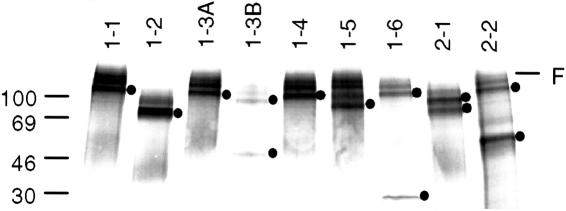
We next examined whether APCAS9 was somatically mutated in colorectal tumors from patients carrying APCAS9. Nine early colorectal adenomas in two patients from a family carrying APCAS9 were examined for somatic mutations in APC exon 15. Single crypts were isolated from each tumor, and genomic DNA was prepared from each crypt, for use in the analysis. All nine tumors had somatic mutations, including one that had LOH at the APC locus, and five of them had somatic mutations in both APC alleles (fig. 2 and table 1). DNA sequencing of APC exon 9 of the tumor having loss of one APC allele showed that the allele lost was APCAS9 (data not shown). Four of these nine tumors had the 4666insA mutation (table 1). To determine which APC allele had 4666insA, a DNA fragment containing this mutation and two intragenic polymorphic nucleotides, 4479 and 5034 (Nagase et al. 1992; Powell et al. 1992), was amplified by PCR, cloned, and sequenced. The allelotype of the wild-type APC allele, with regard to these two polymorphisms, was determined by sequencing of a similar PCR product amplified using DNA from the tumor that had lost the APCAS9 allele. The results showed that 4666insA occurred in APCAS9 in all three tumors from patient 1 (data not shown). The allele carrying this mutation in the tumor from patient 2 could not be determined because he was homozygous for these two polymorphic nucleotides. The allele carrying 4616delAG in a tumor from patient 1 also was found to be APCAS9 (data not shown). Therefore, somatic mutation of the APCAS9 allele could be demonstrated in eight of the nine tumors examined, including three of the four tumors that had only one somatic APC mutation identified.
Figure 2 .
Detection of somatic APC mutation. The result of IVSP analysis of codons 686–1686 of a representative single crypt from each tumor is shown. The use of single isolated crypts in this assay allowed the easy detection of LOH in tumor 1-2. “F” indicates the position of the full-length product presented in tumors 1-1, 1-3A, 1-4, and 1-5. Dots indicate mutant products. The positions of the molecular-weight standards are shown on the left.
Table 1.
Somatic APC Mutations
|
No. of crypts |
||||
| Patienttumor | Somatic Mutation(s) | Codon(s) Affected | Examined | WithMutation |
| 1-1 | 4666insAa | 1556 | 5 | 5 |
| 1-2 | 3896del11, LOHb | 1298–1301 | 11 | 11 |
| 1-3A | 4666insAa | 1556 | 12c | 4 |
| 1-3B | 3158delA, 4392delAG | 1053, 1465–1466 | 12c | 8 |
| 1-4 | 4616delAGa | 1539–1540 | 5 | 3d |
| 1-5 | 4216delAG | 1406 | 5 | 5 |
| 1-6 | 2562delGA, 4666insAa | 855, 1556 | 5 | 5 |
| 2-1 | 3925del5, R1450X | 1309–1310, 1450 | 7 | 6e |
| 2-2 | R1114X, 4666insA | 1114, 1556 | 7 | 6d |
In APCAS9 allele.
Lost allele was the APCAS9 allele.
Isolated from what appeared to be one adenoma. The different mutation patterns suggest that either the adenoma was heterogeneous or two adjacent adenomas were confluent.
Remaining crypts did not have mutations.
Remaining crypt had only 3925del5.
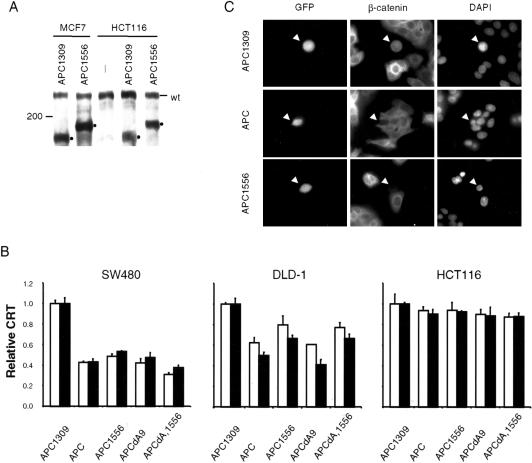
The high frequency of the 4666insA mutation in these tumors—and, specifically, in the APCAS9 allele—suggested that, although this mutation could inactivate APCAS9, it might not significantly affect the function of wild-type APC. To test this hypothesis, the 4666insA mutation was introduced into APC and APCdA9, to generate APC1556 and APCdA,1556, respectively. Immunoblot analysis confirmed that this mutation resulted in the truncation of APC protein (fig. 3A). The ability of these forms of APC to down-regulate CRT was then examined. As expected, APC and APCdA9 effectively regulate CRT in both SW480 and DLD-1 cells but not in HCT116 cells (fig. 3B). The 4666insA mutation did not appear to affect the activity of APC and APCdA9 in SW480 cells. This mutation reduced, but did not abolish, their activity in DLD-1 cells. Consistent with its ability to regulate CRT, APC1556 was able to reduce the β-catenin–protein level in SW480 cells (fig. 3C). These results showed that the 4666insA mutation did not abolish the function of APC or APCdA9.
Figure 3 .
Regulation of β-catenin by APC1556. A, Expression of APC1556. MCF7 and HCT116 cell lines were transfected with pCIN alone (—) or pCIN expressing the indicated mutant APC. APC proteins were detected by immunoblotting. “wt” indicates the endogenous wild-type APC. Dots indicate mutant APC proteins. B, Regulation of CRT by APC1556. SW480, DLD-1, and HCT116 cell lines were transfected with a combination of pRL-TK, pTOPFLASH, and an APC expression vector, as indicated. The unblackened and blackened bars indicate expression of APC by pCIN and by pCMV-NEO-BAM, respectively. The mean and SD of the result of triplicate experiments are shown. The activities of the wild-type APC, APC1556, APCdA9, and APCdA,1556 in the regulation of CRT are compared with that of APC1309. (C) Reduction of β-catenin by APC1556. SW480 cells were transfected with pUHD15-1 and pTBI, expressing indicated APC. Cells were immunostained with a monoclonal antibody against β-catenin. Transfected cells were identified by their expression of green fluorescence protein (GFP), because pTBI also expressed GFP. Nuclei were revealed by staining with 4,6-diamidino-2-phenylindole (DAPI). Arrowheads indicate transfected cells.
Discussion
We have provided evidence that APCAS9 has tumor-suppressor activity (i.e., regulation of CRT) and that it is somatically mutated in colorectal tumors in APCAS9 carriers. Somatic mutations at the APCAS9 allele were identified in eight of the nine tumors examined. This finding supports the premise that all nine tumors had somatic mutations in both APC alleles, with some of these mutations probably in APC regions that we did not examine. The absence of the wild-type IVSP product in those single crypts having two somatic mutations strongly supports the possibility that both somatic mutations occurred in the same cell (fig. 2). Because all tumors examined were <2 mm in diameter, somatic inactivation of both APC alleles was most likely to be necessary prior to colorectal tumorigenesis in these patients. This would explain why patients carrying APCAS9 develop fewer tumors, and at older ages, than do patients with typical FAP.
Somatic mutations of both the wild-type and the germline mutant APC alleles was reported for some colorectal tumors from patients with AFAP who carry APC1–4 (Spirio et al. 1998). However, the rate of double somatic mutations is significantly higher in tumors with APCAS9, which we examined, than in those with APC1–4, which have been reported elsewhere (5/9 vs. 7/35 [Spirio et al. 1998]; P<.05, Fisher’s exact test). The proportion of tumors having LOH as one of the double somatic mutations is significantly lower in tumors with APCAS9 than that reported in tumors with APC1–4 (1/5 vs. 6/7 [Spirio et al. 1998]; P<.05), although the overall rates of LOH in these two groups of tumors are comparable (1/9 vs. 8/66 [Spirio et al. 1998]; P=.7) and are similar to that reported for FAP-associated adenomas (Miyaki et al. 1994). These differences could reflect the biological differences between these two mutant APC alleles. The difference also may be partly due to the greater sensitivity of our method, which analyzes single crypts, for detection of mutations. A drawback of both studies is that each examined tumors from patients in a single family. Additional investigations of tumors from other families should provide more-conclusive information.
Patients with AFAP who carry APCAS9 are predisposed to colorectal tumorigenesis despite their attenuated phenotype. Therefore, the APC gene in these patients must be somatically mutated more easily than it is in the general population. One possibility is that germline mutations increase the mutation rate of APCAS9. Increased somatic mutation frequency has been demonstrated in the APCI1307K allele, and this may explain why APCI1307K carriers have an increased risk of colon cancer (Laken et al. 1997; Gryfe et al. 1998, 1999). Although the possibility that APCAS9 alleles have an increased mutation rate cannot be completely ruled out, we do not have evidence for it. Instead, we propose that APCAS9 alleles are inactivated more easily because they can be inactivated by mutations that do not inactivate wild-type APC. The 4666insA mutation is common in tumors from patients with AFAP who carry either APCAS9 or APC1–4 (Spirio et al. 1998; present study). In contrast, this mutation was rarely found in sporadic colorectal tumors or tumors from patients with typical FAP (Powell et al. 1992; Miyaki et al. 1994). Although the 4666insA mutation occurs at a mononucleotide repeat, it is not caused by increased microsatellite instability in tumors from patients with AFAP (Spirio et al. 1998). We suggest that the 4666insA mutation may occur frequently because it arises within a stretch of six As and that it is rarely found in non–AFAP-associated colorectal tumors because it only slightly impairs the tumor-suppressor function of the wild-type APC. However, this apparently weak mutation may be able to inactivate the residual activity of APCAS9 because of the normally low level of APCdA9 (fig. 4). Additional studies of tumors from patients with AFAP who carry different germline APC mutations will be needed to provide additional support for this hypothesis.
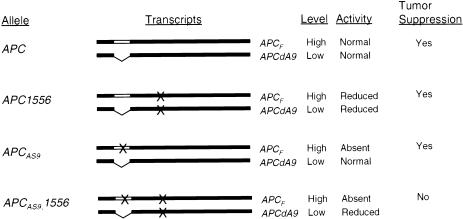
Figure 4 .
Inactivation of APCAS9, but not wild-type APC, by the 4666insA mutation. “APCF” indicates the full-length APC mRNA. “×” indicates mutations. “Tumor Suppression” indicates whether each APC allele can function as a tumor suppressor, which is determined by the combined activities of transcripts of each allele. The mutation 4666insA slightly reduces the activity of proteins encoded by both APCF and APCdA9 and does not abolish the tumor-suppressor function of APC. APCAS9 does not express full-length APC protein, because its APCF expresses a severely truncated protein owing to the mutation in the alternatively spliced region of exon 9. However, APCAS9 expresses APCdA9 identical to that expressed by the wild-type APC and has reduced but sufficient tumor-suppressor activity. Further decrease of the reduced tumor-suppressor activity of APCAS9 by the 4666insA mutation causes APCAS9 to have insufficient tumor-suppressor activity.
APC1556 was found to down-regulate CRT, although it lacks any conductin-binding domains and therefore is unlikely to interact with axin or conductin directly (Behrens et al. 1998). However, APC1556 contains all three 15-amino-acid– and three of seven 20-amino-acid–repeat sequences that interact with β-catenin (Su et al. 1993; Munemitsu et al. 1995). Because axin binds β-catenin and GSK3β directly (Behrens et al. 1998; Hart et al. 1998), APC1556 could associate with β-catenin/axin/GSK3β complex and promote the degradation of β-catenin, albeit less efficiently than do APC proteins that can bind axin directly. This hypothesis is consistent with results of a study comparing the CRT activity in mouse embryonic stem cells expressing different Apc mutants (Smits et al. 1999).
Other mutations downstream of codon 1500 that occur less frequently than the 4666insA mutation also may differentially affect the wild-type APC and APCAS9. The finding that 4616delAG also occurred on the APCAS9 allele supports this hypothesis. It is also supported by observations that somatic mutations of APC that are downstream of codon 1500 are rare in colorectal tumors (Nagase et al. 1992; Powell et al. 1992; Miyaki et al. 1994). Moreover, patients carrying germline APC mutations downstream of codon 1500, including those in a family reported to carry the 4666insA mutation, usually develop AFAP (Friedl et al. 1996; van der Luijt et al. 1996; Walon et al. 1997; Soravia et al. 1998). Future studies of derivatives of human colon cancer cell lines that can be induced to express different forms of APC could further clarify the tumor-suppression function of these APC variants.
These findings provide a model for AFAP in patients carrying APCAS9. We propose that these patients develop fewer tumors, and at older ages, than patients with typical FAP because, although patients with AFAP already have a germline APC mutation, somatic mutation of both APC alleles is necessary prior to tumorigenesis. Nevertheless, patients with AFAP are still predisposed to colorectal tumorigenesis, because APCAS9 is more easily inactivated than is wild-type APC, by mutations that do not significantly affect the tumor-suppressor function of the wild-type APC (fig. 4). This model for AFAP caused by APCAS9 also may explain AFAP caused by APC1–4. Alternative splicing of the first four coding exons of APC has been demonstrated, and the 4666insA somatic mutation has been found in the APC1–4 allele in tumors (Samowitz et al. 1995; Spirio et al. 1998). However, our model does not readily explain AFAP caused by APC3H. Although somatic inactivation of APC3H is probably required for tumor formation, mutation such as 4666insA is unlikely to inactivate APC3H. Most reported APC3H alleles have germline mutations downstream of codon 1556. Therefore, a somatic 4666insA mutation in these alleles will result in APC1556, which still has tumor-suppressor activity. Thus, the data support a model requiring somatic inactivation of both APC alleles, for tumor formation in patients with AFAP, although the mechanism for the increased somatic inactivation of alleles with germline mutation in different regions of APC may be different.
Acknowledgments
We thank the patients for participating in this study. We thank Drs. Hermann Bujard, Hans Clevers, Mien-Chie Hung, Kenneth W. Kinzler, Bert Vogelstein, and Duen-Hwa Yan for providing various plasmids. Dr. Shih-Jen Hwang is acknowledged for assistance in the statistical analysis. We thank Drs. Stanley R. Hamilton and Walter N. Hittelman for critical review and Dr. Maureen E. Goode for editorial review of the manuscript. This research was supported in part by The Gillson Longenbaugh Foundation, a Physicians Referral Service grant from The University of Texas M. D. Anderson Cancer Center, and National Cancer Institute grant CA70371, contract N01-CN-65118-MAO, and Cancer Center Core Grant CA16672.
Appendix
Summary of APC Alleles, mRNA, and cDNA Described in this Paper
| APC1–4 | APC alleles that carry germline mutations in the first four coding exons |
| APCAS9 | APC alleles that carry germline mutations in the alternatively spliced region of exon 9 |
| APC3H | APC alleles that carry germline mutations in the 3′ half of the coding region |
| APCAFAP | APC alleles that usually cause AFAP, including APC1–4, APCAS9, and APC3H |
| APC1556 | APC allele or cDNA that carries the 4666insA mutation |
| APCAS9,1556 | APC alleles that carry germline mutations in the alternatively spliced region of exon 9 and the 4666insA mutation |
| APCdA9 | APC mRNA or cDNA that does not have the alternatively spliced region of exon 9 |
| APCdA,1556 | APC mRNA or cDNA that does not have the alternatively spliced region of exon 9 and carries the 4666insA mutation |
Electronic-Database Information
The accession number and URL for data in this article is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www3.ncbi.nlm.nih.gov/Omim/ (for APC/FAP [MIM 175100])
References
- Baker SJ, Markowitz S, Fearon ER, Willson JK, Vogelstein B (1990) Suppression of human colorectal carcinoma cell growth by wild-type p53. Science 249:912–915 [DOI] [PubMed] [Google Scholar]
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W (1998) Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science 280:596–599 [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W (1996) Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382:638–642 [DOI] [PubMed] [Google Scholar]
- Brensinger JD, Laken SJ, Luce MC, Powell SM, Vance GH, Ahnen DJ, Petersen GM, Hamilton SR, Giardiello FM (1998) Variable phenotype of familial adenomatous polyposis in pedigrees with 3′ mutation in the APC gene. Gut 43:548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Bjerknes M, Amar J (1984) Methods for the determination of epithelial cell kinetic parameters of human colonic epithelium isolated from surgical and biopsy specimens. Gastroenterology 86:78–85 [PubMed] [Google Scholar]
- Dashwood RH, Suzui M, Nakagama H, Sugimura T, Nagao M (1998) High frequency of β-catenin (Ctnnb1) mutations in the colon tumors induced by two heterocyclic amines in the F344 rat. Cancer Res 58:1127–1129 [PubMed] [Google Scholar]
- Friedl W, Meuschel S, Caspari R, Lamberti C, Krieger S, Sengteller M, Propping P (1996) Attenuated familial adenomatous polyposis due to a mutation in the 3′ part of the APC gene. A clue for understanding the function of the APC protein. Hum Genet 97:579–584 [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 89:5547–5551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, Burt R, Hughes JP, Warrington J, McPherson J, Wasmuth J, Le Paslier D, Abderrahim H, Cohen D, Leppert M, White R (1991) Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66:589–600 [DOI] [PubMed] [Google Scholar]
- Gryfe R, Di Nicola N, Gallinger S, Redston M (1998) Somatic instability of the APC I1307K allele in colorectal neoplasia. Cancer Res 58:4040–4043 [PubMed] [Google Scholar]
- Gryfe R, Di Nicola N, Lal G, Gallinger S, Redston M (1999) Inherited colorectal polyposis and cancer risk of the APC I1307K polymorphism. Am J Hum Genet 64:378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P (1998) Downregulation of β-catenin by human axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr Biol 8:573–581 [DOI] [PubMed] [Google Scholar]
- Joslyn G, Carlson M, Thliveris A, Albertsen H, Gelbert L, Samowitz W, Groden J, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, Burt R, Hughes JP, Warrington J, McPherson J, Wasmuth J, Le Paslier D, Abderrahim H, Cohen D, Leppert M, White R (1991) Identification of deletion mutations and three new genes at the familial polyposis locus. Cell 66:601–613 [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Su L-K, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D, Finniear R, Markham A, Groffen J, Boguski MS, Altschul SF, Horii A, Ando H, Miyoshi Y, Miki Y, Nishisho I, Nakamura Y (1991) Identification of FAP locus genes from chromosome 5q21. Science 253:661–665 [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87:159–170 [DOI] [PubMed] [Google Scholar]
- Knudson AG Jr (1985) Hereditary cancer, oncogenes, and antioncogenes. Cancer Res 45:1437–1443 [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a β-catenin–Tcf complex in APC−/− colon carcinoma. Science 275:1784–1787 [DOI] [PubMed] [Google Scholar]
- Laken SJ, Petersen GM, Gruber SB, Oddoux C, Ostrer H, Giardiello FM, Hamilton SR, Hampel H, Markowitz A, Klimstra D, Jhanwar S, Winawer S, Offit K, Luce MC, Kinzler KW, Vogelstein B (1997) Familial colorectal cancer in Ashkenazim due to a hypermutable tract in APC. Nat Genet 17:79–83 [DOI] [PubMed] [Google Scholar]
- Lynch HT, Smyrk T, McGinn T, Lanspa S, Cavalieri J, Lynch J, Slominski-Castor S, Cayouette MC, Priluck I, Luce MC (1995) Attenuated familial adenomatous polyposis (AFAP). A phenotypically and genotypically distinctive variant of FAP. Cancer 76:2427–2433 [DOI] [PubMed] [Google Scholar]
- Lynch HT, Smyrk TC, Watson P, Lanspa SJ, Lynch PM, Jenkins JX, Rouse J, Cavalieri J, Howard L, Lynch J (1992) Hereditary flat adenoma syndrome: a variant of familial adenomatous polyposis? Dis Colon Rectum 35:411–421 [DOI] [PubMed] [Google Scholar]
- Miyaki M, Konishi M, Kikuchi-Yanoshita R, Enomoto M, Igari T, Tanaka K, Muraoka M, Takahashi H, Amada Y, Fukayama M, Maeda Y, Iwama T, Mishima Y, Mori T, Koike M (1994) Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res 54:3011–3020 [PubMed] [Google Scholar]
- Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y (1992) Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet 1:229–233 [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H (1996) XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86:391–399 [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW (1997) Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 275:1787–1790 [DOI] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P (1995) Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA 92:3046–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Miyoshi Y, Horii A, Aoki T, Petersen GM, Vogelstein B, Maher E, Ogawa M, Maruyama M, Utsunomiya J, Baba S, Nakamura Y (1992) Screening for germ-line mutations in familial adenomatous polyposis patients: 61 new patients and a summary of 150 unrelated patients. Hum Mutat 1:467–473 [DOI] [PubMed] [Google Scholar]
- Nagase H, Nakamura Y (1993) Mutations of the APC (adenomatous polyposis coli) gene. Hum Mutat 2:425–434 [DOI] [PubMed] [Google Scholar]
- Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P, Markham A, Kruch AJ, Petersen G, Hamilton SR, Nilbert MC, Levy DB, Bryan TM, Preisinger AC, Smith KJ, Su L-K, Kinzler KW, Vogelstein B (1991) Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 253:665–669 [DOI] [PubMed] [Google Scholar]
- Peifer M, Polakis P (2000) Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 287:1606–1609 [DOI] [PubMed] [Google Scholar]
- Polakis P (1999) The oncogenic activation of β-catenin. Curr Opin Genet Dev 9:15–21 [DOI] [PubMed] [Google Scholar]
- Powell SM, Petersen GM, Krush AJ, Booker S, Jen J, Giardiello FM, Hamilton SR, Vogelstein B, Kinzler KW (1993) Molecular diagnosis of familial adenomatous polyposis. N Engl J Med 329:1982–1987 [DOI] [PubMed] [Google Scholar]
- Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW (1992) APC mutations occur early during colorectal tumorigenesis. Nature 359:235–237 [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P (1996) Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 272:1023–1026 [DOI] [PubMed] [Google Scholar]
- Samowitz WS, Thliveris A, Spirio LN, White R (1995) Alternatively spliced adenomatous polyposis coli (APC) gene transcripts that delete exons mutated in attenuated APC. Cancer Res 55:3732–3734 [PubMed] [Google Scholar]
- Smits R, Kielman MF, Breukel C, Zurcher C, Neufeld K, Jagmohan-Changur S, Hofland N, van Dijk J, White R, Edelmann W, Kucherlapati R, Khan PM, Fodde R (1999) Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev 13:1309–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soravia C, Berk T, Madlensky L, Mitri A, Cheng H, Gallinger S, Cohen Z, Bapat B (1998) Genotype-phenotype correlations in attenuated adenomatous polyposis coli. Am J Hum Genet 62:1290–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks AB, Morin PJ, Vogelstein B, Kinzler KW (1998) Mutational analysis of the APC/β-catenin/Tcf pathway in colorectal cancer. Cancer Res 58:1130–1134 [PubMed] [Google Scholar]
- Spirio L, Olschwang S, Groden J, Robertson M, Samowitz W, Joslyn G, Gelbert L, Thliveris A, Carlson M, Otterud B, Lynch H, Watson P, Lynch P, Laurent-Puig P, Burt R, Hughes JP, Thomas G, Leppert M, White R (1993) Alleles of the APC gene: an attenuated form of familial polyposis. Cell 75:951–957 [DOI] [PubMed] [Google Scholar]
- Spirio LN, Samowitz W, Robertson J, Robertson M, Burt RW, Leppert M, White R (1998) Alleles of APC modulate the frequency and classes of mutations that lead to colon polyps. Nat Genet 20:385–388 [DOI] [PubMed] [Google Scholar]
- Su L-K, Steinbach G, Sawyer JC, Hindi M, Ward PA, Lynch PM (2000) Genomic rearrangements of the APC tumor-suppressor gene in familial adenomatous polyposis. Hum Genet 106:101–107 [DOI] [PubMed] [Google Scholar]
- Su L-K, Vogelstein B, Kinzler KW (1993) Association of the APC tumor suppressor protein with catenins. Science 262:1734–1737 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Fukuda K, Sugimura T, Wakabayashi K (1998) β-catenin is frequently mutated and demonstrates altered cellular location in azoxymethane-induced rat colon tumors. Cancer Res 58:42–46 [PubMed] [Google Scholar]
- van der Luijt RB, Meera Khan P, Vasen HF, Breukel C, Tops CM, Scott RJ, Fodde R (1996) Germline mutations in the 3′ part of APC exon 15 do not result in truncated proteins and are associated with attenuated adenomatous polyposis coli. Hum Genet 98:727–734 [DOI] [PubMed] [Google Scholar]
- van der Luijt RB, Vasen HF, Tops CM, Breukel C, Fodde R, Meera Khan P (1995) APC mutation in the alternatively spliced region of exon 9 associated with late onset familial adenomatous polyposis. Hum Genet 96:705–710 [DOI] [PubMed] [Google Scholar]
- Walon C, Kartheuser A, Michils G, Smaers M, Lannoy N, Ngounou P, Mertens G, Verellen-Dumoulin C (1997) Novel germline mutations in the APC gene and their phenotypic spectrum in familial adenomatous polyposis kindreds. Hum Genet 100:601–605 [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Rago C, Kinzler KW, Vogelstein B (1999) Identification and classification of p53-regulated genes. Proc Natl Acad Sci USA 96:14517–14522 [DOI] [PMC free article] [PubMed] [Google Scholar]