Abstract
Linkage studies were performed in a large family with an autosomal dominant phenotype characterized by nephropathy and hypertension. In this family of Iraqi Jewish origin, the nephropathy develops into progressive renal failure. By performing a genomewide linkage search, we localized the disease gene to chromosome 1q21; the highest LOD score was obtained for the marker at locus D1S305, which yielded a maximum LOD score of 4.71 at a recombination fraction of 0. Recombination mapping defined an interval of ∼11.6 cM, between the markers at loci D1S2696 and D1S2635, that contains the disease gene. Localization of the disease-causing gene in this family represents a necessary step toward isolation of the defective gene and toward a deeper understanding of the mechanisms of hypertension and progressive renal failure.
Introduction
Several distinct clinical phenotypes have been described that lead to autosomal dominantly inherited end-stage renal failure. Examples include Alport syndrome (Jefferson et al. 1997) and non-Alport nephritis (Ben-Ishay et al. 1967; Dockhorn 1967; Albert et al. 1969; Gauthier et al. 1989), nephronophthisis/medullary cystic kidney disease (Goldman et al. 1966), interstitial nephritis (Richmond et al. 1981), adult polycystic kidney disease, and various inherited glomerulopathies (Faubert and Porush 1997). Genetic loci for many of these conditions have been defined (Christodoulou et al. 1998; Winn et al. 1999; Scolari et al. 1999), and the disease genes have been isolated for several disorders in this group. The roles of the defective proteins reveal that diverse mechanisms can produce these phenotypes, including defects in extracellular matrix structural proteins, such as type IV collagen abnormalities in Alport syndrome; defects in membrane proteins, such as the polycystins in adult polycystic kidney disease (European Polycystic Kidney Disease Consortium 1994; Mochizuki et al. 1996); and defects in cytoskeletal organization, as observed for abnormalities in α-actinin-4 in familial focal segmental glomerulosclerosis (Kaplan et al. 2000). However, in many of the aforementioned disorders there are additional, nonrenal manifestations, such as deafness or ocular abnormalities. In forms of nephropathy and hypertension without extrarenal findings (e.g., MIM 161900), neither a locus nor a specific gene defect has yet been defined.
In addition, genes responsible for at least two autosomal dominant forms of hypertension without nephropathy have been found. Defects involving CYP11B1 and CYP11B2 on chromosome 8 are responsible for glucocorticoid-suppressible hyperaldosteronism (Pascoe et al. 1992), and mutations in the gene on chromosome 16 encoding the β subunit of the epithelial sodium channel cause Liddle’s syndrome (Shimkets et al. 1994).
We have ascertained a large Israeli family of Iraqi Jewish origin with an autosomal dominant form of adult-onset nephropathy and hypertension. Hypertension was the presenting symptom in all patients. There was progressive renal failure, and patients eventually required hemodialysis followed by renal transplantation. The aims of the present study were to describe the clinical features in the family and to determine the chromosomal location of the defective gene by a genomewide linkage search.
Subjects and Methods
The Family and the Phenotype
The Institutional Review Board of the Rabin Medical Center approved this study, and all subjects provided written informed consent. The family included 14 affected members over four generations (fig. 1). This study included only family members who were aged >30 years, and all of these underwent complete physical examination, blood pressure measurement, measurement of serum creatinine and BUN levels, and urine testing. We considered as affected those individuals with creatinine levels of >2 mg/dl in two repeat measurements. All affected individuals had either progressive renal failure or marked hypertension (or both), in addition to the high serum creatinine level. We determined that an individual was unaffected only if the serum creatinine and BUN levels were normal in two repeat measurements (serum creatinine level <1.3 mg/dl), urine testing gave normal results, there was no hypertension, and the individual was >30 years old.
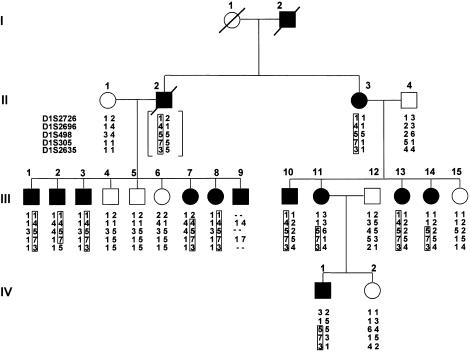
Figure 1.
Pedigree of the family with nephropathy-hypertension, showing the most likely haplotypes for the chromosome 1 markers. Affected individuals are indicated by blackened symbols. Generations are indicated by Roman numerals on the left. Within each generation, identifying numbers for individuals are indicated with Arabic numerals above each symbol. The haplotype linked to the phenotype is boxed. For individual II-2, the genotypes are bracketed to indicate that they were inferred from those of his offspring. The order of and genetic distances, in centimorgans, between the markers were D1S2726–9.5–D1S2696–2.2–D1S498–3.4–D1S305–6.0–D1S2635.
Eleven family members had end-stage renal failure, which developed at ages 19–50 years. Seven affected individuals (II-3, III-1, III-2, III-10, III-11, III-14, and IV-1) have undergone renal transplantation, and three affected members of the family are on either hemodialysis (III-3 and III-7) or peritoneal dialysis (III-8). In addition, we identified two individuals (III-13, aged 34 years, and III-9, aged 38 years) with hypertension (blood pressure 180/130 and 160/110 mm Hg [systolic/diastolic], respectively) and a high blood-creatinine level (2.2 mg/dl and 2.8 mg/dl, respectively), without other clinical evidence of renal failure. No extrarenal manifestations were found in any of the subjects.
All affected family members had marked hypertension (diastolic pressure 105 mm Hg) at the time of diagnosis. In at least two patients (II-3 and III-7), marked hypertension had been evident for several years prior to onset of high blood-creatinine levels and additional clinical findings indicating renal failure. Several affected family members underwent more extensive clinical investigation. Serum electrolytes were normal (sodium levels 143–145 meq/l) and, apart from reduced creatinine clearance, urinalysis did not reveal evidence of any other abnormalities, even in the later stages of the condition. In two individuals (III-3 and IV-1), 24-h urine analysis gave normal results for protein (140–147 mg/24 h), uric acid, sodium, potassium, and calcium and was negative for the presence of blood cells. Their blood-creatinine levels were 5.8 mg/dl and 3.1 mg/dl, respectively. Renal ultrasound findings were normal in all affected individuals examined. No evidence for abnormalities in corticosteroid metabolism was seen in any affected members of the family.
We reviewed the reports of the renal biopsies performed on two affected individuals (III-3 and III-14), both of whom had hypertension and reduced creatinine clearance at the time of biopsy. Patient III-3 underwent renal biopsy at age 27 years, at which time blood pressure was 160/110 mm Hg and serum creatinine level was 1.4 mg/dl. Of 10 glomeruli examined, 7 were normal and 3 were sclerotic, and there was evidence of interstitial fibrosis and mild tubular atrophy. Individual III-14 underwent renal biopsy at age 26 years, when blood pressure was 150/110 mm Hg and serum creatinine level was 1.7 mg/dl. On electron microscopy, apart from mild thinning of the membranes, no significant renal abnormalities were found in seven glomeruli or in the medulla, nor were any abnormalities seen after immunofluorescence staining for IgG, IgA, IgM, C3, C4, C1q, properdin, fibrinogen, and albumin.
Blood Samples
Blood samples for DNA testing were collected from 22 family members. With the exception of IV-1 and IV-2, all the individuals in generation IV were <25 years of age, and most were <18 years old. At the time of the study all these individuals were healthy, but, since there was no way to predict whether they would develop renal problems or hypertension at a later stage, they were excluded from participation in the study.
Genotyping
The LD20 linkage mapping set of fluorescently labeled synthetic oligonucleotide primers was obtained from ABI. Additional fluorescently labeled markers for candidate regions were also obtained from ABI. PCR amplification reactions were performed either singly or in combination, under conditions recommended by the manufacturer. PCR products were pooled and separated on an ABI 373 stretch automated DNA sequencer. Additional markers from chromosome 1 were derived from the Whitehead Institute for Biomedical Research database, and oligonucleotide primers for analysis of these markers were obtained from Research Genetics.
Linkage Calculations
Two-point linkage analyses were conducted for all markers. LOD scores were calculated with the MLINK subroutine of the LINKAGE 5.1 package (Terwilliger and Ott 1994), using a Pentium PC. The nephropathy-hypertension phenotype was modeled as an autosomal dominant, fully penetrant disease with an allele frequency of .0001. Although genotypes could be inferred for II-2, these were not included in the LOD score calculations. Since marker allele frequencies in this population are unknown, the allele frequencies for each marker were set at 1/N, where N was the number of alleles observed in the pedigree.
Results
Markers at the 206 autosomal loci from the ABI Prism LD20 linkage mapping set, with an average spacing of 20 cM, were typed in the family. Two-point LOD scores were calculated for each marker. There were 15 loci on 11 chromosomes that yielded a two-point LOD score >0 at a recombination fraction (θ) of 0.1. For each of these loci, flanking markers were typed. Among the flanking markers, suggestive linkage was obtained only for the marker at locus D1S2635, which yielded a maximum LOD score of 2.72 at θ=0.05.
Genotypes at loci for additional regional polymorphic markers on chromosome 1 confirmed the initial linkage result. Two-point LOD scores for these markers are shown in table 1. The highest LOD score was obtained for the marker at locus D1S305, which was fully informative and yielded a maximum LOD score of 4.71 at θ=0. Genotypes for the chromosome 1 markers are shown on the pedigree in figure 1, with haplotypes assigned by parsimony.
Table 1.
Two-Point LOD Scores Obtained between the Disease and Five Polymorphic Marker Loci Spanning the Nephropathy-Hypertension Region on Chromosome 1q21-q22
|
LOD Score at θ = |
|||||||||||
| Marker | 0 | .01 | .02 | .03 | .04 | .05 | .1 | .15 | .2 | .3 | .4 |
| D1S2726 | −∞ | −2.04 | −1.47 | −1.14 | −.92 | −.76 | −.30 | −.10 | −.003 | .02 | −.04 |
| D1S2696 | −∞ | −2.82 | −1.96 | −1.74 | −1.14 | −.89 | −.21 | .08 | .22 | .24 | .10 |
| D1S498 | .83 | .81 | .80 | .78 | .77 | .75 | .67 | .59 | .50 | .34 | .17 |
| D1S305 | 4.71 | 4.63 | 4.55 | 4.47 | 4.39 | 4.31 | 3.90 | 3.47 | 3.01 | 2.03 | |
| D1S2635 | −∞ | 2.30 | 2.53 | 2.64 | 2.69 | 2.72 | 2.66 | 2.46 | 2.18 | 1.50 | .67 |
Recombination mapping defined a region of ∼11.6 cM, at chromosomal region 1q21, containing the disease gene. The proximal boundary at locus D1S2696 was established by recombinant events between D1S2696 and D1S498, observed for individuals III-11 and III-14. The distal boundary at locus D1S2635 reflected a recombination between D1S305 and D1S2635 for individual III-2.
Discussion
The data presented here have defined an ∼11.6-cM interval at chromosome 1q21 containing a locus for an autosomal dominant form of nephropathy and hypertension that leads to end-stage renal failure. The renal component of the phenotype was significant for the absence of renal cysts, specific urine abnormalities, or extrarenal findings. Renal biopsy in two affected individuals showed evidence of end-stage renal failure but did not reveal a pathognomonic renal lesion. The most consistent and significant finding in all patients was the marked hypertension. Whether the hypertension was the primary cause of the renal failure or was secondary to an underlying renal defect is not clear.
On the basis of the inheritance pattern, the presence of renal failure without extrarenal abnormalities, and the frequent finding of hypertension, the phenotype in this family is most similar to familial nephropathy (MIM 161900). However, there is a wide range of phenotypic expression in families grouped in this entity, suggesting the strong possibility of genetic heterogeneity. With the identification of a locus for familial nephropathy, it will now be possible to assess both the question of locus heterogeneity and the range of clinical presentation among families in which the phenotype is linked to the 1q21 region.
Of note are the striking uniform progression to renal failure and the absence of proteinuria seen in this kindred, which appear to distinguish this family from other families with autosomal dominant focal segmental glomerulosclerosis. Among families with familial nephropathy, another Israeli family of Iraqi Jewish origin, with similar clinical findings, has been reported (Ben-Ishay et al. 1967). In that family, 20 affected individuals were identified among 66 family members examined. The affected individuals showed clinical findings similar to findings in the patients described here except that they also had mild proteinuria. Four patients in the family underwent renal biopsy. One patient showed normal histology, chronic glomerulonephritis was found in two patients, and pyelonephritis with nephrosclerosis was observed in the fourth. The authors concluded that the histological studies were too limited and inconsistent to warrant a conclusive pathologic diagnosis. Similar to the findings in our family, hypertension was present 3–11 years prior to renal failure. Thus the question of whether the hypertension was causative rather than secondary to underlying renal disease could not be resolved.
Christodoulou et al. (1998) localized a gene for autosomal dominant medullary cystic kidney disease (MCKD1) to chromosome 1q21 in two genetically related Cypriot families. The interval containing the MCKD1 gene was flanked by markers D1S498 and D1S2125, a region that overlaps with the interval containing the familial nephropathy locus defined here. However, on the basis of clinical findings, the two disorders appear to represent distinct entities. In the patients with familial nephropathy, there was relatively increased sodium preservation (blood and urine sodium levels were in the normal range, at 143–145 meq/l and 107 mg/24 h, respectively), whereas, in the patients with MCKD1, there was increased sodium excretion and hyponatremia (with hypotension in the later stages). In addition, medullary cysts have not been found in any of the familial nephropathy patients in our kindred. Whether the localization of the disease genes for these two renal phenotypes reflects clustering of two genes important for proper kidney function or results from distinct types of mutations in a single gene remains to be determined.
The NPR1 gene, which encodes atrial natriuretic peptide receptor 1, has been localized to the 1q21-q22 region (Lowe et al. 1990). The product of this gene is involved in blood pressure regulation and is found in the kidney, vasculature, and adrenal glands. Mice homozygous for a knockout mutation in the mouse orthologue had high blood pressure, cardiac hypertrophy, and early death due to cardiac complications (Oliver et al. 1997). The mice did not have nephritis or other obvious abnormalities in the kidneys, although there may not have been time for this to develop. A polymorphic allele of the human NPR1 gene has been associated with essential hypertension (Nakayama et al. 2000). Despite the apparent absence of kidney involvement in both the mouse knockout strain and the patients with essential hypertension, the role of the NPR1 gene product in blood pressure control is intriguing and suggests the possibility that distinct types of mutations in the NPR1 gene could produce different phenotypes. Therefore, NPR1 remains a candidate disease gene for the nephropathy and hypertension phenotype present in the family described here.
This report describes definition of a locus for a form of nephropathy and hypertension that leads to end-stage renal failure. Localization of the disease gene in this family holds the promise of the eventual identification of the defective gene and development of a deeper understanding of the relationship between hypertension and progressive renal failure.
Acknowledgments
We thank Dr. Nurit Magal for kind technical assistance, and we thank the family for their participation and cooperation in this study. This work was supported in part by grant 32/97 from the Israeli Science Foundation and by the Rosie Friedman and Cohn-Kowski Family funds.
Electronic-Database Information
Accession number and URLs for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for familial nephropathy [MIM 161900])
- Whitehead Institute for Biomedical Research/MIT Center for Genome Research, http://www-genome.wi.mit.edu/
References
- Albert MS, Leeming JM, Wigger HJ (1969) Familial nephritis associated with the nephrotic syndrome in a family with severe involvement in females. Am J Dis Child 117:153–155 [DOI] [PubMed] [Google Scholar]
- Ben-Ishay D, Biran S, Ullmann TD (1967) Familial nephritis. Israel J Med Sci 3:106–112 [PubMed] [Google Scholar]
- Christodoulou K, Tsingis M, Stavrou C, Eleftheriou A, Papapavlou P, Patsalis PC, Ioannou P, Pierides A, Constantinou-Deltas C (1998) Chromosome 1 localization of a gene for autosomal dominant medullary cystic kidney disease (ADMCKD). Hum Mol Genet 7:905–911 [DOI] [PubMed] [Google Scholar]
- Dockhorn RJ (1967) Hereditary nephropathy without deafness. Am J Dis Child 114:135–138 [DOI] [PubMed] [Google Scholar]
- European Polycystic Kidney Disease Consortium (1994) The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell 77:881–894 [DOI] [PubMed] [Google Scholar]
- Faubert PF, Porush JG (1997) Familial focal segmental glomerulosclerosis: nine cases in four families and review of the literature. Am J Kidney Dis 30:265–270 [DOI] [PubMed] [Google Scholar]
- Gauthier B, Trachtman H, Frank R, Valderrama E (1989) Familial thin basement membrane nephropathy in children with asymptomatic microhematuria. Nephron 51:502–508 [DOI] [PubMed] [Google Scholar]
- Goldman SH, Walker SR, Merigan, TC Jr, Gardner KD Jr, Bull JMC (1966) Hereditary occurrence of cystic disease of the renal medulla. N Engl J Med 274:984–992 [DOI] [PubMed] [Google Scholar]
- Jefferson JA, Lemmink HH, Hughes AE, Hill CM, Smeets HJ, Doherty CC, Maxwell AP (1997) Autosomal dominant Alport syndrome linked to the type IV collagen alpha 3 and alpha 4 genes (COL4A3 and COL4A4). Nephrol Dial Transpl 12:1595–1599 [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodríguez-Pérez JC, Allen PG, Beggs AH, Pollak MR (2000) Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24:251–256 [DOI] [PubMed] [Google Scholar]
- Lowe DG, Klisak I, Sparkes RS, Mohandas T, Goeddel DV (1990) Chromosomal distribution of three members of the human natriuretic peptide receptor/guanylyl cyclase gene family. Genomics 8:304–312 [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Constantinou-Deltas C, Peters DJM, Somlo S (1996) PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272:1339–1342 [DOI] [PubMed] [Google Scholar]
- Nakayama T, Soma M, Takahashi Y, Rehemudula D, Kanmatsuse K, Furuya K (2000) Functional deletion mutation of the 5′-flanking region of type A human natriuretic peptide receptor gene and its association with essential hypertension and left ventricular hypertrophy in the Japanese. Circ Res 86:841–845 [DOI] [PubMed] [Google Scholar]
- Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N (1997) Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci USA 94:14730–14735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe L, Curnow KM, Slutsker L, Connell JM, Speiser PW, New MI, White PC (1992) Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2. Proc Natl Acad Sci USA 89:8327–8331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JM, Whitworth JA, Kincaid-Smith PS (1981) Familial interstitial nephritis. Clin Nephrol 16:109–113 [PubMed] [Google Scholar]
- Scolari F, Puzzer D, Amoroso A, Caridi G, Ghiggeri GM, Maiorca R, Aridon P, De Fusco M, Ballabio A, Casari G (1999) Identification of a new locus for medullary cystic disease, on chromosome 16p12. Am J Hum Genet 64:1655–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR Jr, Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP (1994) Liddle’s syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79:407–414 [DOI] [PubMed] [Google Scholar]
- Terwilliger JD, Ott J (1994) Handbook of human genetic linkage. Johns Hopkins University Press, Baltimore [Google Scholar]
- Winn MP, Conlon PJ, Lynn KL, Howell DN, Slotterbeck BD, Smith AH, Graham FL, Bembe M, Quarles LD, Pericak-Vance MA, Vance JM (1999) Linkage of a gene causing familial focal segmental glomerulosclerosis to chromosome 11 and further evidence of genetic heterogeneity. Genomics 58:113–120 [DOI] [PubMed] [Google Scholar]