Abstract
During our studies of Romany (Gypsy) families with hereditary motor and sensory neuropathy–Lom, we have identified a large kindred with two independently segregating autosomal recessive neuropathies. The novel disorder, named “hereditary motor and sensory neuropathy–Russe” (HMSNR), presented as a severe disabling form of Charcot-Marie-Tooth disease with prominent sensory loss, moderately reduced motor nerve conduction velocity, and a high threshold for electrical nerve stimulation. A genome scan in two branches of the large kindred detected linkage to the 10q22-q23 region containing the early growth response 2 gene (EGR2), a transcription factor with a key role in peripheral nerve myelination. The results of sequence analysis and the detection of an intragenic polymorphism allowed us to exclude EGR2 as the HMSNR gene. Further analysis done using linkage and recombination mapping refined the position of the HMSNR gene to a small interval on 10q23.2, flanked by markers D10S581 and D10S1742, telomeric to EGR2. In this interval, a conserved seven-marker haplotype is shared by all disease chromosomes, suggesting a single founder mutation. The homozygosity region is contained in bacterial-artificial-chromosome contig 1570 of the Sanger Centre physical map and has an estimated physical size of ∼500 kb.
Introduction
Hereditary motor and sensory neuropathies (HMSNs) are a heterogeneous group of disorders including a growing number of clinically and genetically distinct autosomal recessive (AR) conditions (Ben Othmane et al. 1993; Bolino et al. 1996; Casaubon et al. 1996; LeGuern et al. 1996; Bouhouche et al. 1999; Othmane et al. 1999; Delague et al. 2000; Thomas, in press). Although the common autosomal dominant demyelinating neuropathies are now known to result from mutations in structural myelin components (Lupski 1998; Keller and Chance 1999; Schenone and Mancardi 1999), AR forms are more likely to reveal defects in the mechanisms of cell growth, differentiation, and signaling, as illustrated by the recent identification of the first two genes of this group (Bolino et al. 2000; Kalaydjieva et al. 2000). Understanding the molecular basis of these conditions can therefore be expected to contribute significantly to the current limited understanding of the biology and pathophysiology of the peripheral nervous system.
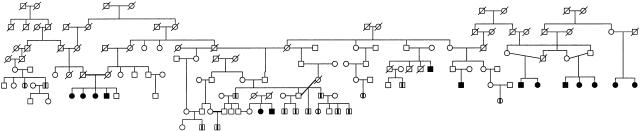
Two of the known AR forms of HMSNs—namely, HMSN-Lom (HMSNL [MIM 601455]) (Kalaydjieva et al. 1996, 1998) and the congenital cataracts/facial dysmorphism neuropathy syndrome (CCFDN [MIM 604168]) (Angelicheva et al. 1999; Tournev et al. 1999)—have been identified and characterized in studies of the Romany (Gypsies). In the course of our previous investigations of HMSNL, we detected a large kindred with multiple affected members (fig. 1). In some branches of this kindred, linkage to the HMSNL region on 8q24 and a phenotype that matched the clinical criteria for HMSNL were shown. In other branches of the kindred, linkage to 8q24 was excluded, suggesting the segregation of two genetically distinct AR neuropathies.
Figure 1.
Extended pedigree in which HMSNR was identified. Blackened symbols denote subjects affected by HMSNR; symbols containing a vertical line denote subjects affected by HMSNL, which is the other neuropathy in this kindred.
Clinically, the novel disorder presented as a severe form of Charcot-Marie-Tooth (CMT) disease. Age at onset of distal lower-limb weakness was 8–16 years. Age at onset of upper-limb involvement was variable, with a range of 10–43 years. The disorder was steadily progressive, leading to severe disability and total paralysis of muscles below the knees and, in many cases, below the elbows, by the 4th or 5th decades of life. Sensory loss affecting all modalities was a prominent feature. Motor nerve conduction was unobtainable in the legs. In the upper limbs, a peculiar combination of moderately reduced motor nerve conduction velocity (31.9±7.05 m/s for the ulnar nerve and 32.0±6.8 m/s for the median nerve) and a greatly increased threshold for electrical nerve stimulation was observed. Sensory action potentials were absent. Sural nerve–biopsy specimens demonstrated a depletion of large myelinated nerve fibers, profuse regenerative activity, and uniformly reduced thickness of the myelin sheath relative to axonal diameter, indicating hypomyelination. The disorder was therefore clinically distinct from the known forms of AR CMT disease. It was named “hereditary motor and sensory neuropathy–Russe” (HMSNR), for the city on the Danube river in northern Bulgaria where most of the affected individuals reside. A detailed description of the disease phenotype will be published elsewhere (P. K. Thomas, I. Tournev, D. Angelicheva, B. Youl, R. H. M. King, M. Nourallah, J. R. Muddle, and L. Kalaydjieva, unpublished data). Here we report the genetic mapping of HMSNR to chromosome 10q23 and the exclusion of the early growth response 2 gene (EGR2) as the candidate gene.
Subjects and Methods
Subjects and Pedigrees
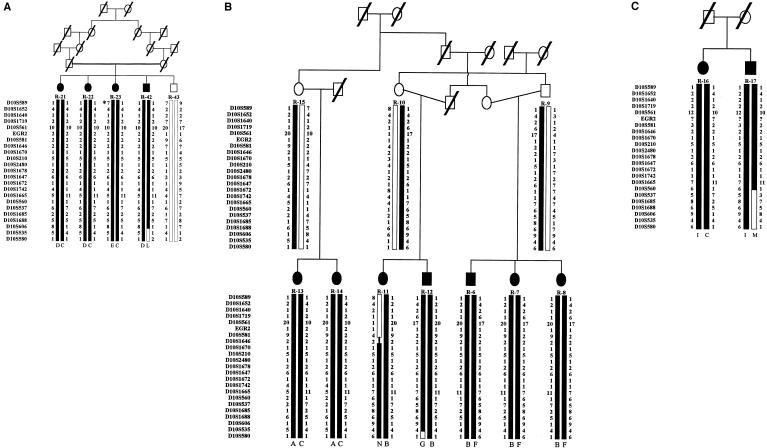
Linkage analysis was conducted in two branches of the extended kindred (figs. 1 and 2a and b). A total of 15 individuals, 11 of whom were affected, were analyzed. After identification of the candidate region, refined genetic mapping and haplotype analysis included four additional affected subjects (including two siblings) from the extended kindred (figs. 1 and 2c). Detailed clinical and electrophysiological examinations were performed, in all instances, by the same clinical team.
Figure 2.
Haplotypes in the region of linkage on 10q22-q23. Marker order is according to the Sanger Centre physical map. For the SNP at nucleotide 1219 of the EGR2 gene, “1” denotes the A allele and “2” denotes the C allele. A, First pedigree used in the genome scan and linkage analysis. For individual R-23, the asterisk (*) denotes a microsatellite mutation, in D10S589, that is assumed to be present on the basis of the conserved flanking haplotypes. B, Second pedigree used in the genome scan and linkage analysis. Different alleles of EGR2 were found to segregate in the two affected branches. Maternal recombination in individual R-11 places the centromeric boundary at D10S581. C, Affected siblings included in the analysis of the 10q22-q23 region. A recombination event in individual R-17 defines marker D10S537 as the telomeric boundary of the HMSNR-gene region.
Informed consent was obtained from all individuals participating in the study. The study complies with the ethical guidelines of the institutions involved. DNA was isolated from peripheral blood lymphocytes as described elsewhere (Miller et al. 1988).
Genotyping Analysis
The ABI Prism Linkage Mapping Sets LMS and LMS version 2 (PE Biosystems), which have an average intermarker distance of 10 cM, were used in the genome scan. Additional markers for the analysis of chromosome 5q included D5S2084, D5S2055, D5S2011, D5S2090, D5S2049, D5S2040, and D5S2057 from the ABI Prism LMS-HD5 Mapping Set.
For the refined mapping of the candidate area on chromosome 10q22-q23, the following additional markers were selected from the Généthon database: D10S1780, D10S578, D10S220, D10S1790, D10S546, D10S589, D10S1640, D10S1719, D10S561, D10S581, D10S1670, D10S1646, D10S210, D10S1647, D10S1672, D10S1742, D10S1678, D10S560, D10S1665, D10S1685, D10S1688, D10S606, D10S535, D10S580, and D10S1730. Marker D10S2480 was chosen from the Southampton map (Genetic Location Database).
PCR amplification was performed in a 5-μl total volume containing 10 ng DNA, 500 μM each dNTP, 4–5 μM or 50 ng each primer, 1 × Qiagen PCR buffer (Qiagen), 1.5 mM MgCl2, and 0.25 U Taq polymerase (Qiagen). PCR amplification was done in a 9600 thermal cycler (PE Biosystems), with use of the following cycling conditions: denaturation at 94°C for 5 min; 15 cycles at 94°C for 20 s, 63°C–55°C (−0.5°C decrease in annealing temperature/cycle) for 60 s, and 72°C for 30 s; 15 cycles at 94°C for 20 s, 55°C for 60 s, and 72°C for 60 s; and final extension at 72°C for 5 min.
The PCR products were separated on an ABI Prism 377 DNA Analyzer (PE Biosystems), with use of denaturing 5.0% polyacrylamide gels (Long Ranger [FMC BioProducts]). The genotyping data were processed using GENESCAN version 3.1, GENOTYPER version 2.5, and GENBASE version 2.01 (PE Biosystems). Haplotypes were constructed manually, with markers ordered according to the physical map of chromosome 10, as provided by the Sanger Centre.
Statistical Analysis
Linkage analysis was performed under the assumptions of AR inheritance, full penetrance, and equal frequencies of marker alleles. For the calculations, consanguinity loops were broken, and the pedigree shown in figure 2b was split into two. The analyses were done using two different disease-gene frequencies: .001 and .01. Two-point LOD scores were calculated using the MLINK program version 5.10 of the LINKAGE package (Ott 1991). Multipoint parametric linkage analysis was performed using GENEHUNTER version 1.0 (Kruglyak et al. 1996). For the genome-scan multipoint calculations, the maps provided in the ABI Prism Linkage Mapping Sets LMS and LMS version 2 (PE Biosystems) were used. Multipoint analysis of the candidate region on chromosome 10q relied on the Généthon map, from which the additional markers were selected, despite some discrepancies with the Sanger Centre physical map.
Sequence Analysis of the EGR2 Gene
The coding regions of EGR2 were amplified from genomic DNA, with the use of overlapping primers as described elsewhere (Timmerman et al. 1999; Lupski Lab Homepage). The 5′ and 3′ UTRs were analyzed using newly designed primers (table 1). PCR reactions were performed in a 50-μl volume containing 50 ng DNA, 50 ng each primer, 500 μM each dNTP, 1.5 mM MgCl2, 1 × Qiagen PCR buffer (Qiagen), and 0.125 U Taq DNA polymerase (Qiagen). A PE 9600 thermal cycler (PE Biosystems) was used with the following cycling conditions: initial denaturation at 94°C for 5 min; 35 cycles at 94°C for 30 s, 66°C for 1 min, and 72°C for 1 min; and final extension at 72°C for 7 min. PCR products were purified through QIAquick Spin Columns (Qiagen) and were sequenced using the PCR primers and Big Dye Terminator Cycle Sequencing Ready Reaction Kits (PE Biosystems). The sequencing reactions were run on an Applied Biosystems model 377 DNA Analyzer (PE Biosystems). The initial analysis included two patients and two unaffected control subjects from Romany families with other genetic disorders. Data were compared for patients and control subjects and were also compared with published EGR2 sequences by use of Sequence Navigator version 1.0.1 (PE Biosystems). Segregation analysis of the newly detected single-nucleotide polymorphism (SNP) in EGR2 was performed through direct sequencing of exon 2.5c (Timmerman et al. 1999).
Table 1.
PCR Primers Used in Sequencing Analysis of the 5′ and 3′ UTRs of EGR2
|
Primer |
||
| UTR | Forward | Reverse |
| 5′-1 | cagaggaaggagggtgtctc | ccctactcactttcttggcagc |
| 5′-2 | cttccgtgaatgcatgtagc | gccgtcacatggctgatttg |
| 5′-3 | caaatcagccatgtgacggc | gttggactgagcctgggatg |
| 3′-1 | cacaacaacactaccacc | gatatgctctgattcacc |
| 3′-2 | cagtgagtgaagtatagcc | gcatgcatcctagtttcagag |
| 3′-3 | gcatttatgatctcagagg | cactatagtcacaaaccatcc |
Results
Linkage Analysis
As a first step, we examined, in families with HMSNR, linkage to chromosomal regions containing known AR HMSN loci, in addition to HMSNL on 8q24, which had already been excluded. Results that were suggestive of linkage were obtained for the demyelinating CMT (CMT4C [MIM 601596]) region on 5q23-q33 (LeGuern et al. 1996), where a two-point LOD score of 2.27 was calculated for marker D5S471 (recombination fraction [θ] 0) and a multipoint LOD score of 2.75 was obtained. Further analysis with a higher-density map (additional markers are those listed in the Subjects and Methods section) reduced the multipoint LOD score to −0.34 (not shown).
A genomewide analysis was then conducted using a 10-cM map density. Evidence of linkage was obtained over a >40-cM region on chromosome 10q, flanked by markers D10S208 and D10S1686 (table 2). The multipoint LOD score peaked (maximum value 4.5) in the interval D10S196–D10S537. The highest two-point LOD score (3.96) was observed at D10S1652, at θ = 0).
Table 2.
Two-Point LOD Scores Obtained in the Refined Mapping of the 10q22-23 Region
|
Two-Point LOD Score at θ = |
||||||||||
| Markera | Sizeb (cM) | .00 | .05 | .10 | .15 | .20 | .25 | .30 | .35 | .40 |
| D10S208 | 60.3 | −8.83 | −2.22 | −1.07 | −.53 | −.24 | −.08 | −.01 | .01 | .01 |
| D10S1780 | 66.9 | −5.47 | −.39 | .15 | .32 | .35 | .31 | .24 | .15 | .08 |
| D10S578 | 66.9 | −5.77 | −.57 | −.01 | .19 | .25 | .24 | .19 | .13 | .07 |
| D10S196 | 72.5 | −1.35 | .22 | .35 | .35 | .30 | .23 | .16 | .10 | .05 |
| D10S220 | 74.2 | −.99 | .57 | .67 | .62 | .52 | .40 | .28 | .17 | .08 |
| D10S1790 | 77.0 | −3.06 | .37 | .66 | .69 | .61 | .49 | .35 | .21 | .10 |
| D10S546 | 81.7 | −1.60 | 1.67 | 1.75 | 1.60 | 1.35 | 1.06 | .76 | .48 | .23 |
| D10S589 | 83.3 | −2.08 | 1.00 | 1.19 | 1.14 | .99 | .79 | .58 | .37 | .19 |
| D10S1652 | 83.3 | 3.96 | 3.48 | 2.99 | 2.51 | 2.03 | 1.56 | 1.10 | .69 | .34 |
| D10S1640 | 83.3 | 3.04 | 2.70 | 2.35 | 1.99 | 1.63 | 1.26 | .90 | .57 | .28 |
| D10S1719 | 83.3 | −.05 | 1.28 | 1.20 | 1.02 | .81 | .60 | .40 | .24 | .11 |
| D10S561 | 84.9 | 2.29 | 3.39 | 3.08 | 2.65 | 2.17 | 1.68 | 1.20 | .75 | .37 |
| D10S581 | 88.6 | 2.04 | 3.18 | 2.90 | 2.51 | 2.06 | 1.60 | 1.14 | .71 | .35 |
| D10S1670 | 88.6 | −1.29 | 1.90 | 1.98 | 1.81 | 1.55 | 1.24 | .92 | .60 | .30 |
| D10S1646 | 89.4 | 2.93 | 2.49 | 2.07 | 1.66 | 1.28 | .93 | .62 | .36 | .17 |
| D10S2480 | 91.4 | 4.09 | 3.53 | 2.98 | 2.45 | 1.93 | 1.44 | 1.00 | .60 | .29 |
| D10S210 | 91.4 | 3.58 | 3.13 | 2.66 | 2.19 | 1.73 | 1.29 | .89 | .54 | .26 |
| D10S1647 | 91.5 | 5.53 | 4.87 | 4.21 | 3.54 | 2.87 | 2.21 | 1.58 | 1.00 | .50 |
| D10S1672 | 91.6 | 4.31 | 3.75 | 3.20 | 2.65 | 2.13 | 1.62 | 1.14 | .71 | .35 |
| D10S1742 | 92.1 | 4.21 | 3.68 | 3.15 | 2.63 | 2.12 | 1.63 | 1.16 | .73 | .36 |
| D10S1678 | 92.2 | 2.25 | 1.93 | 1.63 | 1.33 | 1.06 | .80 | .56 | .35 | .17 |
| D10S560 | 92.2 | 2.79 | 2.40 | 2.01 | 1.64 | 1.29 | .96 | .66 | .40 | .19 |
| D10S1665 | 93.8 | 3.58 | 3.16 | 2.72 | 2.29 | 1.85 | 1.42 | 1.01 | .63 | .31 |
| D10S537 | 93.8 | −.10 | 1.56 | 1.49 | 1.28 | 1.02 | .76 | .51 | .30 | .14 |
| D10S1685 | 94.0 | 1.23 | 2.73 | 2.53 | 2.20 | 1.82 | 1.43 | 1.03 | .65 | .32 |
| D10S1688 | 95.6 | 4.57 | 4.00 | 3.42 | 2.85 | 2.29 | 1.75 | 1.23 | .76 | .37 |
| D10S606 | 96.9 | −1.53 | 1.53 | 1.62 | 1.47 | 1.22 | .95 | .67 | .41 | .19 |
| D10S535 | 98.1 | 1.11 | 2.29 | 2.07 | 1.74 | 1.38 | 1.03 | .70 | .42 | .20 |
| D10S580 | 100.5 | −3.76 | −.18 | .17 | .27 | .27 | .23 | .17 | .10 | .05 |
| D10S1730 | 103.8 | −7.24 | −.91 | −.09 | .24 | .36 | .37 | .31 | .21 | .11 |
| D10S1686 | 109.2 | −∞ | −1.71 | −.72 | −.27 | −.05 | .05 | .07 | .06 | .04 |
Analysis of the EGR2 Gene
The region of linkage contained an obvious candidate, the EGR2/Krox20 gene (Joseph et al. 1988), encoding a transcription factor with a key role in Schwann-cell differentiation and myelination (Topilko et al. 1994). Sequencing analysis of the entire coding sequence and of the 5′ and 3′ UTRs of EGR2 revealed identical wild-type sequence in individuals with HMSNR and in control subjects. Nucleotide positions 971 and 1476 conformed with the EGR2 sequence published under GenBank accession number AF139463 (Warner et al. 1999). An SNP at position 433 (numbering is based on GenBank accession number AF139463)—namely, an AT transversion (Warner et al. 1999)—was nonpolymorphic in our study.
A novel SNP, an AC transversion, was identified at position 1219 (numbering is based on GenBank accession number AF139463), resulting in a silent mutation in codon 362 (R362R). The segregation of this SNP in the two families with HMSNR was studied by PCR amplification and sequencing of exon 2.5c (Timmerman et al. 1999) of EGR2. The results of this analysis showed variation, in the polymorphic alleles inherited by the affected members, both between the two families (fig. 2a and b) and between the branches of the family shown in figure 2b.
Refined Mapping of the HMSNR Region
To refine the position of the HMSNR gene, the 10q22-q23 region was further studied using higher map density with an average intermarker distance of ∼1.5 cM. The highest two-point LOD score (5.53) was calculated at marker D10S1647 (θ = 0), which is located 8.2 cM telomeric of D10S1652, where the initial highest-LOD-score value was observed (table 2). The multipoint LOD scores peaked in the interval D10S1647–D10S560, with a maximum value of 6.43. LOD scores did not change significantly with the use of different gene frequencies.
Haplotype analysis in the affected families produced evidence of four recent recombination events, one of which was proximal (in individual R-11; fig. 2b) and three of which were distal (in individuals R-42, R-12, and R-17; fig. 2a,b, and c, respectively). The recombination event in individual R-11 (fig. 2b), occurring between markers D10S581 and either D10S1646 (uninformative) or D10S1670, placed the proximal boundary of the HMSNR region at D10S581. Of the three recombinations seen in the telomeric part of the region, that which was seen in individual R-17 (fig. 2c) defined marker D10S537 as the probable distal boundary of the HMSNR region. The parents in that family were not available for analysis; therefore, the haplotypes were inferred under assumption of the presence of the most common haplotype on one of the disease chromosomes.
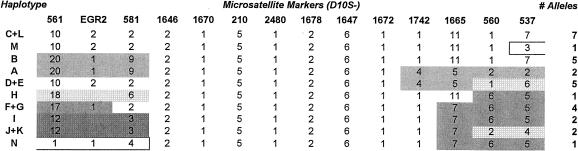
The D10S581–D10S537 interval spans 5.2 cM. For this interval, the current sample of 15 affected individuals from a single large kindred presented with a total of 10 different haplotypes, of which 8 were the product of historical recombinations (fig. 3). Three of the historical recombinations observed in the proximal part of the region (in haplotypes A+B, H, and I+J+K) had breakpoints that mapped to the same interval as did the recent recombination event in individual R-11—namely, that between markers D10S581 and D10S1646. In one of the distal historical recombinations (in haplotypes A and D+E), the breakpoint mapped to the interval D10S1672–D10S1742. Historical recombinations thus place the HMSNR gene in the region defined by markers D10S581 and D10S1742. In this interval, all disease chromosomes from our present sample share a conserved seven-marker haplotype (fig. 3). The region spans a genetic distance of 3.5 cM.
Figure 3.
Marker haplotypes in the 5.2-cM interval (D10S581–D10S537) defined by linkage analysis. Markers D10S561 and EGR2 are included to show the position of EGR2. Marker order is according to the Sanger Centre physical map. Haplotype and EGR2 allele designations are the same as those used in figure 2. Haplotypes N and M are products of the recent recombinations in individuals R-11 and R-17. The two affected members of the large kindred, who were not included in the linkage study, displayed haplotypes D/K and H/J. The unblackened area indicates the assumed ancestral haplotype. Historical recombinations place the HMSNR gene between markers D10S581 and D10S1742.
The HMSNR Region According to the Sanger Centre Physical Map
The markers within the conserved region of homozygosity have been mapped, by the Sanger Centre, to a single bacterial-artificial-chromosome contig (contig 1570) at 10q23.2. According to the physical map of the region, EGR2 is located ∼300 kb centromeric to the proximal boundary of the HMSNR region—that is, centromeric to marker D10S581. The HMSNR homozygosity region has a physical length estimated to be ∼500 kb.
Discussion
The HMSNR phenotype is that of a severe progressive sensorimotor neuropathy with prominent sensory loss, intermediate reduction in nerve conduction velocity, and an increased threshold for electrical stimulation. Both the clinical features and the genetic data indicate that this is a novel disorder that is clinically and genetically distinct from the known forms of AR HMSN (Ohnishi et al. 1989; Gabreëls-Festen et al. 1990, 1991; Ben Othmane et al. 1993; Bolino et al. 1996; LeGuern et al. 1996; Gambardella et al. 1997; Kessali et al. 1997; Bouhouche et al. 1999; Othmane et al. 1999; Delague et al. 2000; Thomas, in press).
After HMSNL (Kalaydjieva et al. 1996) and CCFDN (Angelicheva et al. 1999), this is the third novel AR hypomyelinating/demyelinating neuropathy that has been identified and genetically mapped in Romany groups descended from the Vlax Romany. The clustering of peripheral myelin disorders is reminiscent of the observations in other founder populations (e.g., the occurrence of lysosomal storage disorders among the Ashkenazi Jews) that have given rise to the debate about the role of genetic drift versus selection (Goodman 1979; Motulsky 1995). The clustering of peripheral neuropathies among the Romany has important practical implications. The independent segregation, within the same families, of phenotypically similar but genetically distinct disorders emphasizes the need for both careful evaluation of the phenotype of every affected individual and comprehensive genetic analysis of the entire kindred. Although this is not easily achieved, it should be regarded as an essential requirement for predictive genetic testing. Because a number of affected individuals who are homozygous for a given mutation may also be carriers of another mutation, identification of the genes involved will allow for the study of possible interactions and their modifying effects.
The affected individuals in this study presented with identical phenotypes and belong to a single kindred from a closely knit Romany group with a high frequency of consanguineous marriage. The design of the study, which involved conventional linkage analysis rather than homozygosity mapping, was based on the experience of previous genetic studies of the Romany, including studies of HMSNL (Kalaydjieva et al. 1996), limb-girdle muscular dystrophy type 2C (LGMD2C [MIM 253700]) (Picollo et al. 1996), congenital glaucoma (MIM 231300) (Plasilova et al. 1998), CCFDN (Angelicheva et al. 1999), and congenital myasthenia (MIM 254210) (Abicht et al. 1999). Although genetic homogeneity and a single founder mutation were invariable findings in these studies, the conserved regions of homozygosity were small even within individual consanguineous families. This pattern is likely to result from the history of the Romany; the Romany groups of today are the product of recent fissions of larger founder populations. Old age of a disease-causing mutation and high frequency of the mutation in the ancestral population would lead to a diversity of disease haplotypes introduced at the time of the splits into each of the currently existing endogamous, socially divergent groups. The conserved region of homozygosity on 10q23, with an identical seven-marker haplotype shared by all HMSNR chromosomes, unambiguously points to a single founder mutation. At the same time, the fact that 15 affected individuals from the Russe kindred presented with 10 different haplotypes in the 5-cM linkage region indicates that substantial haplotype diversity existed in the ancestral population of the Vlax Romany. The current findings suggest that HMSNR is probably a common form of AR peripheral neuropathy that is likely to occur in Romany families across Europe.
The novel HMSNR gene was mapped to 10q22-23, in close proximity to the EGR2/Krox20 gene (Joseph et al. 1988), which is an excellent positional and functional candidate gene encoding a transcription factor with an important role in myelination and Schwann-cell differentiation (Topilko et al. 1994). It has been shown to be mutated in different forms of hypomyelinating/demyelinating peripheral neuropathies (MIM 129010) (Warner et al. 1998; Timmerman et al. 1999). Sequencing data as well as the identification of an intragenic SNP presenting with different alleles in the affected individuals allowed us to exclude EGR2. This exclusion is further supported by the physical map of the region, which places EGR2 ∼300 kb centromeric of the proximal boundary of the HMSNR region. Sequencing of this part of the genome is in progress and will result in both the identification of other candidate genes and the positional cloning of HMSNR.
Acknowledgments
We thank all members of the affected kindred for their participation. The study was supported by the Muscular Dystrophy Association of the United States of America, Edith Cowan University, and the Wellcome Trust.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank (for EGR2 mRNA [accession numbers J04076 and AF139463])
- Genetic Location Database, The, http://cedar.genetics.soton.ac.uk/public_html/ldb.html (for marker D10S2480 from the Southampton map)
- Généthon, http://www.genethon.fr/genethon_en.html (for markers for mapping of the candidate area)
- Lupski Lab Homepage, http://imgen.bcm.tmc.edu/molgen/lupski/EGR2.html (for EGR2 exon primers and amplification conditions)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CCFDN [MIM 604168], CMT4A [MIM 214400], CMT4B [MIM 601382], CMT4C [MIM 601596], congenital glaucoma [MIM 231300], congenital myasthenia [MIM 254210], EGR2 [MIM 129010], HMSNL [MIM 601455], and LGMD2C [MIM 253700])
- PE Biosystems Mapping Sets, http://www.pebio.com/ga/gaww0002.htm
- Sanger Centre, The, http://www.sanger.ac.uk/HGP/Chr10/ (for physical maps)
References
- Abicht A, Stucka R, Karcagi V, Herczegfalvi A, Horvath R, Mortier W, Schara U, Ramaekers V, Jost W, Brunner J, Janssen G, Seidel V, Schlotter B, Muller-Felber W, Pongrantz D, Rudel R, Lochmuller H (1999) A common mutation (epsilon 1267delG) in congenital myasthenic patients of Gypsy ethnic origin. Neurology 53:1564–1569 [DOI] [PubMed] [Google Scholar]
- Angelicheva D, Turnev I, Dye D, Chandler D, Thomas PK, Kalaydjieva L (1999) Congenital cataracts facial dysmorphism neuropathy (CCFDN) syndrome: a novel developmental disorder in Gypsies maps to 18qter. Eur J Hum Genet 7:560–566 [DOI] [PubMed] [Google Scholar]
- Ben Othmane K, Hentati F, Lennon F, Ben Hamida C, Blel S, Roses AD, Pericak-Vance MA, Ben Hamida M, Vance JM (1993) Linkage of a locus (CMT4A) for autosomal recessive Charcot-Marie-Tooth disease to chromosome 8q. Hum Mol Genet 2:1625–1628 [DOI] [PubMed] [Google Scholar]
- Bolino A, Brancolini V, Bono F, Bruni A, Gambardella A, Romeo G, Quattrone A, Devoto M (1996) Localization of a gene responsible for autosomal recessive demyelinating neuropathy with focally folded myelin sheaths to chromosome 11q23 by homozygosity mapping and haplotype sharing. Hum Mol Genet 5:1051–1054 [DOI] [PubMed] [Google Scholar]
- Bolino A, Muglia M, Conforti FL, LeGuern E, Salih M, Georgiu DM, Christodoulou K, Hausmanowa-Petrusewicz I, Mandich P, Schenone A, Gambardella A, Bono F, Quattrone A, Devoto M, Monaco AP (2000) Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat Genet 25:17–19 [DOI] [PubMed] [Google Scholar]
- Bouhouche A, Benomar A, Birouk N, Mularoni A, Meggouh F, Tassin J, Grid J, Vandenberghe A, Yahyaoui M, Chkili T, Brica A, LeGuern E (1999) A locus for an axonal form of autosomal recessive Charcot-Marie-Tooth disease maps to chromosome 1q21.2-q21.3. Am J Hum Genet 65:722–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaubon LK, Melanson M, Lopes-Cendes I, Marineau C, Andermann E, Andermann F, Weissenbach J, Prevost C, Bouchard JP, Mathieu J, Rouleau GA (1996) The gene responsible for a severe form of peripheral neuropathy and agenesis of the corpus callosum maps to chromosome 15q. Am J Hum Genet 58:28–34 [PMC free article] [PubMed] [Google Scholar]
- Delague V, Bareil C, Tuffery S, Bouvagnet P, Chouery E, Koussa S, Maisonobe T, Loiselet J, Megarbane A, Claustres M (2000) Mapping of a new locus for autosomal recessive demyelinating Charcot-Marie-Tooth disease to 19q13.1-13.3 in a large consanguineous Lebanese family: exclusion of MAG as a candidate gene. Am J Hum Genet 67:236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabreëls-Festen AAWM, Joosten EMG, Gabreëls FJM, Jennekens FG, Gooskens RH, Stegeman DF (1991) Hereditary motor and sensory neuropathy of neuronal type with onset in early childhood. Brain 114:1855–1870 [DOI] [PubMed] [Google Scholar]
- Gabreëls-Festen AAWM, Joosten EMG, Gabreëls FJM, Stegeman DF, Vos AJ, Busch HF (1990) Congenital demyelinating motor and sensory neuropathy with focally folded myelin sheaths. Brain 113:1629–1643 [DOI] [PubMed] [Google Scholar]
- Gambardella A, Muglia M, Quattrone A (1997) Hereditary demyelinating neuropathy of infancy: a genetically complex syndrome. Brain 120:2113–2114 [DOI] [PubMed] [Google Scholar]
- Goodman RM (1979) Genetic disorders among the Jewish people. Johns Hopkins University Press, Baltimore [Google Scholar]
- Joseph LJ, Le Beau MM, Jamieson GA Jr, Acharya S, Shows TB, Rowley JD, Sukhatme VP (1988) Molecular cloning, sequencing, and mapping of EGR2, a human early growth response gene encoding a protein with “zinc-binding finger” structure. Proc Natl Acad Sci USA 85:7164–7168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaydjieva L, Gresham D, Gooding R, Heather L, Baas F, de Jonge R, Blechschmidt K, Angelicheva D, Chandler D, Worsley P, Rosenthal A, King RH, Thomas PK (2000) N-myc downstream regulated gene 1 is mutated in hereditary motor and sensory neuropathy–Lom. Am J Hum Genet 67:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaydjieva L, Hallmayer J, Chandler D, Savov A, Nikolova A, Angelicheva D, King RH, Ishpekova B, Honeyman K, Calafell F, Shmarov A, Petrova J, Turnev I, Hristova A, Moskow M, Stancheva S, Petkova I, Bittles AH, Georgieva V, Middleton L, Thomas PK (1996) Gene mapping in Gypsies identifies a novel demyelinating neuropathy on chromosome 8q24. Nat Genet 14:214–217 [DOI] [PubMed] [Google Scholar]
- Kalaydjieva L, Nikolova A, Turnev I, Petrova J, Hristova A, Ishpekova B, Petkova I, Shmarov A, Stancheva S, Middleton L, Merlini L, Trogu A, Muddle JR, King RH, Thomas PK (1998) Hereditary motor and sensory neuropathy–Lom, a novel demyelinating neuropathy associated with deafness in Gypsies: clinical, electrophysiological and nerve biopsy findings. Brain 121:399–408 [DOI] [PubMed] [Google Scholar]
- Keller MP, Chance PF (1999) Inherited neuropathies: from gene to disease. Brain Pathol 9:327–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessali M, Zemmouri R, Guilbot A, Maisonobe T, Brice A, LeGuern E, Grid D (1997) A clinical, electrophysiologic, neuropathologic, and genetic study of two large Algerian families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease. Neurology 48:867–873 [DOI] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- LeGuern E, Guilbot A, Kessali M, Ravise N, Tassin J, Maisonobe T, Grid D, Brice A (1996) Homozygosity mapping of an autosomal recessive form of demyelinating Charcot-Marie-Tooth disease to chromosome 5q23-q33. Hum Mol Genet 5:1685–1688 [DOI] [PubMed] [Google Scholar]
- Lupski JR (1998) Charcot-Marie-Tooth disease: lessons in genetic mechanisms. Mol Med 4:3–11 [PMC free article] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky AG (1995) Jewish diseases and origins. Nat Genet 9:99–101 [DOI] [PubMed] [Google Scholar]
- Ohnishi A, Murai Y, Ikeda M, Fujita T, Furuya H, Kuroiwa Y (1989) Autosomal recessive motor and sensory neuropathy with excessive myelin outfolding. Muscle Nerve 12:568–575 [DOI] [PubMed] [Google Scholar]
- Othmane KB, Johnson E, Menold M, Graham FL, Ben Hamida M, Hasegawa O, Rogala AD, Ohnishi A, Pericak-Vance M, Hentati F, Vance JM (1999) Identification of a new locus for autosomal recessive Charcot-Marie-Tooth disease with focally folded myelin on chromosome 11p15. Genomics 62:344–349 [DOI] [PubMed] [Google Scholar]
- Ott J (1991) Analysis of human genetic linkage. John Hopkins University Press, Baltimore [Google Scholar]
- Piccolo F, Jeanpierre M, Leturcq F, Dode C, Azibi K, Toutain A, Merlini L, Jarre L, Navarro C, Krishnamoorthy R, Tome FM, Uritzberea JA, Beckmann JS, Campbell KP, Kaplan JC (1996) A founder mutation in the gamma-sarcoglycan gene of Gypsies possibly predating their migration out of India. Hum Mol Genet 5:2019–2022 [DOI] [PubMed] [Google Scholar]
- Plasilova M, Ferakova E, Kadasi L, Polakova E, Gerinec A, Ott J, Ferak V (1998) Linkage of autosomal recessive primary congenital glaucoma to the GLC3A locus in Roms (Gypsies) from Slovakia. Hum Hered 48:30–33 [DOI] [PubMed] [Google Scholar]
- Schenone A, Mancardi GL (1999) Molecular basis of inherited neuropathies. Curr Opin Neurol 12:603–616 [DOI] [PubMed] [Google Scholar]
- Thomas PK (2000) Autosomal recessive hereditary motor and sensory neuropathy. Curr Opin Neurol (in press) [DOI] [PubMed] [Google Scholar]
- Timmerman V, De Jonghe P, Ceuterick C, De Vriendt E, Lofgren A, Nelis E, Warner LE, Lupski JR, Martin JJ, Van Broeckhoven C (1999) Novel missense mutation in the early growth response 2 gene associated with a Dejerine-Sottas syndrome phenotype. Neurology 52:1827–1832 [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi ABY, Seitainidou T, Babinet C, Charnay P (1994) Krox-20 controls myelination in the peripheral nervous system. Nature 371:796–799 [DOI] [PubMed] [Google Scholar]
- Tournev I, Kalaydjieva L, Youl B, Ishpekova B, Guerguelcheva V, Kamenov O, Katzarova M, Kamenov Z, Raicheva-Terzieva M, King RH, Romanski K, Petkov R, Schmarov A, Dimitrova G, Popova N, Uzunova M, Milanov S, Petrova J, Petkov Y, Kolarov G, Aneva L, Radeva O, Thomas PK (1999) Congenital cataracts facial dysmorphism neuropathy syndrome, a novel complex genetic disease in Balkan Gypsies: clinical and electrophysiological observations. Ann Neurol 45:742–750 [PubMed] [Google Scholar]
- Warner LE, Mancias P, Butler IJ, McDonald CM, Keppen K, Koob KG, Lupski JR (1998) Mutations in the early growth response 2 (EGR2) gene are associated with hereditary myelinopathies. Nat Genet 18:382–384 [DOI] [PubMed] [Google Scholar]
- Warner LE, Svaren J, Milbrandt J, Lupski JR (1999) Functional consequences of mutations in the early growth response 2 gene (EGR2) correlate with severity of human myelinopathies. Hum Mol Genet 8:1245–1251 [DOI] [PubMed] [Google Scholar]