Abstract
Turner syndrome (TS) is associated with a characteristic neurocognitive profile that includes impaired visuospatial/perceptual abilities. We used a molecular approach to identify a critical region of the X chromosome for neurocognitive aspects of TS. Partial deletions of Xp in 34 females were mapped by FISH or by loss of heterozygosity of polymorphic markers. Discriminant function analysis optimally identified the TS-associated neurocognitive phenotype. Only subjects missing ∼10 Mb of distal Xp manifested the specified neurocognitive profile. The phenotype was seen with either paternally or maternally inherited deletions and with either complete or incomplete skewing of X inactivation. Fine mapping of informative deletions implicated a critical region of <2 Mb within the pseudoautosomal region (PAR1). We conclude that haploinsufficiency of PAR1 gene(s) is the basis for susceptibility to the TS neurocognitive phenotype.
Introduction
Turner syndrome (TS), also known as “Ullrich-Turner syndrome” or “monosomy X,” is a genetic disorder that occurs in 1/2,500 female births. The complex phenotype includes ovarian failure, specific physical features (short stature), and a characteristic neurocognitive profile. Unlike other common chromosome disorders—for example, trisomy 21—TS is typically not associated with general mental retardation (Van Dyke et al. 1991). Verbal abilities are generally normal; however, 45,X girls and women, as a group, have specific deficits in visuospatial abilities, visuoperceptual abilities, motor function, nonverbal memory, executive function, and attentional abilities, when compared with normal females matched for age, height, IQ, and socioeconomic status (SES) (Waber 1979; Bender et al. 1984; McCauley et al. 1987; Rovet 1991; Ross et al. 1995; Romans et al. 1998).
The etiology of impaired cognition in TS is unknown. Observed deficits could be due to environmental/experiential, endocrine, or genetic factors. Girls with TS are short and may have other physical abnormalities that could indirectly affect their cognitive development. Sex steroid deficiency may also influence cognitive outcome. For example, impaired memory ability and motor function in children with TS may result from estrogen deficiency and may improve with estrogen replacement therapy (Ross et al. 1998, 1999). Cognitive deficits, such as impaired visuospatial/perceptual skills, may represent more direct genetic effects. These deficits are relatively consistent across wide age ranges in females with TS and are not reversible with estrogen treatment (Downey et al. 1991; Swillen et al. 1993; Romans et al. 1998). Furthermore, women with TS who have received estrogen replacement have improved verbal memory and speeded motor performance but retain a verbal IQ– performance IQ (VIQ-PIQ) discrepancy as well as impaired visuospatial processing, visual memory, and arithmetic skills (Downey et al. 1991; Swillen et al. 1993; Romans et al. 1998).
Precedence for a genetic influence on visuospatial cognitive ability comes from the example of Williams syndrome, which is due to haploinsufficiency of only a few genes in a small region of chromosome 7 (7q11.23) (Frangiskakis et al. 1996). One of these genes, LIM-kinase1, may be responsible for the deficit in visuospatial construction characteristic of this disorder (Mervis et al. 1999). The LIM-kinase1 gene may be one of several genes that influence spatial cognitive ability (Mervis et al. 1999).
TS genes are localized to the sex chromosomes and are thought to escape X inactivation. Thus, like Williams syndrome, haploinsufficiency may also explain the cognitive deficits in TS (Zinn et al. 1993). One way to deduce the underlying genotype-phenotype relationships in TS is to compare the phenotypes of individuals missing various portions of one sex chromosome, in order to assign specific features to “critical regions.” A trait maps to a region if deletion of that region accounts for the variance in that trait. In actuality, most TS traits are probably due to multiple genes, each contributing to the phenotypic variance. For example, deletion of the SHOX gene in the Xp-Yp pseudoautosomal region (PAR1) accounts for much of the characteristic TS growth deficit (Rao et al. 1997), and more proximal Xp gene(s) probably account for most of the remaining variance in stature (Kosho et al. 1999). Our study of the physical traits of TS indicated that subjects with Xp deletion displayed a wide spectrum of TS phenotypes (Zinn et al. 1998), whereas the only TS feature commonly and consistently associated with Xq deletions was ovarian failure (Geerkens et al. 1994). In the present study, we extend our findings on subjects with Xp deletions to the neurocognitive aspects of TS.
Elsewhere we have demonstrated that discriminant function analysis (DFA) can be used to identify children with a defined TS neurocognitive profile (Ross et al. 1997). In the present study, we have extended our DFA to adults. DFA is a statistical method for deriving a linear function that optimally weights parameters to permit sensitive and specific differentiation among groups. Although our choice of which cognitive abilities to test was guided by general knowledge of TS-associated deficits, the DFA did not assume a priori knowledge as to which of these deficits best characterizes TS, nor does DFA explain these deficits. The tasks within the discriminant-function result, therefore, are unlikely to represent a cohesive underlying domain but, rather, they represent a set of measures that optimally distinguishes subjects with TS and controls. The results provide a summary statistic for purposes of phenotype mapping that identifies which subjects with partial monosomy X have the defined TS-associated neurocognitive phenotype.
Using the combination of molecular mapping and detailed neurocognitive profiling, we identified a small interval of distal Xp, deletion of which was sufficient for expression of the defined TS neurocognitive phenotype. These results set the stage for identification of specific X-linked gene(s) that influence visuospatial cognitive ability.
Subjects and Methods
Subjects
This study was approved by the Human Studies Committees at Thomas Jefferson University and The University of Texas Southwestern Medical School. Informed consent and assent were obtained from all participants or, in the case of minors, from a parent or legal guardian. We excluded subjects with sex-chromosome mosaicism, ring X chromosomes, clinical features of autosomal aneuploidy in the case of unbalanced X;autosome translocations, or clinical diagnoses of Goltz, Aicardi, or MLS syndromes in the case of distal Xp deletions. Each subject had a comprehensive evaluation for TS clinical features. Subjects with serum gonadotropin levels in the castrate range (at least twice the upper limit of normal) and amenorrhea (if >16 years of age) were determined to have ovarian failure. Ovarian status was considered indeterminate in subjects <10 years of age with subcastrate gonadotropin levels, because gonadotropins do not reliably rise to these levels in girls with TS until they are ⩾10 years of age (Conte et al. 1975).
Cytogenetic and Molecular Analyses
Repeat karyotypes were obtained for subjects not evaluated cytogenetically within the previous 2 years. For each subject, ⩾20 cells were karyotyped. Lymphoblastoid cell lines were derived from blood samples by standard methods (Gilbert 1995). DNA was prepared from 1 ml of blood, using the Promega Wizard kit. Molecular cytogenetic analyses for mosaicism and deletion mapping, using FISH, and analysis of X inactivation patterns, using the androgen receptor methylation assay, have been described elsewhere (Allen et al. 1992; Zinn et al. 1998). FISH probes not described (Zinn et al. 1998) were prepared from PAC clones selected from the Integrated X Chromosome Database (Leser et al. 1999).
Breakpoints were inferred from data on relatives for two subjects with familial deletions: SW175 (lymphoblast transformation failed) and SW105 (blood sample unavailable). A subset of breakpoints was further mapped, using standard techniques, by genotyping subjects and both parents for polymorphic microsatellite markers obtained from the Genome Database, as described (James et al. 1998).
Neurocognitive Phenotype Evaluation
The neurocognitive and behavioral assessments included tests of general cognition (Wechsler Intelligence Scale for Children–Revised [WISC-R] and [Wechsler Adult Intelligence Scale–Revised [WAIS-R]), achievement (Wide Range Achievement Test [WRAT]), memory (word list recall, Denman story recall, and recall of the Rey complex figure), language (token test, Peabody picture vocabulary test, and Boston naming test), visuospatial ability (judgment of line orientation, motor-free visual perception test, Money street map, test of facial recognition, Warrington faces, and Gestalt closure), visuomotor ability (test of visual-motor integration and recall of the Rey complex figure), and attention and impulsivity (freedom from distractibility from the WISC-R, tests of variables of attention, and the matching familiar figures test). These tests are referenced in previous publications (Ross et al. 1995; Romans et al. 1998). We did not analyze subjects with VIQ <69, who were likely to have pervasive developmental delay.
Statistical Analyses
DFA
We performed DFA (see below), using the results of the battery of cognitive tests on our 45,X subjects with TS and on age-, VIQ-, and SES-matched normal female controls (Ross et al. 1997). Separate analyses were performed for the children, aged 7–16.9 years, and for the adults, aged 17–55 years, because certain tasks are age specific. Results used to develop the TS visuospatial/perceptual neurocognitive (VSPN) score were then used to identify which subjects with Xp deletion had the TS neurocognitive phenotype.
From the above tasks, to select 20 variables that differed significantly (P<.001) between subjects with TS and controls, >80 neurocognitive variables were first tested by analysis of variance, with age and SES as covariates. We then applied a stepwise DFA to those 20 variables, using the Mahalanobis distance formula to maximize the n-dimensional distance between group centroids. This analysis identified specific neurocognitive variables that optimally separated the TS and control groups and estimated the coefficients of the linear discriminant function (DF).
The DFA in children was based on the neurocognitive evaluations of 83 girls with TS and 165 healthy control girls, aged 7–16.9 years (Ross et al. 1997). The variables selected by the DFA for children were the following: WISC-R freedom from distractibility: standard score; WISC-R picture completion: standard score; test of facial recognition: raw score; Rey Osterreith figure copy: raw score; motor free visual perception test: performance quotient; Beery test of visual motor integration: standard score; and the WRAT—Reading (WRAT-R): standard score. The sensitivity of the resulting DF was 0.78, and the specificity was 0.84.
The DFA in adults was based on the neurocognitive evaluations of 51 women with TS and 37 healthy control women, aged 17–55 years. All controls had normal menstrual histories and were evaluated in the follicular phase of their cycles. The groups were well matched for age, VIQ, race, and SES. The variables selected by the DFA for the adults were the following: WAIS-III arithmetic, picture completion, and picture arrangement subtests; Rey figure organization; semantic fluency; Warrington faces; Lafayette pegboard; Money street map—timed performance; and facial affect perception—anger. The sensitivity of the DF, using the above results, was 0.86, and the specificity was 1.00.
Derivation of the VSPN
On the basis of the DFA results, we developed a formula by converting the standardized coefficients to integers and revising the scale of the linear DF separately for the children and the adults. We imposed minimum and maximum limits of ±2 SD for any particular test result, so that no single result would unduly bias the modified DF score, which was the VSPN. The sensitivity and specificity of the VSPN score depend on the cutoff value chosen. We chose, a priori, 0.0 as the VSPN cutoff for both children and adults, so as not to influence, post hoc, the sensitivity and specificity of the VSPN score results. We do note that the cutoffs could be changed to minimize the chance of falsely identifying a subject as having the typical TS neurocognitive phenotype, at the expense of reduced sensitivity, but false positives would be much worse than false negatives for mapping a rare trait.
In accordance with the original children’s DFA (20), the freedom from distractibility z-score result (the highest discriminating variable) was weighted by a factor of 2, and the other six test z-score results were weighted by a factor of unity. The VSPN score was then adjusted for the z score of VIQ, to take into account the effect of general cognitive ability and to reduce the correlation of the VSPN score with VIQ. VIQ did not correlate significantly with the VSPN score (r=.06, P=.14). The sum of the seven weighted z scores yielded VSPN scores in a range of −12 to +12 for the children. The mean VSPN scores for the TS and control populations differed significantly (-3.1±3.6 vs. 2.5±3.3, P<.0001).
The VSPN cutoff score of ⩽0.0 defined the TS neurocognitive phenotype in children. By this criterion, 102/132 of the 45,X subjects and 45/203 of the female controls had the TS neurocognitive profile, yielding a sensitivity of 0.78 and a specificity of 0.78.
For the adults, the Money time z score, semantic fluency z score, Warrington faces z score, and timed pegboard completion–z score results were weighted by a factor of 2 (the highest discriminating variables) and the other five test z-score results were weighted by a factor of unity. The VSPN score was then adjusted for the z score of VIQ, to take into account the effect of general cognitive ability and to reduce the correlation of VSPN score with VIQ. The VSPN score did not correlate with VIQ (r=.13, P=.23). The sum of the seven weighted z scores yielded VSPN scores in the range of −16.0 to +16.0. The mean VSPN score and its component results differed significantly for the TS and control populations (−4.6±3.6 vs. 4.8±4.4, P<.0001).
The VSPN cutoff score of ⩽0.0 defined the TS neurocognitive phenotype in adults. By this criterion, 46/51 45,X subjects and 7/37 female adult controls were identified as having the TS neurocognitive profile, yielding a sensitivity of 0.90 and a specificity of 0.81.
Linear regression analysis
Using multiple regression analysis, we converted adult VSPN scores to the same scale as child scores, so that both sets of data could be incorporated into a single analysis. There were >200 controls and TS subjects in our larger database with both adult and child VSPN scores, and from these subjects we performed a simple regression analysis. To minimize variability, we converted the adult scores to child scores, since there were more children than adults in our analysis. The regression formula for the adult score conversion was [adult score × 0.41 + 2.1], r=.6, P<.0001.
Results
The study population included 34 females with partial monosomy for Xp due to terminal or interstitial deletions or unbalanced translocations (table 1). There was ascertainment bias in favor of short stature or ovarian failure. There may also have been bias toward lower overall cognitive ability (mean VIQ=95±14), since individuals with intellectual impairment are more likely to be karyotyped, but there was no apparent bias with regard to selective cognitive deficits. Subjects were white (28), African American (2), Hispanic (3), and Asian (1) and had an age range of 7–46 years. Thirteen subjects were members of kinships; other subjects were unrelated (fig. 1).
Table 1.
Subjects with Nonmosaic Partial Monosomy Xp: VIQ, VSPN Score, Height z score, and Ovarian Status
| SW No. | Age(years) | Karyotype (p deletion) | Height SDa | Ovarian Failureb | VIQ | VSPN Score | TSCognitivePhenotypeb |
| Deletion of distal Xp in Xp children | |||||||
| 74 | 13 | 46,X,del(X)(p11.21) | −3.3 | − | 91 | −0.2 | + |
| 75 | 13 | 46,X,del(X)(p11.3) | −2.2 | − | 101 | 1.4 | − |
| 80 | 13 | 46,X,del(X)(p11.4) | −2.4 a | + | 103 | −6.4 | + |
| 86 | 11 | 46,X,der(X)t(X;X)(p11;q24) | −1.2 | + | 75 | 0.1 | − |
| 92 | 7 | 46,X,del(X)(p11.23) | −2.4 | Indeterminate | 90 | −9.0 | + |
| 93 | 10 | 46,X,del(X)(p11.23) | −3.5 | + | 123 | 3.7 | − |
| 96 | 16 | 46,X,del(X)(p21.2) | −1.2 | + | 64 | ||
| 97 | 13 | 46,X,del(X)(p21.2) | −0.4 | − | 86 | 4.1 | − |
| 106 | 7 | 46,del(X)(p.22.3) | −1.6 | Indeterminate | 114 | −9.4 | + |
| 112 | 11 | 46,X,del (X)(p11.2) | −3.9 | + | 115 | 0.5 | − |
| 122 | 16 | 46,X,del(X)(p22.1) | −2.7 | + | 106 | 2.5 | − |
| 145 | 14 | 46,X,del(X)(p22.1) | −1.4 | − | 90 | 2.7 | − |
| 146 | 12 | 46,X,del(X)(p22.1) | −0.4 | − | 96 | 1.4 | − |
| 151 | 7 | 46,X,del(X)(p11.2) | −2.5 | Indeterminate | 84 | 0.0 | + |
| 161 | 9 | 46,X,del(X)(p11.23) | −3.3 | + | 106 | −4.0 | + |
| 202 | 11 | 46,X,del(X)(p11.4) | −0.3 | − | 96 | 0.7 | − |
| 216 | 7 | 46,X,der(X)t(X;Y)(p22.3;q11.2)maternal | −2.1 | Indeterminate | 106 | 0.5 | − |
| 241 | 7 | 46,X,der(X)t(X;Y)(p22.3;q11.2)paternal | −1.5 | Indeterminate | 79 | −1.0 | + |
| 242 | 7 | 46,X,der(X)t(X;Y)(p22.3;q11.2)paternal | −1.3 | Indeterminate | 92 | −1.6 | + |
| Deletion of distal Xp in Xp adultsc | |||||||
| 16 | 17 | 46,Xder(X)t(X;1)(p11;q44)maternal | −3.5 | + | 88 | 1.6 | − |
| 46 | 34 | 46,X,del(X)(p21.2) | −0.3 | − | 96 | 3.4 | − |
| 71 | 20 | 46,X,del(X)(p11.2) | −2 | + | 93 | −9.4 | + |
| 85 | 45 | 46,X, del(X)(p11.2) | −3.4 | + | 100 | −1.1 | + |
| 105 | 25 | 46,Xder(X)t(X;1)(p11;q44)maternal | −3.3 | + | 90 | −1.4 | + |
| 109 | 46 | 46,X,del(X)(p11.1) | −4.7 | + | 111 | −3.1 | + |
| 111 | 20 | 46,X,del(X) (p11.21) | −3.3 | + | 118 | 0.2 | − |
| 144 | 36 | 46,X,del(X)(p22.1) | −0.6 | − | 86 | 2.4 | − |
| 157 | 42 | 46,X, del(X)(p22.31p22.33)b | −0.9 | − | 100 | −0.9 | + |
| 174 | 22 | 46,X,der(X)t(X;A)(p22.3;p11.2)maternal | −0.8 | − | 76 | −5.1 | + |
| 175 | 40 | 46,X,der(X)t(X;A)(p22.3;p11.2) | −1.8 | − | 69 | −1.6 | + |
| 211 | 20 | 46,X,del(X)(p11.23) | −3.6 | + | 104 | 0.4 | − |
| 217 | 29 | 46,X,der(X)t(X;Y)(p22.3;q11.2) | −1.4 | − | 96 | 3.7 | − |
| Nondeletion of distal Xp in Xp adultsb | |||||||
| 190 | 40 | 46,X,del(X)(p22.1)b | 0.8 | − | 100 | 12.1 | − |
| 239 | 31.2 | 46,X,del(X)(p11.2p11.4) | −1.7 | + | 89 | 3.2 | − |
MP height z score adjusted for midparental height, unavailable for one subject who was adopted.
A plus sign (+) indicates presence, and a minus sign (−) indicates absence.
Classification based on FISH results (fig. 1).
Figure 1.
Mapping of subjects with nonmosaic Xp deletions. Related subjects are indicated. Bars indicate regions present. Shaded bars indicate subjects with TS neurocognitive phenotype. Approximate physical distances are shown on the left. Deletions are grouped according to results of FISH, with markers shown on the right; order of subjects within groups is arbitrary.
A summary of subjects’ height z score (adjusted for midparental height), ovarian status, and neurocognitive results (VIQ and VSPN scores) is shown in table 1. No VSPN score was calculated for one subject (SW96) with VIQ <69. There was no obvious relationship between VIQ and the position or extent of Xp deletion. When we applied the criterion of ⩽0.0 (children or adult) SD units for the TS-associated neurocognitive phenotype, 8 (44%) of 18 children with the Xp deletion and 7 (54%) of 13 adults with the Xp deletion, with overlapping deletions of distal Xp (15/31 total), were positive (table 1). These included subjects with either large or small deletions; all were missing a portion of distal Xp (fig. 1). By comparison, 78% of 45,X children (n=132) and 90% of 45,X adults (n=51) had the defined neurocognitive phenotype. The proportion of subjects with Xp deletion meeting the VSPN criteria was less than that of 45,X subjects (Fisher’s exact test, 2-tailed, P=.007 and P=.006 for children and adults, respectively).
VIQ and PIQ varied widely (69–114 for VIQ and 77–106 for PIQ) among the subjects who met the VSPN criteria. Thus, individual VIQ results did not predict who had the TS-associated neurocognitive phenotype. For example, SW85 and SW190 both had average VIQ scores of 100, but SW190 had the highest VSPN score (12.1), whereas SW85 had one of the lower VSPN scores (−1.1).
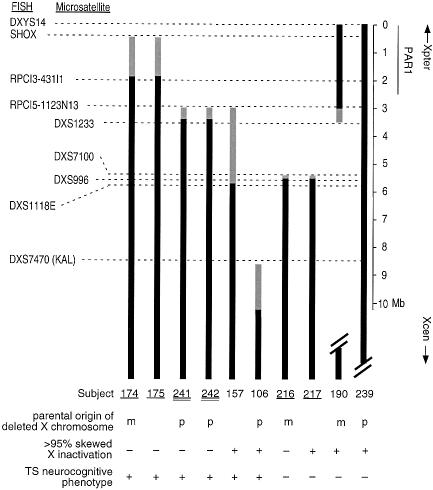
Molecular cytogenetic breakpoints are indicated schematically in figure 1. Twenty-eight breakpoints were reported (Zinn et al. 1998). Seven breakpoints <10 Mb from the telomere were not differentiated by initial FISH analyses. Because deletion of this region was associated with the neurocognitive phenotype, we investigated distal Xp breakpoints (fig. 1, subjects SW157-SW106 and SW190) in more detail. We obtained DNA samples from both parents of SW106, SW241/SW242, SW216, SW217, and SW190 and assayed subjects for loss of heterozygosity of polymorphic microsatellite markers. We also tested additional distal Xp22.3 FISH markers. The results are shown in figure 2. SW174 and SW175 (mother and daughter), both of whom have the TS neurocognitive phenotype (VSPN scores −1.0 and −1.6, respectively), had the smallest deletion. Both had deletions of SHOX but not RPCI3-431I1, which is ∼1.9 Mb from the telomere, and both were fertile with apparently normal ovarian function. Four of the remaining six subjects had the TS neurocognitive phenotype. Thus, six of eight subjects with the most distal deletions of Xp met the statistical criteria for the TS cognitive phenotype. Two pairs of these subjects were mother/daughter pairs and one pair was mz twins. All had concordant cognitive phenotypes. If only one subject from each pair of relatives is counted, the frequency of the TS cognitive phenotype in the distal Xp deletion subjects is 80% (4/5).
Figure 2.
The neurocognitive phenotype maps to distal Xp. Data for deletion mapping, X inactivation, parent of origin (p = paternal, m = maternal), and neurocognitive phenotype are shown for the eight subjects with the smallest terminal deletions and for two subjects with interstitial deletions (fig. 1). Black lines indicate regions not deleted; gray lines indicate uncertainty. Underscored subjects indicate mother-daughter pairs; double-underscored subjects indicate mz twins.
Two adults (SW190 and SW239) had interstitial deletions of Xp that did not include SHOX or PAR1. Neither had the TS neurocognitive phenotype (VSPN scores 12.1 and 3.2, respectively). SW239 had short stature and ovarian failure, whereas SW190 did not. Ovarian failure was clearly neither necessary nor sufficient for the TS cognitive phenotype, since at least three adult subjects with small distal deletions and with the phenotype did not have ovarian failure (SW174, SW175, and SW157).
All the deletions in subjects with the TS neurocognitive phenotype included the SHOX stature locus. To assess whether the cognitive phenotype was related to stature, we performed regression analysis of the VSPN score versus height SD units, using the entire population. There was no significant correlation (r=.19, P=.30), which suggests that the TS cognitive phenotype is due to a genetic effect independent of stature. We also performed regression analysis, merging the children and adults (45,X and partial-deletion populations) of the VSPN score versus age. There was a small but significant relationship between the VSPN score and age in a positive direction (r=.24 and P<.001), accounting for ∼4% of the variance.
We examined the parent of origin of the X chromosome deletions in subjects SW157–SW106. SW174 and SW216 inherited unbalanced translocations from their mothers, and SW241 and SW242 inherited a de novo unbalanced X;Y translocation from their father (case 2, Joseph et al. 1996). Microsatellite marker analysis allowed us to determine the parent of origin of the X deletion for four of the remaining six subjects. The deletion was paternally inherited in three subjects (SW106, SW241, and SW239) and maternally inherited in one (SW190). There was no absolute relationship between the parental origin of the X chromosome deletion and the presence or absence of the TS neurocognitive phenotype (fig. 2).
To determine whether X inactivation differences might play a role in the neurocognitive phenotype, we tested the pattern of X inactivation in our subjects. One subject (SW96) was uninformative for the androgen receptor polymorphism, and blood DNA was unavailable from seven subjects. Of the remaining 26 subjects, all with sequences deleted proximal to Xp22.3 (fig. 1, SW144–SW80) had >95% skewed X inactivation, with the deleted X presumably inactive (data not shown). By contrast, five of the seven subjects with distal Xp22.3 deletions (fig. 1, SW157–SW216) did not have highly skewed inactivation (fig. 2). Of these five, four had the TS neurocognitive profile and one did not.
Discussion
Our data suggest that the basis for the defined visuospatial/perceptual deficit associated with monosomy X is genetic. We used a molecular approach and genotype-phenotype correlations to identify a critical region of the X chromosome for neurocognitive aspects of TS. We studied a population of females with nonmosaic deletions spaced along the short arm of the X chromosome. Subjects missing only distal Xp22.33, at a minimum, manifested the defined TS-associated neurocognitive profile. By contrast, two subjects with interstitial Xp deletions sparing distal Xp22.33 did not have the phenotype. Furthermore, there was no apparent relationship between the TS neurocognitive phenotype and either stature or ovarian status. We conclude that the defined TS neurocognitive phenotype is genetic in etiology and maps to distal Xp22.3. The smallest deletion associated with the TS neurocognitive phenotype was in a mother and daughter missing <2 Mb of terminal Xp. This deletion fell within the 2.6 Mb Xp-Yp PAR1, where the sex chromosomes pair and recombine during male meiosis. Genes within this region escape X inactivation, so that both XY males and XX females express equivalent levels. Thus, deletions of the Y copy of PAR1 could be expected to cause TS-associated neurocognitive deficits in males. However, the phenotype may be sex-limited because of positive androgen effects on visuospatial abilities.
There are seven known genes in the minimal PAR1 critical region. They include PGPL, SHOX, CSF2RA, IL3RA, ANT3, ASMT, and ASMTL. PGPL encodes a putative GTP-binding protein that is ubiquitously expressed (Gianfrancesco et al. 1998). SHOX (or PHOG) encodes a homeodomain protein; haploinsufficiency of SHOX causes short stature or Leri-Weill dyschondrosteosis (Rao et al. 1997; Belin et al. 1998; Shears et al. 1998). One isoform, SHOXa, is expressed in many embryonic tissues, including brain (Rao et al. 1997), and it is tempting to speculate that the gene functions in nervous system development. However, intellectual impairment is not seen in patients with Langer mesomelic dwarfism (Jones 1997), in whom there are homozygous SHOX mutations. CSF2RA and IL3RA encode subunits of cytokine receptors that function in hematopoiesis (Kitamura et al. 1991). ANT3 encodes a ubiquitous ADP/ATP translocase that functions in cellular energy metabolism (Schiebel et al. 1993). ASMT encodes acetylserotonin methyltransferase (hydroxyindole-O-methyltransferase; EC 2.1.1.4 ), which catalyzes the final step in the synthesis of melatonin and is abundant in the pineal gland and retina (Yi et al. 1993). On the basis of an association between psychoses and supranumerary sex chromosomes (Crow et al. 1988) and one linkage study of schizophrenia (Collinge et al. 1991), ASMT was proposed as a candidate gene for psychiatric disorders. ASMTL is a ubiquitously expressed homolog of ASMT, whose precise biochemical function is not known (Ried et al. 1998).
There are several hypotheses to explain the phenotypic effects of TS (Epstein 1988). The simplest explanation is that the phenotype occurs in individuals having half the normal dose of X-linked genes and thus subnormal levels of gene products (Epstein 1993). X-inactivation differences or imprinting could affect the observed phenotype. All but four subjects with the very smallest Xp deletions had completely skewed X inactivation, with the deleted X presumably inactive. Incomplete skewing associated with similar deletions has been reported by others (Schaefer et al. 1993; Thomas et al. 1999). We excluded subjects with clinical abnormalities, such as Goltz syndrome or MLS syndrome, that are thought to be due to nullisomy for part of the deleted X, which is active in a portion of cells (Ballabio et al. 1989). Also, we observed the TS neurocognitive profile in subjects with either complete or incomplete skewing. Thus, X-inactivation differences are unlikely to explain our data. The lack of association with X inactivation is consistent with a pseudoautosomal locus, since PAR1 genes appear to fully escape inactivation. Last, we observed the defined TS neurocognitive phenotype in subjects with either maternal or paternal deletions, indicating that it is not imprinted. This finding is consistent with that of Skuse et al. (1997), who reported evidence of imprinting for social function but not for visuospatial abilities.
Certain study limitations should be addressed. First, the proportion of subjects with the Xp deletion who met the VSPN criteria was less than the proportion of 45,X subjects who met the criteria. This difference may be due to gene(s) elsewhere on the X chromosome that contribute to TS neurocognitive deficits or to hormonal differences. Subjects with partial deletions are less likely than 45,X subjects to have ovarian failure (Zinn et al. 1998) or estrogen deficiency that may also affect neurocognitive abilities (Ross et al. 1998).
Second, our genotype-phenotype analysis assumes that haploinsufficiency of one gene on Xp is a major determinant of the defined TS-associated VSPN deficit. We do not presume to understand the entire genetic basis for the TS cognitive profile. It would be inaccurate to assume that the only deficit in TS is in visuospatial abilities or that each specific domain of deficit is genetically determined. Likewise, it would be an oversimplification to expect a 1:1 relationship between deletion of a specific gene and individual TS deficits because of the multifactorial nature of these deficits and of normal cognitive function. In addition, autosomal genes undoubtedly influence these cognitive abilities. For example, haploinsufficiency of a gene on chromosome 7, possibly LIM-kinase1, impairs visuospatial construction abilities in Williams syndrome (Frangiskakis et al. 1996; Mervis et al. 1999). In addition, other genetic determinants that are not specific for visuospatial ability may have an impact on this trait. Identical deletions in Williams syndrome may be associated with varying visuospatial impairments (Mervis et al. 1999).
There are also study-related issues concerning ascertainment bias and mosaicism. Ascertainment bias is unavoidable in studies of rare chromosomal abnormalities. For example, some of our most informative subjects were ascertained because of chromosomal abnormalities in a relative. However, we are not aware of any bias with regard to selective neurocognitive features. As with other TS phenotype-mapping studies, mosaicism could confound apparent genotype-phenotype correlations. Although mosaicism can never be completely excluded, the lack of correlation with height SD, ovarian failure, or other TS physical features (data not shown) argues that the VSPN score is unlikely to reflect occult mosaicism.
Questions may arise about the use of the DFA, the variables chosen, and testing of individuals in a wide age range. We used DFA in defining the TS-associated neurocognitive phenotype. DFA has been applied to genetic disorders to describe facial features of Down syndrome (Allanson et al. 1993) and TS (Kaiser et al. 1996). The neurocognitive phenotype associated with TS is not a uniform phenotype but, instead, represents increased susceptibility to certain defined deficits compared with the phenotype of normal unaffected individuals. We developed the VSPN score as a tool for description of the phenotype. We then applied this tool to subjects with partial deletions, to define the genes associated with the phenotype.
The VSPN cutoff had relatively high sensitivity but lacked 100% specificity because the TS and control populations have some overlap, particularly with lower-functioning normal individuals. If the specificity is further increased, then the sensitivity is further decreased, and individuals with the defined TS cognitive phenotype and associated gene deletion would not be identified.
There was a small but significant relationship between VSPN score and age. The small positive correlation of VSPN score with age does not change the discriminatory power of the DF, because it applies uniformly to the population.
We performed separate analyses for children and adults because of the wide age range. Many of the cognitive domains and component tasks in the children’s and the adults’ VSPN scores are similar and tend to measure visuospatial perception. Visual memory, visual attention, and visual discrimination are all used in visuospatial perception, which is not a unitary function but rather has multiple components that are both anatomically and functionally distinct. Likewise, visuospatial/perceptual ability is influenced by multiple genes. Some VSPN tasks have an additional visuomotor component, and one has an affect (anger)-recognition component. These areas are typically impaired in children with TS and in adults. Additional domains and associated tasks that have been found to be impaired in subjects with TS did not reach statistical significance in the DFA.
Certain tasks that discriminated between children with TS and controls did not discriminate between the adults with TS and controls. Also, certain domains (spatial construction and attention) found to be impaired in children with TS appear to be less impaired in adults with TS. The reasons for this are likely task related. First, some tasks may have ceiling effects when given to adults. Second, the specific strategy for problem solving in children probably differs from that in adults. Adults are more able to compensate for spatial impairment than are children. Last, there may be more improvement in certain cognitive abilities in adults with TS than in children with TS.
Most tasks in both the children’s and the adult VSPN scores depend, to a great extent, on processing capabilities that are thought to be lateralized to the right cerebral hemisphere. Neuroimaging studies of subjects with TS have suggested a hypothesis of right cerebral dysfunction in TS. However, the WRAT-R test in the children’s VSPN score and semantic fluency in the adult VSPN score depend, at least in part, on the functional capacity of the left parietal region. It is perhaps significant that this is the major left-hemispheric region shown to be abnormal in MRI studies of subjects with TS (Reiss et al. 1995). Other tasks included in the children’s VSPN score may also have substantial left-hemisphere underpinnings, including the Freedom from Distractibility (third factor) from the WISC-R. Also, the Test of Facial Recognition may be coupled to verbal capability, again suggesting left-hemisphere involvement. Last, the WAIS arithmetic subtest from the adult VSPN score is likely right- and left-hemispheric dependent.
Several theories have been proposed to explain how abnormal brain development in TS leads to abnormal cognitive development. Some investigators postulate an anomalous hemispheric maturation, such that the right hemisphere is underdeveloped relative to the usual asymmetry seen in normal, age-matched control females (Money 1973; Netley et al. 1982). Other investigators and our previous findings support an alternative hypothesis for the etiology of the neurocognitive profile of patients with TS, which is consistent with multifocal brain dysfunction (Hier 1980; Pennington et al. 1985; Ross et al. 1995).
The constellation of neurocognitive deficits observed in TS is most likely multifactorial and related to a complex interaction between genetic abnormalities, hormonal deficiencies and replacement, and other unspecified determinants. We do not expect a single gene to affect only a single cognitive domain. Rather, its product influences brain structure and function, which, in turn, are modified by biochemical and experiential influences. Thus, a gene that is highly associated with one specific cognitive function is not precluded from influencing other aspects of cognitive function. Most genes involved in cognitive function probably influence multifocal aspects of brain development. Thus, deletion of a gene on distal Xp may influence the development of visuospatial ability to the greatest extent but also may affect diverse brain regions and, thus, other cognitive abilities. The ultimate cognitive outcome in a female with TS is the result of these features plus the individual’s unique non-TS cognitive profile, environment, and education. This study suggests the origin of the primary genetic event and begins to reduce the genetic complexity of TS to individual gene determinants. Future studies will involve identification of TS neurocognitive gene(s) and determination of whether variation at this locus influences cognitive abilities in the general population.
Acknowledgments
This work was supported in part by National Institutes of Health grants R01 NS35554 and NS32531. We thank Karen Kowal for assistance with phenotype evaluations. We especially thank the patients and families who participated in this study and their referring physicians.
Electronic-Database Information
URLs for data in this article are as follows:
- Genome Database, The, http://www.gdb.org/
- Integrated X Chromosome Database, http://ixdb.molgen.mpg.de/
References
- Allanson JE, O'Hara P, Farkas LG, Nair RC (1993) Anthropometric craniofacial pattern profiles in Down syndrome. Am J Med Genet 47:748–752 [DOI] [PubMed] [Google Scholar]
- Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW (1992) Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 51:1229–1239 [PMC free article] [PubMed] [Google Scholar]
- Ballabio A, Bardoni B, Carrozzo R, Andria G, Bick D, Campbell L, Hamel B, Ferguson-Smith MA, Gimelli G, Fraccaro M (1989) Contiguous gene syndromes due to deletions in the distal short arm of the human X chromosome. Proc Natl Acad Sci USA 86:10001–10005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin V, Cusin V, Viot G, Girlich D, Toutain A, Moncla A, Vekemans M, Le Merrer M, Munnich A, Cormier-Daire V (1998) SHOX mutations in dyschondrosteosis (Leri-Weill syndrome). Nat Genet 19:67–69 [DOI] [PubMed]
- Bender B, Puck M, Salbenblatt J, Robinson (1984) A cognitive development of unselected girls with complete and partial X monosomy. Pediatrics 73:175–182 [PubMed] [Google Scholar]
- Collinge J, Delisi LE, Boccio A, Johnstone EC, Lane A, Larkin C, Leach M, Lofthouse R, Owen F, Poulter M (1991) Evidence for a pseudo-autosomal locus for schizophrenia using the method of affected sibling pairs. Br J Psychiatry 158:624–629 [DOI] [PubMed] [Google Scholar]
- Conte FA, Grumbach MM, Kaplan SL (1975) A diphasic pattern of gonadotropin secretion in patients with the syndrome of gonadal dysgenesis. J Clin Endocrinol Metab 40:670–674 [DOI] [PubMed] [Google Scholar]
- Crow TJ (1988) Sex chromosomes and psychosis: the case for a pseudoautosomal locus. Br J Psychiatry 153:675–683 [DOI] [PubMed] [Google Scholar]
- Downey J, Elkin EJ, Erhardt AA, Meyer-Bahlburg H, Bell J, Morishima J (1991) Cognitive ability and everyday functioning in women with Turner syndrome. J Learn Disabil 24:32–39 [DOI] [PubMed] [Google Scholar]
- Epstein CJ (1988) Mechanisms leading to the phenotype of Turner syndrome. In: Rosenfeld RG, Grumbach MM (eds) Turner syndrome. Marcel Dekker, New York, pp 13–28 [Google Scholar]
- ——— (1993) The conceptual bases for the phenotypic mapping of conditions resulting from aneuploidy. Proc Clin Biol Res 384:1–18 [PubMed]
- Frangiskakis JM, Ewart AD, Morris CA, Mervis CB, Bertrand J, Robinson BF, Klein BP, Ensing GJ, Everett LA, Green ED, Proschel C, Gutowski NJ, Noble M, Atkinson DL, Odelberg SJ, Keating MT (1996) LIM-kinase1 hemizygosity implicated in impaired visuospatial constructive cognition. Cell 86:59–69 [DOI] [PubMed] [Google Scholar]
- Geerkens C, Just W, Vogel W (1994) Deletions of Xq and growth deficit: a review. Am J Med Genet 50:105–113 [DOI] [PubMed] [Google Scholar]
- Gianfrancesco F, Esposito T, Montanini L, Ciccodicola A, Mumm S, Mazzarella R, Rao E, Giglio S, Rappold G, Forabosco A (1998) A novel pseudoautosomal gene encoding a putative GTP-binding protein resides in the vicinity of the Xp/Yp telomere. Hum Mol Genet 7:407–414 [DOI] [PubMed] [Google Scholar]
- Gilbert J (1995) Establishment of permanent cell lines by Epstein-Barr virus transformation. In: Dracopoli N, Haines J, Korf B, Moir D, Morton C, Seidman C (eds) Current protocols in human genetics. Vol 2. John Wiley, New York, pp A.3.H.1–A.3.H.5 [DOI] [PubMed] [Google Scholar]
- Hier DB, Atkins L, Perlo VP (1980) Learning disorders and sex chromosome aberrations. J Mental Defic Res 24:17–26 [DOI] [PubMed]
- James RS, Coppin G, Dalton P, Dennis NR, Mitchell C, Sharp AJ, Skuse DH, Thomas NS, Jacobs PA (1998) A study of females with deletions of the short arm of the X chromosome. Hum Genet 102:507–516 [DOI] [PubMed] [Google Scholar]
- Jones KL (1997) Smith's recognizable patterns of human malformation, 5th ed. W. B. Saunders Company, Philadelphia, pp 442–443 [Google Scholar]
- Joseph M, Cantu ES, Shashidhar Pai G, Willi SM, Papenhausen PR, Weiss L (1996) Xp pseudoautosomal gene haploinsufficiency and linear growth deficiency in three girls with chromosome Xp22;Yq11 translocation. J Med Genet 33:906–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C, Abt K (1996) Recognizing Ullrich-Turner syndrome by discriminant analysis of craniofacial structure. Am J Med Genet 62:113–119 [DOI] [PubMed] [Google Scholar]
- Kitamura T, Sato N, Arai K, Miyajima (1991) Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell 66:1165–1174 [DOI] [PubMed] [Google Scholar]
- Kosho T, Muroya K, Nagai T, Fujimoto M, Yokoya S, Sakamoto H, Hirano T, Terasaki H, Ohashi H, Nishimura G, Sato S, Matsuo N, Ogata T (1999) Skeletal features and growth patterns in 14 patients with haploinsufficiency of SHOX: implications for the development of Turner syndrome. J Clin Endocrinol Metab 84:4613–4621 [DOI] [PubMed] [Google Scholar]
- Leser U, Roest Crollius H, Lehrach H, Sudbrak R (1999) IXDB, an X chromosome integrated database (update). Nucleic Acids Res 27:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley E, Kay T, Ito J, Treder R (1987) The Turner syndrome: cognitive deficits, affective discrimination, and behavior problems. Child Develop 58:464–473 [PubMed] [Google Scholar]
- Mervis CB, Robinson BF, Pani JR (1999) Visuospatial construction. Am J Hum Genet 65:1222–1229 [DOI] [PMC free article] [PubMed]
- Money J (1973) Turner syndrome and parietal lobe functions. Cortex 9:313–326 [DOI] [PubMed] [Google Scholar]
- Netley C, Rovet J (1982) Atypical hemispheric lateralization in Turner syndrome subjects. Cortex 18:377–384 [DOI] [PubMed] [Google Scholar]
- Pennington BF, Heaton RK, Karzmark P, Pendleton MG, Lehman R, Shucard DW (1985) The neuropsychological phenotype in Turner syndrome. Cortex 21:391–404 [DOI] [PubMed] [Google Scholar]
- Rao E, Weiss B, Fukami M, Rump A, Niesler B, Mertz A, Muroya K, Binder, G, Kirsch S, Winkelmann M, Nordsiek G, Heinrich U, Breuning MH, Ranke MB, Rosenthal A, Ogata T, Rappold GA (1997) Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat Genet 16:54–63 [DOI] [PubMed] [Google Scholar]
- Reiss A, Mazzocco M, Greenlaw R, Freund L, Ross J (1995) Neurodevelopmental effects of X monosomy: a volumetric imaging study. Ann Neurol 38:731–738 [DOI] [PubMed] [Google Scholar]
- Ried K, Rao E, Schiebel K, Rappold GA (1998) Gene duplications as a recurrent theme in the evolution of the human pseudoautosomal region 1: isolation of the gene ASMTL. Hum Mol Genet 7:1771–1778 [DOI] [PubMed] [Google Scholar]
- Romans SM, Stefanatos G, Roeltgen DP, Kushner H, Ross JL (1998) The transition to young-adulthood in Ullrich-Turner syndrome: neurodevelopmental changes. Am J Med Genet 79:140–147 [PubMed] [Google Scholar]
- Ross JL, Kushner H, Zinn AR (1997) Discriminant analysis of the Ullrich-Turner syndrome neurocognitive profile. Am J Med Genet 72:275–280 [DOI] [PubMed] [Google Scholar]
- Ross JL, Roeltgen D, Feuillan P, Kushner H, Cutler GB Jr (1998) Estrogen effects on nonverbal processing speed and motor function in girls with Turner syndrome. J Clin Endocrinol Metab 83:3198–3204 [DOI] [PubMed] [Google Scholar]
- ——— (2000) Use of estrogen in young girls with Turner syndrome: effects on memory. Neurology 54:164–170 [DOI] [PubMed]
- Ross JL, Stefanatos G, Roeltgen D, Kushner H, Cutler GB Jr (1995) Ullrich-Turner syndrome: neurodevelopmental changes from childhood through adolescence. Am J Med Genet 58:74–82 [DOI] [PubMed] [Google Scholar]
- Rovet JF (1991) The cognitive and neuropsychological characteristics of females with Turner syndrome. In: Bender B, Berch D (eds) Sex chromosome abnormalities and behavior: psychological studies. Westview Press, Boulder, CO, pp 39–77 [Google Scholar]
- Schaefer L, Ferrero GB, Grillo A, Bassi MT, Roth EJ, Wapenaar MC, van Ommen GJ, Mohandas TK, Rocchi M, Zoghbi HY, Ballabio A (1993) A high resolution deletion map of human chromosome Xp22. Nat Genet 4:272–279 [DOI] [PubMed] [Google Scholar]
- Schiebel K, Weiss B, Wohrle D, Rappold G (1993) A human pseudoautosomal gene, ADP/ATP translocase, escapes X-inactivation whereas a homologue on Xq is subject to X-inactivation. Nat Genet 3:82–87 [DOI] [PubMed]
- Shears DJ, Vassal HJ, Goodman FR, Palmer RW, Reardon W, Superti-Furga A, Scambler PJ, Winter RM (1998) Mutation and deletion of the pseudoautosomal gene SHOX cause Leri-Weill dyschondrosteosis. Nat Genet 19:70–73 [DOI] [PubMed]
- Skuse DH, James RS, Bishop DV, Coppin B, Dalton P, Aamodt-Leeper G, Bacarese-Hamilton M, Creswell C, McGurk R, Jacobs PA (1997) Evidence from Turner's syndrome of an imprinted X-linked locus affecting cognitive function. Nature 387:705–708 [DOI] [PubMed] [Google Scholar]
- Swillen A, Fryns JP, Kleczkowska A, Massa A, Vanderschueren-Lodeweyckx M, Van Den Berghe H (1993) Intelligence, behavior, and psychosocial development in Turner syndrome. Genet Counsel 4:7–18 [PubMed] [Google Scholar]
- Thomas NS, Sharp AJ, Browne CE, Skuse D, Hardie C, Dennis NR (1999) Xp deletions associated with autism in three females. Hum Genet 104:43–48 [DOI] [PubMed] [Google Scholar]
- Van Dyke DL, Wiktor A, Roberson JR, Weiss L (1991) Mental retardation in Turner syndrome. J Pediatr 118:415–417 [DOI] [PubMed] [Google Scholar]
- Waber D (1979) Neuropsychological aspects of Turner syndrome. Dev Med Child Neurol 21:58–70 [DOI] [PubMed] [Google Scholar]
- Yi H, Donohue SJ, Klein DC, McBride OW (1993) Localization of the hydroxyindole-O-methyltransferase gene to the pseudoautosomal region: implications for mapping of psychiatric disorders. Hum Mol Genet 2:127–131 [DOI] [PubMed] [Google Scholar]
- Zinn AR, Page DC, Fisher EMC (1993) Turner syndrome: the case of the missing sex chromosome. Trends Genet 9:90–93 [DOI] [PubMed] [Google Scholar]
- Zinn AR, Tonk VS, Chen Z, Flejter WL, Gardner HA, Guerra R, Kushner H, Schwartz S, Sybert VP, Van Dyke DL, Ross JL (1998) Evidence for a Turner syndrome locus or loci at Xp11.2-p22.1. Am J Hum Genet 63:1757–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]