Abstract
While developing a high-pressure liquid chromatography assay for cefepime in plasma, we observed significant drug degradation at 20 and 37°C but not at 4°C. This plasma-related degradation persisted after protein removal. This warrants caution regarding cefepime assays for pharmacokinetic and pharmacodynamic studies of cefepime in vitro and in vivo.
While a high-pressure liquid chromatography (HPLC) method to measure levels of cefepime in plasma was being validated, significant plasma-dependent and temperature-dependent degradation of cefepime was observed. Since this might bias cefepime determination in clinical samples, the degradation process was further explored. Experiments were performed in the presence or absence of plasma and at temperatures of 4, 20, and 37°C. In the presence of plasma, maintaining the temperature at 4°C was critical to avoid degradation. At higher temperatures, conforming either to bench-top storage (20°C) or incubation at physiological temperature (37°C), rapid degradation occurred.
Methods and experimental design.
Cefepime was obtained from Bristol-Myers Squibb AG (Sermoneta, Italy). Deionized water (>16 MΩ · cm) was used. Healthy volunteers' plasma (stored at −30°C) was obtained from 450-ml individual blood collection bags (Fenwal, Baxter, Volketswil, Switzerland) containing 63 ml of a solution of citric acid (15.6 mM), sodium citrate (90 mM), sodium dihydrogenophosphate (16.1 mM), dextrose (160 mM), and adenine (2 mM).
All samples were initially prepared at 4°C. Before HPLC determination, plasma was deproteinized either by precipitation followed by back extraction or by ultrafiltration. For precipitation, 500 μl of plasma was mixed with 500 μl of acetonitrile, allowed to stand for 10 min, and centrifuged at 15,000 × g for another 10 min. For back extraction, 500 μl of the deproteinized supernatant was mixed with 1 ml of methylene chloride, allowed to stand for 10 min, and centrifuged as described above. The supernatant was injected into the HPLC. Ultrafiltration was performed as described elsewhere (10).
Cefepime was separated on a C18 column (LC18, 15 cm by 4.6 mm; Supelco, Bellefonte, Pa.), using a Merck-Hitachi HPLC LaChrom system (Hitachi Instruments, Ichige Hitachinaka, Japan) with a diode array detection. The mobile phase (1 ml/min) comprised 92% volume of 0.2 M borate adjusted to pH 6.8 (20°C) with NaOH and 8% volume of methanol. Samples were sealed to prevent evaporation and kept at a controlled temperature (Peltier cooler; Labsource, Reinach, Switzerland) until their automated injection (50 μl). Cefepime was quantified by computing the area under the peak from the 260-nm absorption signal. Calibration curves, consisting of seven standards (0.5 to 200 mg/liter) were prepared daily and assayed both at the beginning and the end of the procedure. Linearity, extraction recovery, intrarun and interrun coefficients of variation and accuracy, limit of detection (LOD), and limit of quantification (LOQ) were determined (14).
Assay validation.
The basic performance of the method was excellent. The retention time was 6.06 ± 0.20 min (mean ± standard deviation of 57 runs). Linearity within 0.5 to 200 mg/liter was not rejected by F test computed from six standard curves (P = 0.94). Their individual coefficient of determination was consistently > 0.999; the pooled linear regression provided a slope of 2.16 ± 0.01 absorption units · s · mg−1 · liter (mean ± standard deviation). The intercept of 1.22 ± 0.71 absorption units · s was not significantly different from 0 (t test; P = 0.09). Standards measured at the beginning and end of a 10-h run were similar (analysis of variance with standard concentration as covariate; P = 0.41). At 8, 80, and 160 mg/liter the intrarun coefficients of variation and accuracy, assessed from five replicates, were all below 10%, as well as the interrun coefficients of variation, computed from four replicates. The interrun accuracy was between 2.7 and 10.3%, depending on the concentration. The LOD, defined as the concentration of cefepime giving a peak with a height three times the background noise, was 0.34 mg/liter. The LOQ, defined as the lowest concentration having a coefficient of variation of less than 15% (14), was 0.5 mg/liter. When the International Union of Pure and Applied Chemistry and International Conference on Harmonisation topic Q2B criteria (15) were used, LOD and LOQ were slightly different, at 1.1 and 3.3 mg/liter, respectively.
Degradation of cefepime in human plasma.
Preliminary tests on the ex vivo stability of cefepime diluted in serum or plasma revealed plasma- and temperature-dependent degradation, as detected by both bioassay and HPLC (data not presented). Since plasma deproteinization is a prerequisite for HPLC dosage, we tested whether this step had an effect on subsequent drug degradation. Degradation of a 100-mg/liter cefepime sample was followed over 10 h at 20°C, after either protein precipitation or ultrafiltration. With degradation rates of 0.085 h−1 0.076 h−1, respectively, both conditions were similar (t test; P = 0.11). Subsequent experiments were performed by adding the drug after removing plasma proteins.
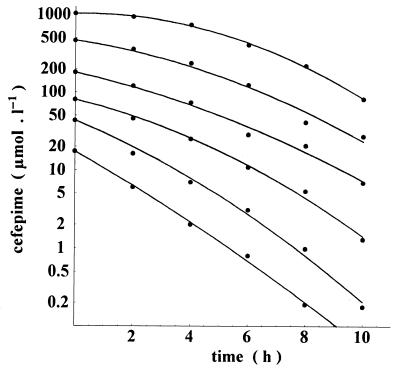
Figure 1 depicts the degradation kinetics of a range of cefepime concentrations (from 10 to 500 mg/liter) incubated at 37°C. The rate of degradation correlated inversely with drug concentration: less than 2 h was sufficient for half the amount of cefepime to vanish with a starting concentration of 10 mg/liter (20.80 μmol/liter), while more than 6 h was required for a similar degradation at a starting concentration of 500 mg/liter (1,040 μmol/liter).
FIG. 1.
Cefepime degradation in deproteinized human plasma at 37°C. Starting concentrations ranged from 10 mg/liter (20.8 μmol/liter) to 500 mg/liter (1,040 μmol/liter). Depending on the initial concentration, the degradation rate was between 0.101 h−1 (1,040 μmol/liter) and 0.189 h−1 (20.8 μmol/liter).
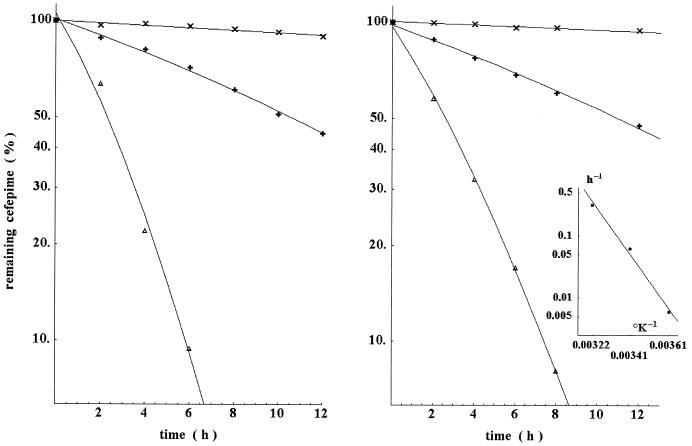
Figure 2 indicates that degradation was progressively abolished by diluting deproteinized plasma with water. Moreover, Fig. 2 also indicates that degradation increased with the temperature.
FIG. 2.
Left panel, effect of diluting deproteinized plasma in water on cefepime degradation. Samples were serially diluted in water before addition of cefepime (100-mg/liter final concentration) and incubation at 37°C. Symbols represent no dilution (Δ) and fourfold (+) and 16-fold (×) dilutions. The resulting degradation rates for these conditions were 0.407, 0.068, and 0.008 h−1, respectively. Further dilutions of plasma resulted in nonmeasurable degradation over the experimental time frame. Right panel, effect of temperature on the degradation of cefepime in deproteinized human plasma. Samples containing a final cefepime concentration of 100 mg/liter were incubated at 4°C (×), 20°C (+), and 37°C (Δ). The degradation rates for these kinetics were 0.0059, 0.062, and 0.31 h−1, respectively. Inset, according to the Arrhenius law, the energy of activation computed from these constants was 20,520 cal/mol.
This degradation was neither related to the plasma source nor to the anticoagulant solution used in the blood collection bags. Using freshly collected plasma from a volunteer, with either citrate (10.7 mM) or lithium heparinate (15 IU/ml) as an alternative to the collection bag mixture, a similar drug degradation was observed. Conversely, cefepime was incubated with the various compounds added to plasma (citrate, heparin, and the solution used in the blood collection bags), which were diluted in water at their equivalent concentrations in plasma. In these conditions, 24 h of incubation resulted in <10% degradation at 20°C and <20% degradation at 37°C.
Cefepime is stable below −20°C for long-term storage in both water and serum (1, 7). It is also stable when kept in H2O solution at room temperature (9) or in plasma at 4°C (7). However, it is unstable in plain serum during bench-top storage (7).
In our study, cefepime degradation (i) was abolished by diluting the plasma in water, (ii) occurred in spite of prior plasma deproteinization, and (iii) was faster at lower than at higher drug concentrations, as already reported (7). Dynamic changes in pH could have influenced the degradation kinetics. In one experiment, pH increased from 8.1 to 8.8 over 24 h (after a first abrupt increase from 7.5 to 8.1 associated with deproteinization) during incubation of 100 mg of cefepime per liter at 37°C in deproteinized plasma. In this regard, adding a buffer to the samples before HPLC, as in the method described by Breilh et al., merits consideration (6). An increase of pH has also been recently reported during cefepime degradation in buffered saline solution (12). On the other hand, evidence for both acid- and base- catalyzed degradation of cefepime has been reported earlier (9).
Eventually, the question arises as to plasma-related degradation of cefepime in vivo. Even though 80 to 90% of cefepime is recovered unchanged in urine (2, 4, 5), the fast renal clearance may mask a slower degradation process in plasma, similar to that observed in this study. Indeed, the ratio of renal clearance to total clearance was shown to fall below 0.6 in young infants (13) and in patients with an impaired renal function (8). Moreover, the release of the C-3′ substituent N-methylpyrrolidine associated with the hydrolysis of cefepime at physiological pH has been reported elsewhere (8), and this metabolite has been detected in urine from animals and humans treated with cefepime, especially in the case of renal failure (3, 8). In the present study, three new HPLC peaks were regularly observed during degradation. They eluted at 3.2, 3.7, and 9.5 min, i.e., outside the main cefepime peak. Therefore, characterizing them precisely will require further studies.
In conclusion, these results present evidence of a nonenzymatic degradation of cefepime in plasma in vitro. While the specific mechanism responsible for this phenomenon is incompletely known, the observation has implications for the drug-dosing technology and eventually for in vitro susceptibility tests in plasma. In this study, HPLC allowed reliable titration, fulfilling the requirement for clinical use, with an LOQ 16 times lower than the MIC cutoff for bacterial susceptibility of the compound (11), but a critical prerequisite for this performance was that all manipulations prior to HPLC separation were performed at 4°C.
Acknowledgments
We thank Saskia Bolay and Marlyse Giddey for outstanding technical assistance and Bernard Testa for stimulating and fruitful discussions.
REFERENCES
- 1.Barbhaiya, R. H., S. T. Forgue, W. C. Shyu, E. A. Papp, and K. A. Pittman. 1987. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob. Agents Chemother. 31:55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbhaiya, R. H., S. T. Forgue, C. R. Gleason, C. A. Knupp, K. A. Pittman, D. J. Weidler, and R. R. Martin. 1990. Safety, tolerance, and pharmacokinetic evaluation of cefepime after administration of single intravenous doses. Antimicrob. Agents Chemother. 34:1118-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbhaiya, R. H., C. A. Knupp, S. T. Forgue, G. R. Matzke, C. E. Halstenson, J. A. Opsahl, and K. A. Pittman. 1991. Disposition of the cephalosporin cefepime in normal and renally impaired subjects. Drug Metab. Dispos. 19:68-73. [PubMed] [Google Scholar]
- 4.Barbhaiya, R. H., S. T. Forgue, C. R. Gleason, C. A. Knupp, K. A. Pittman, D. J. Weidler, H. Movahhed, J. Tenney, and R. R. Martin. 1992. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob. Agents Chemother. 36:552-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbhaiya, R. H., C. A. Knupp, and K. A. Pittman. 1992. Effect of age and gender on pharmacokinetics of cefepime. Antimicrob. Agents Chemother. 36:1181-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breilh, D., C. Lavallée, A. Fratta, D. Ducint, P. Cony-Makhoul, and M. C. Saux. 1999. Determination of cefepime and cefpirome in human serum by high-performance liquid chromatography using an ultrafiltration for antibiotics serum extraction. J. Chromatogr. B 734:121-127. [DOI] [PubMed] [Google Scholar]
- 7.Elkhaïli, H., L. Linger, H. Monteil, and F. Jehl. 1997. High-performance liquid chromatographic assay for cefepime in serum. J. Chromatogr. B Biomed. Sci. Appl. 690:181-188. [DOI] [PubMed] [Google Scholar]
- 8.Forgue, S. T., P. Kari, and R. Barbhaiya. 1987. N-Oxidation of N-methylpyrrolidine released in vivo from cefepime. Drug Metab. Dispos. 15:808-815. [PubMed] [Google Scholar]
- 9.Fubara, J. O., and R. E. Notari. 1998. Influence of pH, temperature and buffers on cefepime degradation kinetics and stability predictions in aqueous solutions. J. Pharm. Sci. 87:1572-1576. [DOI] [PubMed] [Google Scholar]
- 10.Majcherczyk, P. A., P. Moreillon, L. A. Decosterd, D. Sanglard, J. Bille, M. P. Glauser, and O. Marchetti. 2002. Single-step extraction of fluconazole from plasma by ultra-filtration for the measurement of its free concentration by high performance liquid chromatography. J. Pharm. Biomed. Anal. 28:645-651. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing: eleventh informational supplement, M100-S11, vol. 21, no. 1. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.Rabouan-Guyon, S. M., A. F. Guet, P. Y. Courtois, and D. M. C. Barthes. 1997. Stability study of cefepime in different infusion solutions. Int. J. Pharm. 154:185-190. [Google Scholar]
- 13.Reed, M. D., T. S. Yamashita, C. A. Knupp, J. M. Veazey, Jr., and J. L. Blumer. 1997. Pharmacokinetics of intravenously and intramuscularly administered cefepime in infants and children. Antimicrob. Agents Chemother. 41:1783-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah, V. P., K. K. Midha, S. Dighe, I. J. McGilveray, J. P. Skelly, A. Yacobi, T. Layloff, C. T. Viswanathan, C. E. Cook, R. D. McDowall, K. Pittman, and S. Spector. 1991. Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Conference report. Eur. J. Drug Metab. Pharmacokinet. 16:249-255. [DOI] [PubMed] [Google Scholar]
- 15.Valassis, I. N., M. Parissi-Poulou, and P. Macheras. 1999. Quantitative determination of cefepime in plasma and vitreous fluid by chromatography. J. Chromatogr. B 721:249-255. [DOI] [PubMed] [Google Scholar]