Abstract
Familial primary pulmonary hypertension is a rare autosomal dominant disorder that has reduced penetrance and that has been mapped to a 3-cM region on chromosome 2q33 (locus PPH1). The phenotype is characterized by monoclonal plexiform lesions of proliferating endothelial cells in pulmonary arterioles. These lesions lead to elevated pulmonary-artery pressures, right-ventricular failure, and death. Although primary pulmonary hypertension is rare, cases secondary to known etiologies are more common and include those associated with the appetite-suppressant drugs, including phentermine-fenfluramine. We genotyped 35 multiplex families with the disorder, using 27 microsatellite markers; we constructed disease haplotypes; and we looked for evidence of haplotype sharing across families, using the program TRANSMIT. Suggestive evidence of sharing was observed with markers GGAA19e07 and D2S307, and three nearby candidate genes were examined by denaturing high-performance liquid chromatography on individuals from 19 families. One of these genes (BMPR2), which encodes bone morphogenetic protein receptor type II, was found to contain five mutations that predict premature termination of the protein product and two missense mutations. These mutations were not observed in 196 control chromosomes. These findings indicate that the bone morphogenetic protein–signaling pathway is defective in patients with primary pulmonary hypertension and may implicate the pathway in the nonfamilial forms of the disease.
Familial primary pulmonary hypertension (PPH [MIM 178600]) is a rare (1/105–1/106) autosomal dominant disorder that has reduced penetrance and that has been mapped to a 3-cM region on chromosome 2q33 (locus PPH1) (Morse et al. 1996, 1997; Nichols et al. 1997; Deng et al. 2000). It is characterized by monoclonal plexiform lesions of proliferating endothelial cells in pulmonary arterioles (Lee et al. 1998). These lesions lead to elevated pulmonary-artery pressures, right-ventricular failure, and death (Rich et al. 1987). The disease can occur from infancy throughout life; it has a mean age at onset of 36 years, and the ratio of affected females to affected males is 2:1. Without intervention, the median survival is <3 years after diagnosis (D'Alonzo et al. 1991), although recent advances, such as long-term prostacyclin therapy (Barst et al. 1996) and transplantation (Pasque et al. 1995), have significantly improved both the quality of life and the chances of survival in some patients. Although familial PPH (FPPH) is rare, cases secondary to known etiologies are more common and include those associated with the appetite-suppressant drugs, including phentermine-fenfluramine (Douglas et al. 1981; Abenhaim et al. 1996).
We have collected a number of multiplex families with PPH, using experimental protocols approved by the institutional review board of the College of Physicians and Surgeons at Columbia University. Methods used for clinical examination, as well as the diagnostic criteria, have been described elsewhere (Morse et al. 1997). The families with FPPH were ascertained on the basis of physician referrals (meetings, literature, Internet, and e-mail), self-referrals via the Internet, and blood samples from the 1994 and 1996 Pulmonary Hypertension Association meetings (Stone Mountain, GA). The families were predominantly white, and most originated from the United States, Canada, and Europe. During the past 2–3 years, three individuals originally classified as sporadic PPH had to be reclassified as FPPH, when another family member developed the disease. Also, several asymptomatic individuals known to carry the PPH1 gene have developed the disease within the past 4 years. Using DNA that was extracted from either whole-blood samples or formalin-fixed, paraffin-embedded tissue, we genotyped 35 of these families (72 affected individuals and 319 normal individuals and carriers), with 27 microsatellite markers located in the 3-cM minimal genetic region, as described elsewhere (Deng et al. 2000). With the genetic model and marker order determined by radiation-hybrid mapping (Deng et al. 2000), a 10-marker multipoint analysis using GENEHUNTER version 2.0 (Kruglyak et al. 1996) gave a nearly constant LOD score of 10, across the region (data not shown). At a recombination fraction of 0, the maximum LOD scores of the two-point analyses using MLINK from FASTLINK version 4.1p (Cottingham et al. 1993) were more variable, ranging from 0.6 to 8.6, with the higher scores clustering toward the telomeric end (data not shown). Given the low prevalence of the disorder and the consequent possibility that some of the families were from a common founder, we reconstructed, when possible, the 27-marker-microsatellite disease haplotype from each family and visually inspected these haplotypes in order to detect any shared segments. No obvious shared DNA segments were found, so we used the haplotype-analysis program TRANSMIT version 2.5 (1999) (available from the “David Clayton's Genetic Programs” Web page; also see Clayton 1999) to look in a more rigorous fashion. Suggestive evidence of sharing (P=.07) was found with the 345-/214-bp haplotype of markers GGAA19e07 and D2S307. Since these markers were in the telomeric cluster, we began our mutation scan in this region.
We investigated the genetic variation in the coding sequence of three nearby candidate genes by examining 22 individuals from 19 families with FPPH and 2 normal controls, using denaturing high-performance liquid chromatography (dHPLC) with a WAVE™ Nucleic Acid Fragment Analysis System (Transgenomics), according to the manufacturer's directions and as described elsewhere (Underhill et al. 1997; O'Donovan et al. 1998). These individuals were chosen on the basis of the amount of DNA available. PCR-amplification products (maximum size 602 bp) were run with as many as three melting profiles, for fragments with multiple melting domains. DNA sequence determination of fragments containing potential variants was performed by cycle sequencing using Big Dye™ terminators (Applied BioSystems), and sequencing products were resolved on Long Ranger™ gels (BioWhittaker Molecular Applications) and were detected with an ABI model 377 DNA sequencer. DNA sequence traces were analyzed by Vector NTI suite 5.5 (Informax). The first two genes, CD28 and CTLA4, were candidates because of their involvement in immune system regulation (Morse and Barst 1994). No variation in CD28 was observed. In CTLA4, we found one previously unreported single-nucleotide polymorphism (SNP)—that is, 49A→G, with an allele frequency of .50—that causes a nonconservative change in protein structure (A17T). The polymorphism was homozygous in some of the patients and in one of the controls. Since it did not fit known inheritance patterns for the disease, this SNP was ruled out as a potential disease mutation.
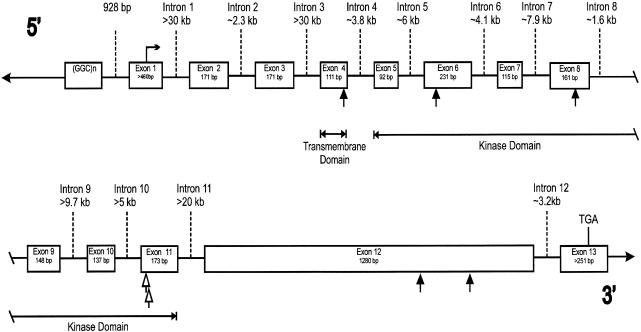
The third positional candidate, the gene encoding the bone morphogenetic protein (BMP) receptor type II (BMPR-II [gene BMPR2, also known as “T-ALK,” “CL4-1,” and “BRK-3”]), a member of the transforming growth factor β (TGF-β)–receptor superfamily, was suggested on the basis of the role of the BMP-signaling pathway in lung morphogenesis (Warburton et al. 2000). The cDNA sequence of this ∼4-kb gene encoding a 1,038-amino-acid protein has been described elsewhere (Kawabata et al. 1995; Liu et al. 1995; Nohno et al. 1995; Rosenzweig et al. 1995). To deduce the genomic structure of BMPR2 (fig. 1 and table 1), we found genomic sequences homologous to exons 1 and 8–13, by querying the National Center for Biotechnology Information high-throughput genome sequence (HTGS) database, using BLAST (Altschul et al. 1990). The intron size and DNA sequence of the other intron-exon boundaries were determined by amplifying and sequencing PCR products by means of oligonucleotide primers designed to amplify either across neighboring exons or out to a nearby Alu repeat, with the structure of mouse BMPR2 (Beppu et al. 1997) being used as a guide. We then designed oligonucleotide primers to amplify the exons from genomic DNA of the patients (table 2). These PCR fragments were screened by dHPLC, and the DNA sequences of those fragments containing apparent variation were determined. Primers to amplify exons 2–7 were designed prior to having the intron/exon structure, and hence a total of 204 bp of known exonic sequence was not screened.
Figure 1.
Intron/exon structure of the human BMPR2 gene. Intron and exon sizes are as indicated. Protein start and stop codons are indicated by the horizontal arrow and “TGA,” respectively. Mutations that cause premature termination of BMPR2 are shown as blackened arrows; unblackened arrows denote mutations in Arg491. The transmembrane and kinase domains are encoded by the indicated exons.
Table 1.
BMPR2 Gene: Intron/Exon Boundaries, Known Size of Exons, and Approximate Sizes of Introns
| Exon (Size) | Exon 3′ | 5′ Intron Sequence | Intron (Size) | 3′ Intron Sequence | Exon 5′ |
| 1 (>460 bp) | GCT G | gtgagtagctccggc.. | 1 (>30 kb) | ..tttcctttattttag | CT TCG |
| Ala A | la Ser | ||||
| 2 (171 bp) | CAA G | gcaagtgatactttc.. | 2 (∼2.3 kb) | ..catattgatttatag | GA TAT |
| Gln G | ly Cys | ||||
| 3 (171 bp) | CTC A | gtaagtaaagtaacc.. | 3 (>30 kb) | ..tttgttttcttttag | GT CCA |
| Leu S | er Pro | ||||
| 4 (111 bp) | ACA G | gtaaaaattaccatt.. | 4 (∼3.8 kb) | ..ttcctgttcttatag | GA GAC |
| Thr G | ly Asp | ||||
| 5 (92 bp) | TTG GAG | gtaagtttgccgtta.. | 5 (∼6 kb) | ..ttaaaacacttgcag | CTG ATT |
| Leu Glu | Leu Ile | ||||
| 6 (231 bp) | CCC AAT | gtaagttcttcatag.. | 6 (∼4.1 kb) | ..ttttcctctatatag | GGA TCT |
| Pro Asn | Gly Ser | ||||
| 7 (115 bp) | GGA G | gtaagatagtcaata.. | 7 (∼7.9 kb) | ..aaattatccaaacag | AT CAT |
| Gly A | sp His | ||||
| 8 (161 bp) | AGC GAG | gtgagtgtatacaaa.. | 8 (∼1.6 kb) | ..actctaatttatcag | GTT GGC |
| Ser Glu | Val Gly | ||||
| 9 (148 bp) | CCA G | gtaaaaactactgtc.. | 9 (>9.7 kb) | ..tctacaaatccacag | GG GAA |
| Pro G | ly Glu | ||||
| 10 (137 bp) | AGC CTG | gtaagaaaaaactaa.. | 10 (>5 kb) | ..tactttgtcttacag | GCA GTG |
| Ser Leu | Ala Val | ||||
| 11 (173 bp) | GAA CG | gtaagaccctaaggg.. | 11 (>20 kb) | ..ctttctttctttaag | C AAC |
| Glu Ar | g Asn | ||||
| 12 (1,280 bp) | CAG A | gtaagtggagggatc.. | 12 (∼3.2 kb) | ..cacttttattttcag | TA GGT |
| Gln I | le Gly | ||||
| 13 (>251 bp) |
Table 2.
Oligonucleotide Primer Sequences Used to Screen BMPR2
|
Primera |
|||
| Exon | 5′ | 3′ | Size of PCR Product (bp) |
| 1 | AACTAGTTCTGACCCTCGCCCC | GGACGCATGGCGAAGGGCAA | 602 |
| 2b | TAGCTTCGCAGAATCAAGAA | TGCCTTGTTTTACAAGATTT | 177 |
| 3b | TAGGATGTTGGTCTCACATT | TACTGAGTGGTGTTGTGTCA | 177 |
| 4b | TAGGTCCACCTCATTCATTT | TACCTGTCAACATTCTGTAT | 117 |
| 5b | TAGGAGACCGTAAACAAGGT | TACCTCCAACAGTTTCAGAT | 98 |
| 6b | CAGCTGATTGGCCGAGGTCG | TACATTGGGATAGTACTCCA | 237 |
| 7b | TAGGGATCTTTATGCAAGTA | TACCTCCTCGTGGTAATTCT | 121 |
| 8 | GCAGAAAAATAATACTACTTCTATA | GATGTTTTAATTAAATTATCATTTC | 319 |
| 9 | AGAATATGCTACGTTCTCTC | ACACTAGATAGCAATGAACTAAAGG | 336 |
| 10 | GTATCAGAAATACCCCTGTT | TTAGGCAACTCCAAAAACTAT | 328 |
| 11 | GGTAAACTGAAAAGCTCAATAC | CATTGAACTATTAGGCTGGT | 345 |
| 12-1 | GATCCCCTTTCTTTCTTTAAGC | CTGTTTAAGAGAGTGCTCCATG | 510 |
| 12-2 | GAACCTCAAGGAAAGCTCTG | AGCATGGGAGTTAACACTGT | 436 |
| 12-3 | ACCTCATGTGGTGACAGTCA | ATTGGAATTAGTTCGGCCAC | 316 |
| 12-4 | ATTCCAGTCCTGATGAGCAT | AGTTATTTAAATGGCCCCAA | 343 |
| 13 | TTACATCCCTTACCCGTTAT | TTAAAGCAAGTCTTTGTTGC | 454 |
Exonic DNA sequences are underlined.
Amplified by primers within the exon.
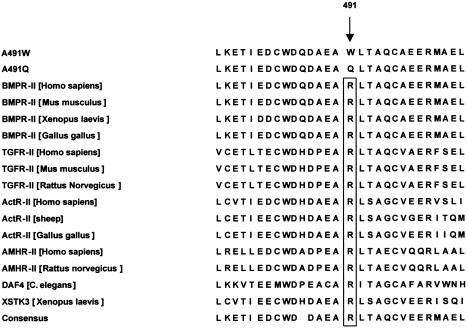
In 9 of the 19 families screened, we observed mutations that are likely to disrupt the function of the receptor. Five of these mutations predict premature termination of BMPR-II, in exons 4, 6, 8, and 12, and each was seen in only one family (fig. 1 and table 3). In addition, in exon 11 in three families, an SNP that causes a nonconservative change in amino acid sequence—that is, from arginine, conserved in all known type II TGF-β–superfamily receptors (fig. 2), to tryptophan—was seen (fig. 1 and table 3). These three families do not share a microsatellite marker haplotype. The same arginine was changed to glutamine (1472G→A) in the proband of another family, PPH019 (fig. 1 and table 3), but this proband's parents are genotypically normal. This proband (from whom we have a DNA sample) had both a son who died of PPH in childhood (presumptive; no postmortem was performed) and a deceased uncle with a history of portal hypertension and unspecified cardiac problems. Since the 1472G→A mutation had not been transmitted through either parent, more history on the “affected” uncle was obtained, and he was found to have longstanding alcoholism—and we think that this, rather than right-sided heart failure secondary to PPH, was responsible for his portal hypertension. The observation of this new mutation suggests that mutations in BMPR2 might also cause sporadic cases of PPH.
Table 3.
BMPR2 Mutations Observed in PPH
| Family or Families | No. of A/C/Ua | Exon | DNA Sequence Variationb | Protein Sequence Variation |
| PPH001, PPH008, PPH021 | 4/5/13 | 11 | 1471C→T | R491W |
| PPH010 | 2/0/1 | 8 | 1099–1103delGGGGA | E368fsX1 |
| PPH015 | 6/1/8 | 12 | 2579delT | N861fsX10 |
| PPH017 | 3/0/6 | 4 | 507–510delCTTTinsAAA | C169X |
| PPH018 | 3/2/4 | 12 | 2617C→T | R873X |
| PPH019 | 1/0/5 | 11 | 1472G→A | R491Q |
| PPH022 | 2/0/0 | 6 | 690–691delAGinsT | K230fsX21 |
No. of DNA samples available for analysis of affected (A), known carrier (C), and unaffected (U) individuals in each family or set of families, determined by segregation pattern and DNA sequencing/dHPLC, except in the case of family PPH019 (in which only DNA sequencing was used; see text).
Sequences are referenced to GenBank BMPR2 cDNA sequence number NM_001204 (see NCBI GenBank Overview); the numbering is based on the use of “+1” to denote the A of the starting methionine codon.
Figure 2.
Sequence alignment of the type II TGF-β–superfamily receptors surrounding R491 in BMPR-II. The mutation in families PPH001, PPH008, and PPH021 was aligned with all known type II receptors (>50), and 14 of these are displayed. TGFR-II = TGF-β–receptor type II; ActR-II = activin receptor type II; AMHR-II = anti–mullerian hormone type II receptor; DAF-4 = development-regulatory growth factor type IV; XSTK3 = Xenopus activin receptor.
Except for family PPH019 (see above), the pattern of mutations observed when all additional members of the other eight families were screened by dHPLC and DNA sequencing was identical to the segregation pattern of PPH. None of the putative mutations were observed in 96 additional samples (a total of 196 chromosomes total, including 4 from the screening). In both samples, we also observed a synonymous SNP (2811G→A) with a minor-allele frequency of .21. Since the nine mutations appeared to be functional, and since no such mutations were observed in the controls, we applied Fisher's exact test to the data, and we observed a significant difference (P<.0001), in mutation prevalence, between cases and controls.
The mutation in exon 4 is in the transmembrane domain, and those in exons 6, 8, and 11 are in the kinase domain, of this serine/threonine kinase receptor (fig. 1). In functional studies of the homologous TGF-β receptor type II (TβR-II), cell lines lacking endogenous TβR-II were transfected with TβR-II constructs lacking either the complete kinase domain or amino acids 490–508 (homologous to amino acids 452–471 in BPMR-II) (Wieser et al. 1993). These constructs were unable to restore the ability to inhibit growth, stimulate fibronectin production, and drive transcription from a TGF-β–responsive element, in response to exogenous TGF-β that was observed when the cell lines were transfected with the wild-type TβR-II (Wieser et al. 1993). Furthermore, the construct lacking amino acids 490–508 was unable to function as a kinase in vitro (Wieser et al. 1993). Therefore, at least three of these mutations (premature terminations in exons 4, 6, and 8) should encode a nonfunctional receptor that is unable both to phosphorylate a type I receptor and to propagate the signal from a BMP ligand, since they will lack this region of the kinase domain. The lack of kinase activity would be consistent with a disease model of haploinsufficiency. Alternatively, the prematurely terminated products could act as a dominant negatives, as has been observed for TβR-II (Wieser et al. 1993) and endoglin (Lux et al. 2000). Given that BMPR-II is likely to be present, on the cell surface, as a dimer (Gilboa et al. 2000), only 25% of such complexes might be functional.
The two mutations in exon 11 change Arg491. This arginine is highly conserved in all type II TGF-β–superfamily receptors (fig. 2) and appears to be homologous to the invariant Arg280 in subdomain XI in other protein kinases (Hanks et al. 1988). Mutation of the homologous amino acid in transfected TβR-II (R582A) greatly reduces the ability of exogenous TGF-β to stimulate transcription of a TGF-β–responsive element in TβR-II–deficient cells (Brand and Schneider 1995). Last, arginine is the amino acid that is most frequently changed in disease mutations (see the “Statistics for Missense/Nonsense Mutations” Web page of the Institute of Medical Genetics, University of Wales College of Medicine, Cardiff). Taken together, these results strongly suggest that Arg491 is important to the function of BMPR-II. The mutations in exon 12 occur in the intracellular C-terminal domain, of unknown function, that is unique to BMPR-II. Possible functions for this portion of the molecule include the binding of downstream effector proteins or a role in dimerization and/or trafficking.
We screened 93% (2,913/3,117 bp) of the known publicly available coding sequence, the majority of the intron/exon boundaries, and 518 bp in the 5′ and 3′ UTRs of BMPR2, but we failed to find a causative mutation in 10 of the 19 families. There are several possible explanations for why this may have occurred. The causative mutations may occur in known or currently unknown coding sequences, intronic or regulatory regions of BMPR2, or other genes in the BMP-signaling pathway. Several of the linked families are large enough to produce LOD scores suggestive of linkage to 2q33 (individual LOD scores >2), indicating that we may not have screened the entire gene. It is possible that some of the families have disease mutations in the 204 bp of known exonic sequence that we missed in our screen, and we currently are rescreening exons 2–7 in the 10 families. There is also the possibility that some of the coding sequence of BMPR2 in lung tissue is currently unknown and that we therefore have not screened it. mRNA transcripts of 5, 6.5, 8, and 11.5 kb have been observed on northern blots, with the longest transcript predominating in lung tissue (Kawabata et al. 1995; Nohno et al. 1995; Rosenzweig et al. 1995), so we may have missed some alternatively spliced exons in our screen.
We have screened only a small portion of the intronic and regulatory sequences in these families, so mutations in these regions are possible. One regulatory region that we have examined is a (GGC)8–16 trinucleotide repeat at the 5′ end of the gene, at positions −928 to −963, which we amplified by means of the oligonucleotide primers TGAGCGAATCACAACCCCCCG and GAGTTCCGTCAGGAGCCCAG. Using PCR, we have not observed evidence of either an apparent increase in homozygosity or expansion of the repeat, either of which would be consistent with the suggestion of anticipation in PPH (Loyd et al. 1995); but detection of this event might require a Southern blot, and we have a limited quantity of DNA from the affected individuals (many of whom are deceased) in many of our families.
Last, it is possible that the mutations in these 10 families occur in other genes in the BMP-signaling pathway. The microsatellite data are consistent—but not conclusive—with linkage to PPH1 in all 19 families, but it is possible that the families with little linkage information could have no linkage to 2q33. An analogous situation has been observed in hereditary hemorrhagic telangectasia (HHT [MIM 187300]), another autosomal dominantly inherited vascular disorder with defects in the TGF-β–signaling pathway, where mutations have been observed in two genes, endoglin (HHT1) (McAllister et al. 1994) and the type I receptor ALK1 (HHT2) (Johnson et al. 1996).
So how do these mutations cause PPH? It is unlikely that they act as in a dominant-negative fashion, by inhibiting the apoptotic effect of the TGF-β pathway, because BMPR-II does not associate with type I receptors of the TGF-β family in transient-expression assays using mammalian cells (Liu et al. 1995), even though this occurs in vitro (Kawabata et al. 1995; Liu et al. 1995; Nohno et al. 1995). It is also unlikely that these mutations completely abolish the BMP-signaling pathway, because mice homozygous for a mutation in the kinase domain of BMPR2 die at day 9.5, prior to gastrulation (heterozygotes are grossly normal) (Beppu et al. 2000). This phenotype is very different from what we observe in PPH, and suggests that only 25%–50% of the function of the BMP pathway is required for it to perform a role in early development, given the analogy to TβR-II, which has been discussed above. The BMP pathway induces apoptosis in some cell types (Soda et al. 1998; Kimura et al. 2000), so a partial block of signal transmission might have a slow proliferative effect and could be caused by either dominant-negative protein interactions or reduced signal transmission due to haploinsufficiency of BMPR-II. As discussed above, both of these mechanisms have been observed with prematurely terminated protein products. Similarly, for both the missense mutations at Arg491 and the C-terminal mutations, either model is possible. In either case, both mechanisms lead to a partial block of BMP-signal transmission, which is likely to cause the development of PPH. This is similar to what has been observed for HHT (Lux et al. 2000), and determination of the mechanism of the mutations at PPH1 will require study of BMPR-II expression.
BMP signaling may occur through both the “Smad” (Massague 1998) and mitogen-activated protein kinase (Kimura et al. 2000) cascades, and both are inhibited by Smad6, which can be induced by vascular shear stress (Topper et al. 1997). Either (a) the reduced apoptotic signals from the BMP pathway, caused by mutations in either BMPR2 or other molecules in the signaling cascades, or (b) shear stress via Smad6, possibly after an initial nidus of vascular injury, might underlie many forms of PPH, including those associated with HIV or appetite-suppressant drugs.
Acknowledgments
We wish to thank the patients with PPH and their families for participating in this study and would like to dedicate this work to the memories of the family members who have been lost to PPH. We also wish to thank Drs. S. Rich, I. Aditia, D. Chitayat, B. Groves, M. Hoeper, E. Horn, M. Humbert, I. Lang D. Langleben, R. Schilz, and G. Simonneau for graciously encouraging a number of families with PPH to enter the study. This work was supported by National Heart, Lung and Blood Institute grant HL60056-1 (to J.H.M.), National Institute of Diabetes and Digestive and Kidney Diseases grant DK31813 (to S.E.H.), the Clinical Trials Department of Columbia University (support to J.A.K.), and United Therapeutics Corporation (support to J.H.M.).
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- BLAST, http://www.ncbi.nlm.nih.gov/BLAST (for search of HTGS database)
- David Clayton's Genetic Programs, http://www.mrc-bsu.cam.ac.uk/pub/methodology/genetics (for TRANSMIT version 2.5 [1999])
- Institute of Medical Genetics, University of Wales College of Medicine, Cardiff, http://www.uwcm.ac.uk/uwcm/mg/docs/haha1.html (for disease mutations)
- NCBI GenBank Overview, http://www.ncbi.nlm.nih.gov/Genbank/GenbankOverview.html (for BMPR2 cDNA sequence number NM_001204)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for PPH [MIM 178600] and HHT [MIM 187300])
References
- Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, Higenbottam T, Oakley C, Wouters E, Aubier M, Simonneau G, Begaud B (1996) Appetite-suppressant drugs and the risk of primary pulmonary hypertension: International Primary Pulmonary Hypertension Study Group. N Engl J Med 335:609–616 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410 [DOI] [PubMed] [Google Scholar]
- Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH (1996) A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension: The Primary Pulmonary Hypertension Study Group. N Engl J Med 334:296–302 [DOI] [PubMed] [Google Scholar]
- Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K (2000) BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol 221:249–258 [DOI] [PubMed] [Google Scholar]
- Beppu H, Minowa O, Miyazono K, Kawabata M (1997) cDNA cloning and genomic organization of the mouse BMP type II receptor. Biochem Biophys Res Commun 235:499–504 [DOI] [PubMed] [Google Scholar]
- Brand T, Schneider MD (1995) Inactive type II and type I receptors for TGF beta are dominant inhibitors of TGF beta-dependent transcription. J Biol Chem 270:8274–8284 [DOI] [PubMed] [Google Scholar]
- Clayton D (1999) A generalization of the transmission/disequilibrium test for uncertain-haplotype transmission. Am J Hum Genet 65:1170–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottingham RW Jr, Idury RM, Schäffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263 [PMC free article] [PubMed] [Google Scholar]
- D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT (1991) Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 115:343–349 [DOI] [PubMed] [Google Scholar]
- Deng Z, Haghighi F, Helleby L, Vanterpool K, Horn EM, Barst RJ, Hodge SE, Morse JH, Knowles JA (2000) Fine mapping of PPH1, a gene for familial primary pulmonary hypertension, to a 3-cM region on chromosome 2q33. Am J Respir Crit Care Med 161:1055–1059 [DOI] [PubMed] [Google Scholar]
- Douglas JG, Munro JF, Kitchin AH, Muir AL, Proudfoot AT (1981) Pulmonary hypertension and fenfluramine. Br Med J (Clin Res Ed) 283:881–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L, Nohe A, Geissendorfer T, Sebald W, Henis YI, Knaus P (2000) Bone morphogenetic protein receptor complexes on the surface of live cells: a new oligomerization mode for serine/threonine kinase receptors. Mol Biol Cell 11:1023–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T (1988) The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42–52 [DOI] [PubMed] [Google Scholar]
- Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, Guttmacher AE, Jackson CE, Attisano L, Kucherlapati R, Porteous ME, Marchuk DA (1996) Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 13:189–195 [DOI] [PubMed] [Google Scholar]
- Kawabata M, Chytil A, Moses HL (1995) Cloning of a novel type II serine/threonine kinase receptor through interaction with the type I transforming growth factor-beta receptor. J Biol Chem 270:5625–5630 [DOI] [PubMed] [Google Scholar]
- Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T (2000) BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J Biol Chem 275:17647–17652 [DOI] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM (1998) Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest 101:927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Ventura F, Doody J, Massague J (1995) Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol Cell Biol 15:3479–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd JE, Butler MG, Foroud TM, Conneally PM, Phillips JA, Newman JH (1995) Genetic anticipation and abnormal gender ratio at birth in familial primary pulmonary hypertension. Am J Respir Crit Care Med 152:93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A, Gallione CJ, Marchuk DA (2000) Expression analysis of endoglin missense and truncation mutations: insights into protein structure and disease mechanisms. Hum Mol Genet 9:745–755 [DOI] [PubMed] [Google Scholar]
- Massague J (1998) TGF-beta signal transduction. Annu Rev Biochem 67:753–791 [DOI] [PubMed] [Google Scholar]
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J (1994) Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 8:345–351 [DOI] [PubMed] [Google Scholar]
- Morse JH, Barst RJ (1994) Immunological disturbances in primary pulmonary hypertension. Semin Resp Crit Care Med 15:222–229 [Google Scholar]
- Morse JH, Jones AC, Barst RJ, Hodge SE, Wilhelmsen KC, Nygaard TG (1997) Mapping of familial primary pulmonary hypertension locus (PPH1) to chromosome 2q31-q32. Circulation 95:2603–2606 [DOI] [PubMed] [Google Scholar]
- Morse JH, Jones A, DiBenedetto A, Hodge SE, Nygaard TG (1996) Genetic mapping of primary pulmonary hypertension: evidence for linkage to chromosome 2 in a large family. Circulation 94:1–468964107 [Google Scholar]
- Nichols WC, Koller DL, Slovis B, Foroud T, Terry VH, Arnold ND, Siemieniak DR, Wheeler L, Phillips JA, Newman JH, Conneally PM, Ginsburg D, Loyd JE (1997) Localization of the gene for familial primary pulmonary hypertension to chromosome 2q31-32. Nat Genet 15:277–280 [DOI] [PubMed] [Google Scholar]
- Nohno T, Ishikawa T, Saito T, Hosokawa K, Noji S, Wolsing DH, Rosenbaum JS (1995) Identification of a human type II receptor for bone morphogenetic protein-4 that forms differential heteromeric complexes with bone morphogenetic protein type I receptors. J Biol Chem 270:22522–22526 [DOI] [PubMed] [Google Scholar]
- O'Donovan MC, Oefner PJ, Roberts SC, Austin J, Hoogendoorn B, Guy C, Speight G, Upadhyaya M, Sommer SS, McGuffin P (1998) Blind analysis of denaturing high-performance liquid chromatography as a tool for mutation detection. Genomics 52:44–49 [DOI] [PubMed] [Google Scholar]
- Pasque MK, Trulock EP, Cooper JD, Triantafillou AN, Huddleston CB, Rosenbloom M, Sundaresan S, Cox JL, Patterson GA (1995) Single lung transplantation for pulmonary hypertension: single institution experience in 34 patients. Circulation 92:2252–2258 [DOI] [PubMed] [Google Scholar]
- Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK (1987) Primary pulmonary hypertension: a national prospective study. Ann Intern Med 107:216–223 [DOI] [PubMed] [Google Scholar]
- Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K (1995) Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci USA 92:7632–7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda H, Raymond E, Sharma S, Lawrence R, Cerna C, Gomez L, Timony GA, Von Hoff DD, Izbicka E (1998) Antiproliferative effects of recombinant human bone morphogenetic protein-2 on human tumor colony-forming units. Anticancer Drugs 9:327–331 [DOI] [PubMed] [Google Scholar]
- Topper JN, Cai J, Qiu Y, Anderson KR, Xu YY, Deeds JD, Feeley R, Gimeno CJ, Woolf EA, Tayber O, Mays GG, Sampson BA, Schoen FJ, Gimbrone MA Jr, Falb D (1997) Vascular MADs: two novel MAD-related genes selectively inducible by flow in human vascular endothelium. Proc Natl Acad Sci USA 94:9314–9319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill PA, Jin L, Lin AA, Mehdi SQ, Jenkins T, Vollrath D, Davis RW, Cavalli-Sforza LL, Oefner PJ (1997) Detection of numerous Y chromosome biallelic polymorphisms by denaturing high-performance liquid chromatography. Genome Res 7:996–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV (2000) The molecular basis of lung morphogenesis. Mech Dev 92:55–81 [DOI] [PubMed] [Google Scholar]
- Wieser R, Attisano L, Wrana JL, Massague J (1993) Signaling activity of transforming growth factor beta type II receptors lacking specific domains in the cytoplasmic region. Mol Cell Biol 13:7239–7247 [DOI] [PMC free article] [PubMed] [Google Scholar]