Abstract
The genomic structure and composition of an avian metapneumovirus (aMPV) recently isolated from wild Canada geese (goose 15a/01) in the United States, together with its replication, virulence, and immunogenicity in domestic turkeys, were investigated. The sizes of seven of the eight genes, sequence identity, and genome organization of goose aMPV were similar to those of turkey aMPV subtype C (aMPV/C) strains, indicating that it belonged to the subtype. However, the goose virus contained the largest attachment (G) gene of any pneumovirus or metapneumovirus, with the predicted G protein of 585 amino acids (aa) more than twice the sizes of G proteins from other subtype C viruses and human metapneumovirus and more than 170 aa larger than the G proteins from the other aMPV subtypes (subtypes A, B, and D). The large G gene resulted from a 1,015-nucleotide insertion at 18 nucleotides upstream of the termination signal of the turkey aMPV/C G gene. Three other aMPV isolates from Canada geese had similarly large G genes, whereas analysis of recent aMPV strains circulating in U.S. turkeys did not indicate the presence of the goose virus-like strain. In vitro, the goose virus replicated to levels (2 × 105 to 5 × 105 50% tissue culture infective dose) comparable to those produced by turkey aMPV/C strains. More importantly, the virus replicated efficiently in the upper respiratory tract of domestic turkeys but with no clinical signs in either day-old or 2-week-old turkeys. The virus was also horizontally transmitted to naïve birds, and turkey infections with goose 15a/01 induced production of aMPV-specific antibodies. Challenging day-old or 2-week-old turkeys vaccinated with live goose aMPV resulted in lower clinical scores in 33% of the birds, whereas the rest of the birds had no detectable clinical signs of the upper respiratory disease, suggesting that the mutant virus may be a safe and effective vaccine against aMPV infection outbreaks in commercial turkeys.
Avian metapneumovirus (aMPV) is one of two paramyxoviruses belonging to the Metapneumovirus genus, the other virus being Human metapneumovirus (hMPV) (33, 42). Since the first detection in South Africa in 1978, aMPV-induced infections in turkeys have recurred worldwide, including in the United States since 1996 (20). Apart from infecting turkeys, aMPV has been associated with swollen-head syndrome of chickens (28, 31). Coughing, sneezing, nasal discharge, and swollen sinuses characterize the turkey aMPV disease in market birds, whereas poor egg quality and low productivity are observed in breeding turkeys (17, 18). Economic losses associated with aMPV infection are the result of poor weight gain and mortality, particularly in cases with secondary bacterial or viral infections, and processing plant carcass condemnation due to air sacculitis (21, 37). Nine years after the first outbreak, epidemics of aMPV turkey infections have persisted in the United States, primarily in the state of Minnesota, where 36.3% to 54.8% of commercial turkey flocks are infected annually, resulting in losses of approximately 15 million dollars per year (9, 37). In addition, the virus appears to be spreading, with severe outbreaks reported in Wisconsin and Iowa in 2004 and viral RNA detected in birds from North Dakota, South Dakota, and Ohio (4; Y. M. Saif, personal communication).
Metapneumoviruses have an enveloped virion containing a single-stranded, negative-sense RNA genome consisting of eight genes, with their products organized in the order 3′-N-P-M-F-M2-SH-G-L-5′, with a total length of between 13,134 (aMPV subtype C [aMPV/C]) and 13,378 (hMPV) nucleotides (12, 27). The aMPV strains isolated from the United States were a distinct subtype C, genetically different from the subtypes A (aMPV/A), B (aMPV/B), and D (aMPV/D) circulating in Europe, Asia, Africa, and South America (2, 22, 23, 24, 34, 38, 46, 47, 48). Approximately 80% of the aMPV outbreaks in the United States occur in spring (March to May) and autumn (October to November), corresponding to periods of wild bird migration, resulting in the hypothesis that wild birds may be involved with the transmission of virus between commercial turkey farms (4, 11, 38, 39). In support of this hypothesis, aMPV RNA was isolated from the nasal turbinates of wild sparrows, geese, blue-winged teal, and starlings and shown to share 90 to 95% nucleotide sequence identity with viruses isolated from domestic turkeys (3, 4, 37). In addition, an infectious aMPV was isolated from sentinel ducks housed in close proximity to an aMPV-infected turkey farm, and the virus also had high nucleotide sequence identity with turkey viruses (39). We recently isolated four strains of aMPV (goose 15a/01, goose 15b/01, goose 15c/01, and goose 15d/01) from asymptomatic wild Canada geese captured in Minnesota (3). Here, we analyzed the genetic composition of one of the goose isolates (goose 15a/01) and investigated its replication, virulence, and immunogenicity in commercial turkeys.
Studies have suggested that the aMPV/C strains have significant genetic and antigenic differences that may preclude use of a vaccine generated from one subtype to protect turkeys against a different aMPV subtype (6, 26). This became evident when serologic tests developed for subtype A and B viruses could not detect the initial subtype C isolates in the United States (35). Subsequently, Cook and others (6) demonstrated that subtype A and B vaccines could protect against subtype C challenge but not vice versa. Birds with convalescent-phase subtype C virus infections were partially protected against subtype A challenge and not at all protected against subtype B challenge. The study also found that neutralizing monoclonal antibodies against the attachment (G) glycoprotein from subtype A and subtype B viruses did not neutralize subtype C viruses (7). The phylogenetic comparisons of aMPV/C with other pneumoviruses revealed a closer relationship to hMPV than to aMPV/A, aMPV/B, or aMPV/D (27, 43).
In Europe and the United States, effective attenuated subtype-specific aMPV vaccines were initially produced by serial passage of the virus in nonturkey cells. For example, 25 passages of subtype A virus in monkey Vero cells produced an attenuated virus that did not induce clinical signs but was protective against virulent virus challenge, whereas 98 passages of the virus in turkey tracheal organ cultures did not attenuate the virus (44, 45). In the United States, a Vero cell-attenuated aMPV/C, passaged 63 times and producing no clinical signs, was recently licensed by the U.S. Department of Agriculture for vaccination of commercial turkeys (30). However, similar to the Vero cell-passaged subtype A vaccine used in Europe, the U.S. vaccine does not induce a strong humoral response, is only partially protective against an experimental virulent challenge, and, under field conditions, appears to reduce the severity of clinical signs but not the number of infected birds (19, 25, 30). Studies have shown that the two major surface proteins of paramyxoviruses, fusion (F) and G, are the most important antigens for the induction of protective immune response (15, 16, 29). In hMPV, antibodies against the F protein were protective across the A and B virus subtypes, whereas antibodies against the G protein protected against only infection with virus from the same subtype. As a result, the experimental vaccines for hMPV contain the F or G proteins (40, 41). Because the goose 15a/01 isolate was not associated with disease in Canada geese, and comparison of its genome sequence with those of the recently published subtype C turkey viruses revealed a conserved F gene but a major insertion at the G gene, we investigated whether this wild bird isolate might be avirulent but immunoprotective in commercial turkeys.
MATERIALS AND METHODS
Virus.
An aMPV isolate from asymptomatic juvenile Canada geese sampled in 2001 (goose 15a/01) as described previously was used for this research (3). The stock virus was passaged five times in specific-pathogen-free chicken embryo fibroblasts, followed by seven passages in monkey Vero cells before one freeze-thaw cycle and clarification to release infectious virus. The presence of goose 15a/01 in either cell type was confirmed by reverse transcriptase PCR (RT-PCR), immunofluorescence assay, and detection of cytopathic changes in cells (cell rounding and syncytial formation). The virus was titrated by serial dilutions onto Vero cells and found to be at 103.3 50% tissue culture infectious dose (TCID50) per milliliter.
Nucleotide sequencing of goose 15a/01.
The nucleotide sequences of nucleoprotein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), and second matrix protein (M2) of the goose 15a/01 strain were previously determined (4). Here, primers to isolate the short hydrophobic protein (SH), G, and RNA-dependent RNA polymerase (L) genes were designed from the turkey aMPV/C (Colorado strain) sequence (GenBank no. AY579780) (22). The PCR products were gel purified (QIAquick gel extraction kit; QIAGEN, Valencia, CA) and ligated into pCR2.1 cloning vector (TA cloning kit; Invitrogen, Carlsbad, CA). The cloning vector containing the insert was used to transform one-shot INVαF′ cells (Invitrogen, Carlsbad, CA), which were grown overnight. Transformed cells were lysed, and plasmid containing the insert was harvested using a QIAprep spin miniprep kit (QIAGEN Inc., Valencia, CA) and sent for automated sequencing at the University of Minnesota Advanced Genetic Analysis Center. Each derived plasmid insert was sequenced three times; in addition, live virus was sent to the USDA/ARS Southeast Poultry Research Laboratory for independent confirmation of the large G gene. The GenBank accession numbers for sequences used for comparison are as follows: APU39295, APU22110, X58639, D00850, X63408, S40185, AY734531, and APU65312 for subtype A genes; AF325442, AF325443, APU37586, Y14290, AJ492378, AJ492378, and AY728268 for subtype B genes; AY579780 for subtype C turkey virus genes; and NC_004148 for hMPV genes.
Inoculation of day-old turkeys with goose 15a/01.
A total of 62 day-old aMPV-free female turkeys, obtained from the Jennie-O Turkey Store hatchery (Barron, WI), were randomly divided into two groups and housed in the University of Minnesota animal housing units on the St. Paul campus. Handling of animals, including feeding and euthanasia, was in accordance with the U.S. Department of Agriculture and University of Minnesota animal care guidelines. One group (n = 28) was intranasally inoculated with 102.3 TCID50 of goose 15a/01 (in a 200-μl volume), whereas the other group (n = 22) was sham inoculated with 200 μl of Vero cell supernatant. All birds were monitored daily for clinical signs of aMPV infection. Nasal turbinates, tracheas, and lungs were collected from four birds each time at 5, 7, and 14 days postinoculation (p.i.) for virus detection and histopathologic examination. To determine whether goose 15a/01 could be horizontally transmitted in turkeys, naïve day-old turkeys (n = 12), identified by wing bands, were comingled with vaccinated birds (n = 28) 24 h after inoculation. Nasal turbinates from contact birds were collected at 5, 7, 10, and 14 days p.i. for virus detection and histopathologic analysis. The GenBank accession number of the goose 15a/01 isolate is DQ009484.
Inoculation of 2-week-old turkeys with goose 15a/01.
A total of 116 day-old aMPV-free female turkeys were obtained from the Jennie-O Turkey Store hatchery (Barron, WI) and reared in isolation for 2 weeks at the University of Minnesota animal housing units on the St. Paul campus. Handling of animals, including feeding and euthanasia, were in accordance with the U.S. Department of Agriculture and University of Minnesota animal care guidelines. At 2 weeks of age, one group (n = 63) was inoculated with 102.3 TCID50 of goose 15a/01 (in a 200-μl volume) via the oculonasal route. The remaining birds (n = 53) were sham inoculated with 200 μl of Vero cell supernatant as negative controls. The birds were monitored daily for clinical signs. Nasal turbinates, tracheas, and lungs were collected from four birds each time at 2, 4, 6, 8, 10, and 14 days p.i. for virus detection and histopathologic examination.
Ability of goose 15a/01 to protect against virulent aMPV challenge.
Turkeys infected with goose 15a/01 at 1 day or 2 weeks of age were assessed for their resistance against challenge with a virulent aMPV strain. Two weeks after inoculation with goose 15a/01, the remaining birds were intranasally challenged with 104 TCID50 of virulent turkey isolate 2a/97 and monitored daily for clinical signs. Sham-inoculated birds from both trials were similarly challenged with turkey 2a/97. The turkey 2a/97 isolate was previously shown to induce clinical signs in susceptible turkeys (13). The virulent-challenge model involved using bacterial coinfection to enhance the clinical signs of turkey 2a/97 aMPV infection. Pasteurella multocida CU vaccine strain (Schering-Plough Animal Health Corp., Omaha, Nebraska) and Bordetella avium (Veterinary Diagnostic Laboratory, University of Minnesota) were grown in tryptic soy broth. At 3 days post-aMPV challenge, birds were inoculated with 200 μl of tryptic soy broth containing 109 and 108 CFU of B. avium and P. multocida, respectively. Nasal turbinates, tracheas, and lungs were collected on days 2, 4, 7, 10, and 14 postchallenge for analysis of virus replication and histopathologic changes.
Clinical sign evaluation.
Clinical signs were monitored daily and evaluated using the following criteria: nasal discharge from one naris was given a score of 1, bilateral nasal discharge a 2, and turbid, bilateral discharge a 3. Swollen sinuses and foamy eyes each received a score of 1. The mean clinical score was calculated by dividing the summation of clinical signs by the total number of birds in the experimental group for each day. The percentage of birds showing clinical signs was calculated by dividing the number of birds showing clinical signs per day by the total number of birds remaining in the group.
Histopathologic analysis.
Sections of nasal turbinates were fixed in 10% buffered neutral formalin, embedded in paraffin wax, sectioned, and stained with hematoxylin and eosin. The sections of nasal turbinates were examined for inflammatory changes, and the severity of lesions was graded, using a 0 to 3 scale, by a pathologist blinded to treatment. The grade of 0 indicated no significant changes, whereas a grade of 3 indicated the most severe changes.
Immunohistochemical staining.
Formalin-fixed tissues were embedded in paraffin blocks, serially sectioned (3 to 4 μm thick), and immunohistochemically stained as previously described (14). Briefly, the deparaffinized sections were incubated with rabbit anti-aMPV polyclonal antibody, followed by incubation with biotinylated secondary antibody. The tissues were washed and incubated with streptavidin-biotin-horseradish peroxidase before the addition of 3-amino-9-ethylcarbazole-peroxidase chromogen as a substrate.
Detection of aMPV-specific IgG.
The aMPV-specific immunoglobulin G (IgG) was detected using an enzyme-linked immunosorbent assay as described previously (5, 10). Briefly, microtiter plates were coated with either viral (Colorado isolate grown in Vero cells) or control (noninfected Vero cells) antigen, and test serum diluted 1:40 was added to the wells. After washing, a horseradish peroxidase-labeled goat anti-turkey IgG conjugate (Kirkegaard & Perry, Gaithersburg, MD) was added to each well, followed by 0.4% ortho-phenylenediamine substrate (Sigma, St. Louis, MO). Color intensities were read at 490/405 nm and the results expressed as the optical density difference between virus antigen-coated and control antigen-coated wells for each serum sample. Samples were considered positive if the optical density difference was greater than 0.2.
Virus detection in turkey tissues.
The TaqMan RT-PCR was used to detect the presence of aMPV RNA in nasal turbinates of turkeys inoculated with goose 15a/01 or goose 2a/97 after challenge. The procedure uses primers specific for the M gene of aMPV/C (3). Nasal turbinates were also cultured for infectious aMPV isolation. Turbinate homogenates were centrifuged at 850 × g for 10 min, filtered though 0.2-μm paper, and passaged five times in Vero cells. Examination for cytopathic changes, direct immunofluorescence, and RT-PCR were used to confirm the presence of the virus after passage.
RESULTS
Molecular characterization of goose 15a/01.
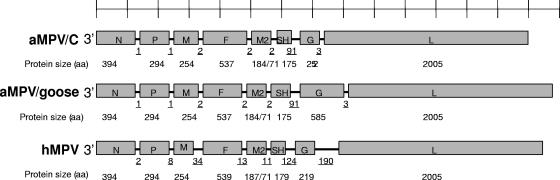
The genomic organization of the goose 15a/01 genome was identical to that of aMPV/C, with a gene product order of N-P-M-F-M2-SH-G-L (Fig. 1). In addition, except for the SH and G genes, the rest of the goose isolate genes were comparable in size to aMPV/C genes, and they had the conserved transcription start signal (GGGACAAGU); hence, the isolate was classified as a subtype C virus. The SH gene was 90 nucleotides (nt) larger than that of the Colorado strain, with the additional nucleotides located after the translation stop signal resulting in an open reading frame (ORF), as in the Colorado strain, corresponding to 175 amino acids (aa). The F gene cleavage site of Arg-Lys-Ala-Arg/Phe-Val-Leu was also conserved in the goose isolate, as in other aMPV/C strains. The goose 15a/01 N, P, M, F, M2, and SH genes had 95.1% to 99.3% nucleotide sequence and corresponding 90.5% to 99.3% amino acid sequence identities to aMPV isolates from domestic turkeys. The goose 15a/01 L gene consisted of 6,174 nt, 2 nt shorter than the turkey virus, but encoded a 2,005-aa protein, which was the same size as the turkey virus polymerase (Fig. 1). The two polymerase genes from goose and turkey subtype C viruses shared 98.3% nucleotide sequence and 99.3% amino acid sequence identities. In a comparison of the N, P, M, F, and L genes only, goose isolate 15a had 64.3% to 71.5% nucleotide sequence identity with aMPV/A and aMPV/B and 69.3% to 76.5% nucleotide sequence identity with hMPV (Table 1). In contrast, the M2, SH, and G gene ORFs, which had high levels of homology with aMPV/C turkey isolates (93.0% to 98.1%), had 4.9% to 64% nucleotide sequence identity with subtypes aMPV/A and aMPV/B and between 17.2% and 77.3% with hMPV (Table 1).
FIG. 1.
Genomic organization of some metapneumoviruses. Gene and protein sizes of the newly isolated goose aMPV/C are compared with those of turkey aMPV/C (Colorado) and hMPV (CAN97-83) isolates to illustrate the large size of the goose virus attachment gene. Underlined numbers indicate the numbers of nucleotides in each intergenic region.
TABLE 1.
Sequencing comparison of goose aMPV/C to aMPV subtypes A and B and hMPV
| MPV strain | % Homologya
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | P | M | F | M2-1 | M2-2 | SH | G | L | |
| aMPV/A | 65.1 (70.3) | 64.8 (54.3) | 71.0 (77.6) | 66.8 (72.3) | 64.0 (70.7) | 27.8 (21.1) | 6.3 (18.4) | 6.6 (18.7) | 64.8 (64.1) |
| aMPV/B | 66.7 (70.3) | 64.3 (54.1) | 71.5 (76.8) | 67.0 (72.1) | 13.1 (72.3) | 10.3 (23.9) | 4.9 (18.9) | 13.5 (14.5) | NA |
| aMPV/C | 97.3 (98.5) | 96.4 (97.3) | 97.6 (98.9) | 97.3 (98.1) | 96.9 (98.9) | 98.1 (98.6) | 95.9 (92.5) | 93.0 (88.5) | 98.3 (99.3) |
| hMPV | 75.6 (88.3) | 69.3 (66.7) | 76.5 (87.4) | 71.4 (81.6) | 77.3 (84.2) | 64.2 (54.9) | 17.2 (26.3) | 43.0 (25.6) | 73.3 (80.4) |
Percent nucleotide sequence homology between the goose isolate (2001) and other aMPV subtypes. Predicted amino acid sequence homology is shown in parentheses. N, nucleoprotein; P, phosphoprotein; M, matrix protein; F, fusion protein; M2-1, second matrix protein first ORF; M2-2, second matrix protein second ORF; SH, short hydrophobic protein; G, attachment glycoprotein; L, RNA-dependent RNA polymerase; NA, sequence not available.
The most significant difference between the goose 15a/01 isolate and other metapneumoviruses was in the attachment (G) gene. The goose virus contained a G gene of 1,798 nt that encoded a 585-aa protein (Fig. 2), one of the largest known attachment proteins in the Paramyxoviridae family. The goose virus G predicted protein was more than twice the size of the G proteins of other subtype C viruses (252 aa) or hMPV (between 219 and 236 aa). The goose virus G protein was 171 aa, 194 aa, 196 aa, and 287 aa larger than those encoded by aMPV/B, aMPV/A, aMPV/D, and human respiratory syncytial virus G genes, respectively. Comparison of the first 738 nt of the goose virus G protein, encoding 246 aa (Fig. 2, unshaded portion), with the turkey aMPV/C strain showed 93% nucleotide sequence identity and corresponding 88.5% amino acid sequence identity, whereas comparison with other paramyxoviruses revealed a 92 to 95% nucleotide sequence identity with the other aMPV subtypes (aMPV/A, aMPV/B, and aMPV/D), 94 to 96% with hMPV, and 89% with human respiratory syncytial virus. Interestingly, the enlargement of the G gene was due to an insert of 1,015 nt after a GGAG signal (nt positions 749 to 752) that terminates with another GGAG signal (nt positions 1765 to 1767) (Fig. 2). Immediately after the downstream GGAG, the nucleotide sequence corresponding to the final 18 nt of the turkey aMPV/C G gene ORF and encoding N-terminal amino acids Val-Ser-Leu-Arg-Ala-Ser was present in the goose G protein, just upstream of the G gene termination signal (AGUUAAUUAAAAA). However, frameshifting produced different N-terminal amino acids for the goose 15a/01 protein. A BLAST search of the unique segment of the G gene ORF (nt 739 to 1737) encoding 339 aa indicated that it was a unique sequence. Primers specific for the novel G insert were unable to amplify any sequence from either chicken embryo fibroblasts or Vero cells, the two cell types used to grow this virus. The identification of the nucleotide sequences and size of the G gene were independently confirmed by sequencing RNA isolated from live virus in Q. Yu's laboratory at the U.S. Department of Agriculture, Athens, GA.
FIG. 2.
Nucleotide sequence of the G attachment gene from the goose 15a/01 virus. The conserved metapneumoviral gene start (GGGACAAGT) and stop (AGTTAATTAAAAA) signals delineate the gene. A unique 1,015-nt insertion 18 nt upstream of the gene termination signal (shaded portion), flanked by GGAG sequences at both ends (in bold), accounts for the large size of the G gene (1,798 nt) and protein (585 aa).
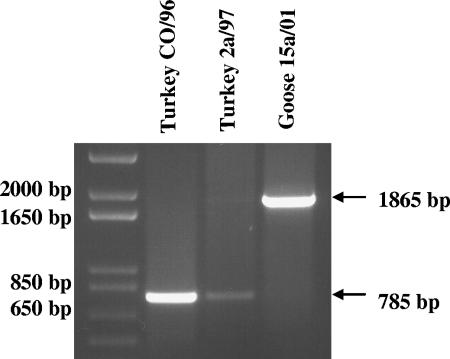
The large size of the goose 15a/01 G gene was confirmed by RT-PCR of the genomic viral RNA using forward primer 5′-ACAAGTCAACATGGAGGTCA-3′ generated from the conserved 5′end of the G gene from alignment of turkey aMPV/C (Colorado strain) and goose 15a/01 and degenerate reverse primer 5′-GGCAAGAYCCTATTGCATTG-3′ generated similarly from the 5′ end of the L gene. As shown in Fig. 3, the RNA isolated from turkey aMPV/C gave a PCR product of 785 nt, whereas viral RNA isolated from goose 15a/01 produced a 1,865-nt product, confirming the larger size of the goose 15a/01 G gene.
FIG. 3.
Agarose gel electrophoresis comparing the RT-PCR products of the G gene from turkey and goose aMPV/C strains. Genomic viral RNA was isolated from the turkey CO/96, turkey 2a/97, and goose 15a/01 strains, reverse transcribed, and examined by PCR using a forward primer derived from a conserved 3′ end of the G gene and a reverse primer derived from a relatively conserved region at the 3′ end of the L gene. The smaller product sizes for the two turkey aMPV/C strains (785 base pairs) contrast with the larger product from the goose virus (1,865 base pairs), confirming the presence of the large attachment gene.
Isolation and sequencing of the G genes from the other three Canada goose viruses (goose 15b/01, goose 15c/01, and goose 15d/01) revealed a G gene of the same length as that of goose 15a/01, with 98 to 99.9% nucleotide sequence identities. To determine whether the goose viruses were circulating in commercial turkeys, the G genes from recent turkey viruses (turkey 16a/01, turkey 17a/01, and turkey 18a/01) were isolated and found to have gene sizes (783 nt) similar to those of other turkey aMPV/C isolates.
The intergenic regions of the goose 15a/01 sequence were identical to those of the turkey aMPV/C isolates, with the N-P and P-M junctions having a single C, the M-F, F-M2, and M2-SH containing two U's, and the SH-G region comprising 91 nucleotides starting with UCAU. The goose 15a/01 SH-G intergenic region had 91.2% nucleotide sequence identity with that of the turkey aMPV/C (Table 2). The G-L intergenic region was a GAA. Because of the large size of the G gene, the genome size of goose 15a/01 was 14,071 nt (without the trailer or leader sequences), making it the largest of any member of the Metapneumovirus genus.
TABLE 2.
Sequencing comparison of SH-G intergenic region of goose aMPV/C to aMPV subtype C (Colorado)
| Strain | Sequencea |
|---|---|
| aMPV/goose | TCATGAGTATGTCTGGACAGTGCCAAGGCTAGG AAAAACTAACACGGGAACAGGTAATCCAAT GATTACAAATGATCAGAGAAGGAAAAAC |
| aMPV/Colorado | TCATGAATATGTCTGGACAGTGCCAAGGCCAAG AAAAACCAACACGAGAACAGGTGATCCAAT GATTAAAAACGATCAGAGAAGGAAAAAC |
Boldface letters indicate sequence differences.
Infection of domestic turkeys with goose 15a/01.
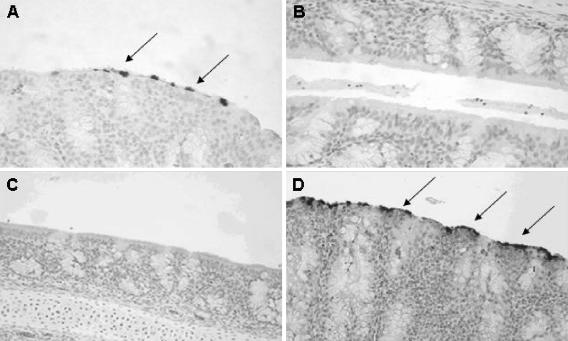
To determine whether goose 15a/01 could replicate in domestic turkeys, day-old and 2-week-old turkeys were intranasally inoculated with 2 × 102.3 virions of goose 15a/01 per bird and monitored daily for clinical signs. There were no respiratory clinical signs characteristic of aMPV disease in either day-old (n = 28) or 2-week-old (n = 63) turkeys inoculated with goose 15a/01 during the 14 days. Virus replication in turkey turbinates was confirmed by detection of viral RNA using RT-PCR, isolation of infectious particles (Table 3), and immunohistochemical detection of intracellular viral antigens (Fig. 4). For birds infected at 1 day of age, aMPV RNA was detected in the turkey turbinates of all birds tested between 1 and 8 days p.i. (n = 8) and in two of four birds tested at 10 to 14 days p.i. (Table 3). In contrast, no viral RNA was detected in sham-inoculated birds (n = 12). For birds infected at 2 weeks of age, aMPV RNA was detected in turbinate tissues in 5/12 birds tested; no viral RNA was detected in sham-inoculated controls. In addition, infectious aMPV particles were isolated from infected birds at 2 to 8 days p.i. (Table 3). The aMPV antigens were localized in the nasal turbinate epithelia of goose 15a/01-infected birds 2 to 8 days p.i. (Fig. 4A), whereas no antigens were evident in sham-inoculated controls (Fig. 4B).
TABLE 3.
Goose 15a/01 virus replication in turkey nasal turbinatesa
| Days p.i. | No. of birds with infection at:
|
Virus isolation | |||
|---|---|---|---|---|---|
| 1 day old
|
2 wk old
|
||||
| Sham inoculated | Infected | Sham inoculated | Infected | ||
| 2 to 5 | 0/4 (0) | 4/4 (3) | 0/2 (0) | 2/4 (1) | Yes |
| 6 to 8 | 0/4 (0) | 4/4 (4) | 0/2 (0) | 2/4 (2) | Yes |
| 10 to 14 | 0/4 (0) | 2/4 (0) | 0/2 (0) | 1/4 (0) | No |
| Total | 0/12 (0) | 10/12 (7) | 0/6 (0) | 5/12 (3) | |
Data are presented as number of animals positive for aMPV RNA by RT-PCR/total number tested. The number of RNA-positive birds also positive for viral antigens by immunohistochemistry is presented in parentheses. Whether or not the virus isolation was successful, which was only attempted in birds inoculated at 2 weeks old, is also indicated.
FIG. 4.
Immunohistochemical localization of aMPV antigens in the nasal turbinates of infected turkeys. (A) Nasal turbinate tissues from day-old turkeys intranasally inoculated with goose 15a/01 for 5 days show aMPV-reactive epithelial cells (dark spots), whereas (B) no reactivity is observed in sham-inoculated turkey poults. Infection of day-old or 2-week-old turkeys with goose 15a/01 for 2 weeks protected the birds from challenge with the turkey 2a/97 strain of aMPV/C. This resulted in (C) no detectable viral antigens in goose virus-vaccinated birds 5 days after challenge, whereas (D) nonvaccinated birds challenged with the virulent turkey strain demonstrated extensive aMPV antigens in nasal turbinate epithelial cells. Tissue sections were lightly counterstained with Mayer's hematoxylin. Arrows indicate cells positive for aMPV antigens.
Histopathologic analysis showed minor lesions for birds infected with goose 15a/01 at 1 day of age characterized by infiltration of the turbinate lamina propria with small numbers of lymphocytes, macrophages, and plasma cells. The Harderian glands had dilated ductules and infiltrations by small numbers of lymphocytes and macrophages. Summation of average lesion scores for birds infected at hatch resulted in an average score of 0.9 over the 2-week sampling period, compared to a score of 0.7 in sham-inoculated control birds. There were no histopathologic differences in birds inoculated at 2 weeks of age, both groups having an average score of 1.16 over the 2-week experiment.
Horizontal transmission of goose 15a/01 in turkeys.
Comingling naïve birds and infected birds provided evidence that the goose 15a/01 isolate could spread to susceptible birds. The ability of a vaccine virus to spread horizontally to noninfected birds is important in the poultry industry, because it reduces the cost and enhances the efficacy of vaccination. Noninfected day-old poults (n = 12) were introduced after 24 h into the room containing day-old goose 15a/01-infected turkeys. Four of the 12 (33.3%) uninfected comingled birds had detectable viral RNA in their nasal turbinates between 4 and 14 days after introduction. Three of the six birds tested (50%) had nasal turbinates that stained positive for aMPV antigens. Similar results were obtained in two independent experiments (data not shown).
Goose 15a/01-induced humoral immune response in turkeys.
Infection with goose15a/01 elicited a weak humoral response, similar to findings observed with other metapneumoviruses (19, 30). Six of 18 (33%) of the day-old turkeys infected with goose 15a/01 had detectable aMPV-specific antibodies 14 days p.i. (average titer = 15.6), whereas 4 of the 5 birds tested (80%) were positive at 30 days p.i. (average titer = 48). No antibodies were detected in the sham-inoculated turkeys.
Goose 15a/01 virus as live vaccine against virulent aMPV challenge.
Birds vaccinated with goose 15a/01 at 1 day of age and challenged with virulent turkey 2a/97 strain 14 days later demonstrated less-severe clinical signs, and they had no detectable viral RNA or antigens in their nasal turbinates (Table 4; Fig. 4 and 5). Maximal clinical scores were observed at 8 to 9 days postchallenge, with an average clinical sign score of 3.3 (±1.5) in the nonvaccinated turkeys compared to an average score of 0.6 (±0.90) in the vaccinated group (Fig. 5A). Similarly, 92% of nonvaccinated birds challenged with virulent virus showed clinical signs 8 days postchallenge, compared to 33% in the vaccinated/challenged group (Fig. 5B). No viral RNA was detected in nasal turbinate tissues from birds vaccinated at 1 day of age at any time after challenge (Table 4). In contrast, all nonvaccinated/challenged birds (n = 16) were positive for aMPV RNA in turbinate tissues between 2 and 14 days after challenge (Table 4). Viral antigens were detected in the nasal turbinates of nonvaccinated birds challenged with virulent turkey aMPV (Fig. 4D), whereas none were found in goose virus-vaccinated birds (Fig. 4C).
TABLE 4.
Detection of virus replication in goose 15a/01-vaccinated turkeys following challenge with virulent aMPVa
| Days postvaccination | No. of birds testing positive that were vaccinated at:
|
|||
|---|---|---|---|---|
| 1 day old
|
2 wk old
|
|||
| Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | |
| 2 to 5 | 16/16 (2/2) | 0/22 (0/2) | 1/1 (1/4) | 1/2 (0/4) |
| 6 to 8 | 2/2 (1/2) | 0/2 (0/2) | 2/2 (0/2) | 2/2 (0/2) |
| 10 to 14 | 2/2 (0/2) | 0/2 (0/2) | 3/4 (1/4) | 3/3 (1/4) |
| Total | 20/20 (3/6) | 0/26 (0/6) | 6/7 (2/10) | 6/7 (1/10) |
Data are presented as number positive for aMPV RNA by RT-PCR/total number tested, with number positive for viral antigens by immunohistochemistry/total number tested in parentheses.
FIG. 5.
Clinical signs scoring of day-old or 2-week-old turkeys inoculated with avirulent goose 15a/01 and challenged with virulent turkey 2a/97 virus 2 weeks later. The clinical signs were scored for each bird as described in Materials and Methods (on a scale of 0 to 4) averaged per time point after challenge and also presented as the percentage of birds showing disease at each time point after challenge. (A) Average clinical sign score and (B) percent showing clinical signs for birds vaccinated at 1 day old. (C) Average clinical score and (D) percent showing clinical signs for birds vaccinated at 2 weeks old.
In birds vaccinated at 2 weeks of age, an average clinical sign score of 3.7 (±1.72) was observed in the nonvaccinated birds, compared to 1.3 (±1.66) in the goose 15a/01-vaccinated/challenged birds at 9 days after challenge (Fig. 5C). Over 92% of nonvaccinated birds displayed clinical signs, compared to 33% in the goose virus-vaccinated birds at 9 days postchallenge (Fig. 5D). Six of the seven (85.7%) birds sampled in the vaccinated/challenged group and a similar number of nonvaccinated turkeys were positive for aMPV RNA.
DISCUSSION
In 2001, we isolated a wild bird virus, aMPV/C (goose 15a/01), from Canada geese captured in the state of Minnesota (3). Here, we completed genome analysis of this virus and investigated its replication, virulence, and immunogenicity in turkeys. The goose virus genome was organized in a pattern similar to those of the turkey aMPV/C strains, and most genes were of comparable sizes; thus, it was one of the subtype C viruses. However, the virus had a large G gene, the largest among both pneumoviruses and metapneumoviruses (4). The G protein of goose 15a/01 (585 aa) was more than twice the size of G proteins from other subtype C viruses and hMPV and more than 170 aa larger than G proteins from other aMPV subtypes. The large size of the goose 15a/01 G gene appears to have resulted from a 1,015-nt insertion 18 nt upstream of the termination signal of the turkey aMPV/C G gene. The insertion of such a large and unique sequence is unprecedented for paramyxoviral attachment genes, and the implications of this insertion in the function of the gene are unclear.
Analysis of paramyxoviral G proteins reveals that the surface glycoprotein is the most variable gene of pneumoviral and metapneumoviral proteins, both in terms of length and nucleotide sequence identity. For example, the G protein of hMPV (219 aa) is almost half the size of the G protein of aMPV/B (414 aa), and among aMPVs, the G protein of aMPV/B (414 aa) is more than 1.5 times the size of that of turkey aMPV/C (252 aa). All of these viruses cause similar respiratory diseases, which suggests that the size of the G protein does not obviate virus replication and induction of disease in a susceptible host. However, the size of the G protein may affect virulence, tissue tropism, and host species susceptibility to the virus. For example, the three aMPV subtypes, A, B, and D, circulating in Europe have comparably sized G proteins (391 aa, 414 aa, and 389 aa for subtypes A, B, and D, respectively) and they induce diseases of comparable severity in turkeys, including replication and induction of pathological changes in nasal turbinate cilia and induction of swollen-head syndrome in chickens (8, 28, 31, 36). In contrast, the turkey aMPV/C strains have a G protein of 252 aa, and they do not induce ciliastasis but have been shown to cause disease in chickens (37). It may be important to note that despite the size variations, the structural profiles of turkey aMPV/C G proteins, including hydrophobicity profiles, and the lengths of the transmembrane and extracellular domains were similar to those of the other aMPV subtypes (27). So far, the sequencing of data from hMPV strains has confirmed the high variability of the G gene, with 52 to 58% nucleotide sequence identity between subtypes but 74 to 100% within a subtype. However, the G protein sizes in these human counterparts of aMPV were comparable, ranging between 217 and 236 aa (1, 32). For the goose 15a/01 strain, the large G gene does not appear to affect replication in vitro, as the virus replicated to levels (2 × 105 to 5 × 105 TCID50) comparable to those observed with turkey aMPV/C strains in Vero cells. However, the size may contribute to the inability of the virus to induce respiratory disease in turkeys.
We have demonstrated that the goose 15a/01 strain replicates in the upper respiratory tract of domestic turkeys, with no detectable clinical signs of disease. In addition, the virus was actively shed, as demonstrated by virus detection in the upper respiratory tract of comingled naïve birds. The ability of goose 15a/01 to be horizontally transmitted makes it an ideal vaccine, resulting in a larger number of birds being vaccinated even when vaccine administration is not ideal, resulting in greater flock immunity. This is an important factor in a highly competitive industry that has a small profit margin. Birds vaccinated with the goose 15a/01 strain also demonstrated a humoral response with the production of protective levels of aMPV antibodies within 14 days, and the antibody titer increased progressively during the first 30 days in over 80% of the birds. However, the role of humoral immunity in protection against metapneumoviral infections remains unclear. The commercially available aMPV vaccines in Europe and the United States induce low levels of neutralizing IgG antibody isotypes, yet the birds are protected against a virulent challenge. This suggests that either cell-mediated immune response plays a more important role in controlling these pathogens or other immunoglobulins such as IgA may be more important in locally protecting the upper respiratory tract. Unfortunately, methods for detecting cell-mediated immune responses in turkeys remain unreliable.
Vaccinating both day-old and 2-week-old turkeys with live goose 15a/01 reduced both the number of birds showing clinical signs and the severity of clinical signs following virulent challenge. Vaccination at hatch is the preferred application time because of cost and other logistics in turkey production plants. We demonstrated that vaccination at hatch is safe and resulted in sterilizing immunity, as no challenge virus was detectable by RT-PCR, nor antigens by immunohistochemical staining of nasal turbinate tissues. In contrast, all nonvaccinated birds had extensive virus replication in the turbinates following challenge, resulting in severe clinical signs. Incidentally, birds vaccinated at 2 weeks of age had levels of virus replication in the turbinates comparable to those of the nonvaccinated birds, perhaps suggesting that vaccinating birds at hatch was more effective. In both day-old and 2-week-old birds, the severity of histological lesions in nasal turbinates was decreased in vaccinated birds compared to nonvaccinated birds following challenge with a virulent virus. The large G protein of the goose 15a/01 strain may explain its lack of virulence in turkeys. Furthermore, since this mutant virus does not appear to spread long-term in commercial turkeys, the unique G gene insert would serve as a good vaccine marker.
Acknowledgments
U.S. Department of Agriculture NRICGP grant 02393 and the Minnesota Agriculture Experiment Station Rapid Response Funds supported this research.
We thank Laurie Brewer, Humphrey Lwamba, Binu Velayudhan, Dale Lauer, and Anmei Cai for their technical assistance and critique.
REFERENCES
- 1.Bastien, N., L. Liu, D. Ward, T. Taylor, and Y. Li. 2004. Genetic variability of the G glycoprotein gene of human metapneumovirus. J. Clin. Microbiol. 42:3532-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bäyon-Auboyer, M. H., C. Arnauld, D. Toquin, and N. Eterradossi. 2000. Nucleotide sequences of the F, L and G protein genes of two non-A/non-B avian pneumoviruses (APV) reveal a novel APV subgroup. J. Gen. Virol. 81:2723-2733. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, R. S., B. McComb, H.-J. Shin, M. K. Njenga, K. V. Nagaraja, and D. A. Halvorson. 2002. Detection of avian pneumovirus in wild Canada geese (Branta canadensis) and blue-winged teal (Anas discors). Avian Dis. 46:1025-1029. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, R. S., M. K. Njenga, J. Nezworski, B. T. Velayudhan, K. V. Nagaraja, D. H. Zeman, N. Dyer, T. Graham, D. C. Lauer, and D. A. Halvorson. 2004. Evidence of avian pneumovirus spread among wild birds and domestic turkeys in central North America. Avian Dis. 48:902-908. [DOI] [PubMed] [Google Scholar]
- 5.Chiang, S., A. M. Dar, S. M. Goyal, M. A. Sheikh, J. C. Pedersen, B. Panigrahy, D. Senne, D. A. Halvorson, K. V. Nagaraja, and V. Kapur. 2000. A modified enzyme-linked immunosorbent assay for the detection of avian pneumovirus antibodies. J. Vet. Diagn. Investig. 12:381-384. [DOI] [PubMed] [Google Scholar]
- 6.Cook, J. K. A., F. Orthel, S. J. Orbell, M. A. Woods, and M. B. Huggins. 1996. An experimental turkey rhinotracheitis (TRT) infection in breeding turkeys and the prevention of its clinical effects using live-attenuated and inactivated TRT vaccines. Avian Pathol. 25:231-243. [DOI] [PubMed] [Google Scholar]
- 7.Cook, J. K. A., M. B. Huggins, S. J. Orbell, and D. A. Senne. 1999. Preliminary antigenic characterization of an avian pneumovirus isolated from commercial turkeys in Colorado, U.S.A. Avian Pathol. 28:607-617. [DOI] [PubMed] [Google Scholar]
- 8.Gough, R. E. 1994. Isolation of an avian pneumovirus from broiler chickens. Vet. Rec. 134:353-354. [DOI] [PubMed] [Google Scholar]
- 9.Goyal, S. M., D. Lauer, K. Friendshuh, and D. A. Halvorson. 2003. Seroprevalence of avian pneumovirus in Minnesota turkeys. Avian Dis. 47:244-250. [DOI] [PubMed] [Google Scholar]
- 10.Goyal, S. M., and M. A. Sheikh. 1999. Analysis of protection conferred by two killed vaccines, p. 51. In Proceedings of the North Central Avian Disease Conference. University of Minnesota, Minneapolis, Minn.
- 11.Graham, D. A., A. German, D. Abernethy, S. J. McCullough, R. J. Manvell, and D. J. Alexander. 1999. Isolation of ortho- and paramyxoviruses from wild birds in Northern Ireland during the 1997 Newcastle epizootic. Vet. Rec. 145:20-21. [DOI] [PubMed] [Google Scholar]
- 12.Ishiguro, N., T. Ebihara, R. Endo, X. Ma, H. Kikuta, H. Ishiko, and K. Kobayashi. 2004. High genetic diversity of the attachment (G) protein of human metapneumovirus. J. Clin. Microbiol. 42:3406-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jirjis, F. F., S. Noll, D. A. Halvorson, K. V. Nagaraja, E. L. Townsend, A. M. Sheikh, and D. P. Shaw. 2000. Avian pneumovirus infection in turkeys: experimental reproduction of the disease. Avian Dis. 44:222-226. [PubMed] [Google Scholar]
- 14.Jirjis, F. F., S. L. Noll, D. A. Halvorson, K. V. Nagaraja, and D. P. Shaw. 2001. Immunohistochemical detection of avian pneumovirus in formalin-fixed tissues. J. Vet. Diagn. Investig. 13:13-16. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, P. R., and P. L. Collins. 1988. The fusion glycoproteins of human respiratory syncytial virus of subgroups A and B: sequence conservation provides a structural basis for antigenic relatedness. J. Gen. Virol. 69:2623-2628. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, P. R., Jr., R. A. Olmsted, G. A. Prince, B. R. Murphy, D. W. Alling, E. E. Walsh, and P. L. Collins. 1987. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J. Virol. 61:3163-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, R. C., C. Baxter-Jones, G. P. Wilding, and D. F. Kelly. 1986. Demonstration of a candidate virus for turkey rhinotracheitis in experimentally inoculated turkeys. Vet. Rec. 119:599-600. [PubMed] [Google Scholar]
- 18.Jones, R. C., R. A. Williams, C. Baxter-Jones, C. E. Savage, and G. P. Wilding. 1988. Experimental infection of laying turkeys with rhinotracheitis virus: distribution of virus in the tissues and serological response. Avian Pathol. 17:841-850. [DOI] [PubMed] [Google Scholar]
- 19.Jones, R. C., C. J. Naylor, A. Al-Afaleq, K. J. Worthing, and R. Jones. 1992. Effect of cyclophosphamide immunosuppression on the immunity of turkeys to viral rhinotracheitis. Res.Vet. Sci. 53:38-41. [DOI] [PubMed] [Google Scholar]
- 20.Kleven, S. H. 1997. Report of the committee. Transmissible diseases of poultry and other avian species, p. 486-491. In Proceedings of the 101st annual meeting of the U.S. Animal Health Association. U.S. Animal Health Association, Richmond, Va.
- 21.Kumar, M. 1999. Incidence of APV in the field, p. 18-20. In Proceedings of the 50th North Central Avian Disease Conference. University of Minnesota, Minneapolis, Minn.
- 22.Li, J., R. Ling, J. S. Randhawa, K. Shaw, P. J. Davis, K. Juhasz, C. R. Pringle, A. J. Easton, and D. Cavanagh. 1996. Sequence of the nucleocapsid protein gene of subgroup A and B avian pneumoviruses. Virus Res. 41:185-191. [DOI] [PubMed] [Google Scholar]
- 23.Ling, R., P. J. Davis, Q. Yu, C. M. Wood, C. R. Pringle, D. Cavanagh, and A. J. Easton. 1995. Sequence and in vitro expression of the phosphoprotein gene of avian pneumovirus. Virus Res. 36:247-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling, R., A. J. Easton, and C. R. Pringle. 1992. Sequence analysis of the 22K, SH, and G genes of turkey rhinotracheitis virus and their intergenic regions reveals a gene order different from that of other pneumoviruses. J. Gen. Virol. 73:1709-1715. [DOI] [PubMed] [Google Scholar]
- 25.Lippert, R. 2003. Morgan project, a success. Gobbles 60:4-7. [Google Scholar]
- 26.Lwamba, H. C., D. A. Halvorson, K. V. Nagaraja, E. A. Turpin, D. Swayne, B. S. Seal, and M. K. Njenga. 2002. Antigenic cross-reactivity among avian pneumoviruses of subgroups A, B, and C at the matrix but not nucleocapsid proteins. Avian Dis. 46:725-729. [DOI] [PubMed] [Google Scholar]
- 27.Lwamba, H. C. M., R. Alvarez, M. G. Wise, Q. Yu, D. Halvorson, M. K. Njenga, and B. S. Seal. 2005. Comparison of the full-length genome sequence of avian metapneumovirus subtype C with other paramyxoviruses. Virus Res. 107:83-92. [DOI] [PubMed] [Google Scholar]
- 28.Maharaj, S. B. 1994. Isolation of an avian pneumovirus-like agent from broiler breeder chickens in South Africa. Vet. Rec. 134:525-526. [DOI] [PubMed] [Google Scholar]
- 29.Olmsted, R. A., N. Elango, G. A. Prince, B. R. Murphy, P. R. Johnson, B. Moss, R. M. Chanock, and P. L. Collins. 1986. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc. Natl. Acad. Sci. USA 83:7462-7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patnayak, D. P., A. M. Sheikh, B. R. Gulati, and S. M. Goyal. 2002. Experimental and field evaluation of a live vaccine against avian pneumovirus. Avian Pathol. 31:377-382. [DOI] [PubMed] [Google Scholar]
- 31.Pattison, M., and N. J. Chette. 1989. Observation on swollen head syndrome in broiler and broiler breeder chickens. Vet. Rec. 125:229-231. [DOI] [PubMed] [Google Scholar]
- 32.Peret, T. C. T., Y. Abed, J. Anderson, D. D. Erdman, and G. Boivin. 2004. Sequence polymorphism of the predicted human metapneumovirus G glycoprotein. J. Gen. Virol. 85:679-686. [DOI] [PubMed] [Google Scholar]
- 33.Pringle, C. R. 1999. Virus taxonomy—1999. The universal system of virus taxonomy, updated to include the new proposals ratified by the International Committee on Taxonomy of Viruses during 1998. Arch. Virol. 144:421-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randhawa, J. S., C. R. Pringle, and A. J. Easton. 1996. Nucleotide sequence of the matrix protein gene of a subgroup B avian pneumovirus. Virus Genes 12:179-183. [DOI] [PubMed] [Google Scholar]
- 35.Senne, D. A., and R. K. Edson. 1997. Avian pneumovirus update, p. 190. In Proceedings of the134th Annual Convention of the American Veterinary Medical Association. Spectrum Press, Reno, Nev.
- 36.Shin, H.-J., B. McComb, A. Back, D. P. Shaw, D. A. Halvorson, and K. V. Nagaraja. 2000. Susceptibility of broiler chicks to infection by avian pneumovirus of turkey origin. Avian Dis. 44:797-802. [PubMed] [Google Scholar]
- 37.Shin, H.-J., M. K. Njenga, B. McComb, D. A. Halvorson, and K. V. Nagaraja. 2000. Avian pneumovirus (APV) RNA from wild and sentinel birds in the United States has genetic homology with RNA from APV isolates from domestic turkeys. J. Clin. Microbiol. 38:4282-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin, H.-J., K. T. Cameron, J. A. Jacobs, E. A. Turpin, D. A. Halvorson, S. M. Goyal, K. V. Nagaraja, M. C. Kumar, D. C. Lauer, B. S. Seal, and M. K. Njenga. 2002. Molecular epidemiology of subgroup C avian pneumoviruses isolated in the United States and comparison with subgroup A and B viruses. J. Clin. Microbiol. 40:1687-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin, H.-J., K. V. Nagaraja, B. McComb, D. A. Halvorson, F. F. Jirjis, D. P. Shaw, B. S. Shaw, B. S. Seal, and M. K. Njenga. 2002. Isolation of avian pneumovirus from mallard ducks that is genetically similar to viruses isolated from neighboring commercial turkeys. Virus Res. 83:207-212. [DOI] [PubMed] [Google Scholar]
- 40.Skiadopoulos, M. H., S. Biacchesi, U. J. Buchholz, J. M. Riggs, S. R. Surman, E. Amaro-Carambot, J. M. McAuliffe, W. R. Elkins, M. St. Claire, P. L. Collins, and B. R. Murphy. 2004. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J. Virol. 78:6927-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang, R. S., J. H. Schickli, M. MacPhail, F. Fernandes, L. Bicha, J. Spaete, R. A. M. Fouchier, A. D. M. E. Osterhaus, R. Spaete, and A. A. Haller. 2003. Effects of human metapneumovirus and respiratory syncytial virus antigen insertion in two 3′ proximal genome positions of bovine/human parainfluenza virus type 3 on virus replication and immunogenicity. J. Virol. 77:10819-10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Hoogen, B. G., J. C. de Jong, J. Groen, T. Kuiken, R. de Groot, R. A. Fouchier, and A. D. Osterhaus. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Hoogen, B. G., T. M. Bestebroer, A. D. M. E. Osterhaus, and R. A. M. Fouchier. 2002. Analysis of the genomic sequence of a human metapneumovirus. Virology 295:119-132. [DOI] [PubMed] [Google Scholar]
- 44.Williams, R. A., C. E. Savage, and R. C. Jones. 1991. Development of a live attenuated vaccine against turkey rhinotracheitis. Avian Pathol. 20:45-55. [DOI] [PubMed] [Google Scholar]
- 45.Williams, R. A., C. E. Savage, K. J. Worthington, and R. C. Jones. 1991. Further studies on the development of a live attenuated vaccine against turkey rhinotracheitis. Avian Pathol. 20:585-596. [DOI] [PubMed] [Google Scholar]
- 46.Yu, Q., P. J. Davis, T. Barret, M. M. Binns, M. E. G. Boursnell, and D. Cavanagh. 1991. Deduced amino acid sequence of the fusion glycoprotein of turkey rhinotracheitis virus has greater identity with that of human respiratory syncytial virus, a pneumovirus, than that of paramyxoviruses and morbilliviruses. J. Gen. Virol. 72:75-81. [DOI] [PubMed] [Google Scholar]
- 47.Yu, Q., P. J. Davis, J. Li, and D. Cavanagh. 1992. Cloning and sequencing of the matrix protein (M) gene of turkey rhinotracheitis virus reveal a gene order different from that of respiratory syncytial virus. Virology 186:426-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu, Q., P. J. Davis, T. D. K. Brown, and D. Cavanagh. 1992. Sequence and in vitro expression of the M2 gene of turkey rhinotracheitis pneumovirus. J. Gen. Virol. 73:1355-1363. [DOI] [PubMed] [Google Scholar]