Abstract
West Nile virus has spread rapidly across the United States, and there is currently no approved human vaccine or therapy to prevent or treat disease. Passive immunization with antibodies against the envelope protein represents a promising means to provide short-term prophylaxis and treatment for West Nile virus infection. In this study, we identified a panel of 11 unique human single-chain variable region antibody fragments (scFvs) that bind the envelope protein of West Nile virus. Selected scFvs were converted to Fc fusion proteins (scFv-Fcs) and were tested in mice for their ability to prevent lethal West Nile virus infection. Five of these scFv-Fcs, 11, 15, 71, 85, and 95, protected 100% of mice from death when given prior to infection with virus. Two of them, 11 and 15, protected 80% of mice when given at days 1 and 4 after infection. In addition, four of the scFv-Fcs cross-neutralized dengue virus, serotype 2. Binding assays using yeast surface display demonstrated that all of our scFvs bind to sites within domains I and II of West Nile virus envelope protein. These recombinant human scFvs are potential candidates for immunoprophylaxis and therapy of flavivirus infections.
West Nile virus (WNV) is a mosquito-borne Flavivirus that causes febrile illness and neurological disease in humans, horses, and birds (18). Endemic to Africa, the Middle East, Europe, and Asia, WNV was first detected in the United States in 1999 (6). Since its introduction into North America, WNV has spread throughout the lower 48 states, as well as into Canada, Mexico, and the Caribbean (10). In humans, WNV infection can develop into meningitis or encephalitis and may lead to death, particularly among the elderly and immunocompromised (6).
As WNV disseminates across the country, the need for effective vaccines and therapies continues to grow. Currently there is no WNV vaccine approved for human use, and the only treatments are supportive. In vitro studies have found ribavirin and alpha2b interferon to be effective against the virus (3, 14, 31), and several human case studies have found that alpha interferon may improve the clinical outcome of WNV infection (15, 25). Antibodies, including both monoclonal antibodies (MAbs) and polyclonal immune sera, represent another potential direction for influencing disease outcome. Several human case reports have suggested that the administration of immune pooled intravenous immunoglobulin (Ig) may aid in the recovery from WNV infection (1, 11, 12, 26); however, there have been no controlled clinical trials demonstrating the efficacy of intravenous Ig as a treatment for WNV.
In a murine model of WNV, antibody therapy has been shown to be effective both as prophylaxis and as treatment for infection. Mice administered human anti-WNV gamma globulin prior to infection were protected from disease, while those given gamma globulin after infection had spread to the central nervous system had an improved clinical outcome (5, 7). Similarly, hamsters administered immunoglobulin 24 h prior to infection were completely protected from infection (28). The WNV envelope (E) glycoprotein is a likely candidate protein to target using passive immunization (23, 30), and monoclonal antibodies produced against the E protein have been found to protect mice from lethal infection (19). Augmenting the level of antibodies against the WNV E protein may prove to be an important treatment strategy for infection, particularly for elderly and immunocompromised patients with immune system deficiencies.
Several MAbs provide cross-protection in animal models against related flavivirus infections (8, 23). The development of cross-protective antibodies is of particular interest because it would be possible to prevent and/or treat several different infections with the same therapeutic antibody preparation. Because many of the flaviviruses are recognized by cross-reactive antibodies, a cross-protective therapy could be used in the absence of virus-specific diagnostics and will reduce the time before treatment can be initiated.
The use of nonhuman antibodies in patients has safety considerations, including cross-species sensitization and potential contamination with blood-borne pathogens. Many of these problems can be overcome by the use of specific humanized antibodies. Recombinant human antibodies created in an in vitro system, such as the phage display system used in this study, provide many advantages over conventional antibody development techniques. These antibodies can be quickly and easily developed at a high titer, are free of blood-borne pathogens, and can be produced without the need for immunization. In addition, human antibodies can be administered without complications of serum sickness or other immune responses to the presence of nonhuman antigens (24).
Although there is a large body of data on murine flavivirus MAbs, there have been few flavivirus MAbs identified from humans or other primates (9). More information regarding human MAbs is clearly needed to develop optimal prophylaxis and treatment strategies for human disease. In this study, we have developed and evaluated the efficacy of recombinant human single-chain variable fragments (scFv) fused to an IgG1 Fc domain and a polyclonal E protein antiserum as both prophylaxis and treatment for lethal WNV infection. We have also evaluated the ability of these antibodies to neutralize related flaviviruses. This study is the first study to characterize human antibodies against WNV and is the first study to use a phage display screen to identify human antibodies against WNV.
MATERIALS AND METHODS
Screening of phage libraries.
scFvs against the WNV E protein were identified using a phage display screen. Two human, nonimmune phage display libraries were screened; both were created from the B cells of normal, non-WNV-immune humans and contain between 12 and 15 billion unique phage displayed in the phagemid vector pFarber as fusion proteins with phage coat protein III (27; http://research.dfci.harvard.edu/nfcr-ctae/research/mehta.php). Maxisorp immunotubes (Nalge Nunc International) were coated overnight with recombinant WNV E protein antigen (rWNV-E) that was expressed in Drosophila melanogaster S2 cells and highly purified (17, 32) at a concentration of 15 μg/ml in phosphate-buffered saline (PBS), pH 7.4. Phage (5 × 1012) were added to the tubes and allowed to bind for 2 h at room temperature. Nonspecifically absorbed phages were removed by extensive washing (15 times with PBS-0.05% Tween 20, 15 times with PBS), and bound phage were eluted in 100 mM triethylamine. Eluted phage were allowed to infect Escherichia coli TG1 cells, and pooled phage were rescued by VCS M13 helper phage and concentrated by polyethylene glycol-NaCl precipitation (27). Four rounds of selection were performed. Following the second, third, and fourth rounds of selection, individual TG1 colonies were screened by enzyme-linked immunosorbent assay (ELISA).
For ELISA screening, 96-well microtiter plates were coated overnight with rWNV-E (10 μg/ml) in PBS, pH 7.4. Plates were blocked with PBS-2% milk for 1 h. After extensive washing with PBS-Tween 20, plates were incubated with anti-M13-horseradish peroxidase (HRP) conjugate (Amersham) to detect the M13 tag on the scFvs, developed with Sure Blue Microwell peroxidase (KPL), and stopped after 10 min with tetramethylbenzidine stop solution (KPL), and the optical density at 450 nm (OD450) was measured. Phage that bound to rWNV-E with an OD450 of >1.0 were scored as positive. Phage clones that bound to rWNV-E were sequenced, and their corresponding amino acid sequences were aligned.
Expression and purification of scFvs and scFv-Fc fusion proteins.
Antibody genes of rWNV-E-specific scFvs were excised from the phagemid vector by NotI-NcoI digestion and ligated into the prokaryotic expression vector pSyn (4), which adds C-terminal c-myc and His6 tags. E. coli XL-1 Blue cells were transformed with the plasmids, individual colonies were screened by restriction digestion, and the insert DNA sequences were verified. For scFv expression, bacteria were grown in 2× YT (tryptone-yeast extract) medium containing 0.1% glucose and 100 μg/ml ampicillin and were induced overnight with 1 mM isopropyl-β-d-thiogalactopyranoside at 30°C. Bacterial cultures were pelleted and resuspended in PBS containing Complete Protease Inhibitor Cocktail tablets (Roche), and the cultures were sonicated for 2 min. The sonicate was centrifuged to remove insoluble debris, the protein was precipitated from the supernatant with 4.1 M ammonium sulfate, and the precipitated protein was purified on a chelating Sepharose (Amersham) column. Purified scFvs were dialyzed overnight against PBS, concentrated, and stored at −70°C.
Purified scFvs were tested for their binding activity against rWNV-E by ELISA. Ninety-six-well microtiter plates were coated overnight with rWNV-E (1 μg/ml in PBS). Plates were blocked with PBS-2% milk, followed by incubation with 10-fold dilutions of the scFvs for 1 h at room temperature. Monoclonal anti-His-HRP (1:4,000; Invitrogen Corporation, Carlsbad, CA) was added for 1 h, and the plates were developed and read as described above.
For production of scFv-Fc fusion proteins, antibody genes were excised from the phagemid vector by NotI-SfiI digestion and cloned into the human IgG1 expression vector pcDNA 3.1 Hinge. scFv-Fc fusion proteins were expressed in 293T cells by transient calcium phosphate transfection and purified by protein A-Sepharose (Amersham) affinity chromatography. scFv-Fc fusions were screened for binding activity against rWNV-E by ELISA as described above for purified scFvs using antihuman IgG-HRP (Sigma) as a secondary antibody.
Serum and rabbit IgG preparation.
A New Zealand White rabbit was immunized with 50 μg of rWNV-E in complete Freund's adjuvant and boosted twice with the same antigen in incomplete Freund's adjuvant at 3-week intervals, and the serum was collected. The IgG fraction was purified from the whole rabbit sera by protein G affinity chromatography (Amersham). Nonimmune rabbit serum was obtained from animals with no history of flavivirus exposure and with no reactivity to the E protein as measured by ELISA and Western blotting. Normal, nonimmune human IgG1 was obtained from Sigma.
The F(ab′)2 fraction was prepared from the purified anti-rWNV-E IgG fraction by digestion with immobilized pepsin using the Immunopure F(ab′)2 preparation kit (Pierce). Intact IgG and Fc fragments were removed from the digests by protein A column chromatography, and the F(ab′)2 fraction was further purified by Sephacryl S-100 column chromatography in PBS. Protein concentration was determined by bicinchoninic acid protein assays (Pierce).
PRNT assay.
For the plaque reduction neutralization (PRNT) assay, Vero cells (ATCC CCL-81) were seeded in six-well plates at a density of 2 × 105 cells/ml 24 h before infection. For WNV (strain 2741, a lineage I isolate) (2, 20) and St. Louis encephalitis virus (SLEV; strain Parton P-3) neutralization, cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (FCS) and 100 μg/ml penicillin-streptomycin at 37°C in 5% CO2. For dengue virus serotype 2 (DENV-2; strain New Guinea C) neutralization, cells were maintained in Dulbecco's modified Eagle's medium-F-12 supplemented with 10% FCS, 100 μg/ml penicillin-streptomycin, and 100 μg/ml amphotericin B (Fungizone) at 37°C in 5% CO2.
Serial dilutions of IgG, scFvs, or scFv-Fcs were mixed with 100 PFU virus and incubated for 1 h at 37°C in 5% CO2. The virus-antibody mixture was added to the cell monolayers and incubated for another hour. Cells were overlaid with 3 to 4 ml of 1% agarose in cell culture medium, and after 4 days a second overlay of 2.5 ml 1% agarose-medium containing 0.01% neutral red was added to visualize plaques. The neutralization assay for DENV-2 was conducted as described above, but cells were incubated for 6 days before the second overlay.
Affinity measurements by Biacore.
The binding kinetics and affinity of the scFvs for rWNV-E were measured by surface plasmon resonance (Biacore 3000, Uppsala, Sweden). scFvs (30 to 50 μg/ml) were covalently immobilized to a nitrilotriacetic acid (NTA) sensor chip (Biacore) via their histidine tag. The running buffer used contained 0.01 M HEPES (pH 7.4) with 0.15 M NaCl2, 50 μM EDTA, and 0.005% Surfactant P20 (Biacore). The NTA surface was activated with 500 μM NiCl2 in running buffer. All experiments were run at a flow rate of 20 μl/minute in HBS-EP buffer (Biacore). The chip surface was regenerated with 0.01 M HEPES with 0.15 M NaCl, 0.35 M EDTA, and 0.005% Surfactant P20, pH 8.3. The binding kinetic parameters were measured by varying the molar concentration (0.704 to 440 nM) of rWNV-E and analyzed using BIA-EVALUATION software (Biacore).
To measure the binding affinity of the scFv-Fcs to rWNV-E, scFv-Fcs (30 μg/ml) were first captured using goat anti-human IgG (30 μg/ml in 10 mM sodium acetate, pH 5.0; Bethyl Laboratories, Montgomery, TX) that was covalently coupled to a CM4 sensor chip (Biacore) using an amine coupling kit (Biacore). Assays with the scFv-Fcs were run at a flow rate of 20 μl/minute in HBS-EP buffer (Biacore), and the chip surface was regenerated with 10 mM glycine, pH 1.8. The binding kinetics were measured and analyzed as above.
Mouse passive immunization and viral challenge.
Groups of 5 to 10 female C57BL/6 mice (Jackson Laboratories) between 4 and 6 weeks of age were used in all experiments. Mice were infected with 102 to 103 PFU WNV intraperitoneally. In experiments with rabbit antibodies, mice were injected intraperitoneally with the indicated doses of serum or IgG at times ranging from 1 day prior to 5 days post-WNV infection. Human IgG1, scFvs, and scFv-Fc constructs were administered subcutaneously either 24 h before or up to 96 h after virus inoculation. Survival was recorded daily until no further deaths had occurred for at least 7 days or for 21 days after infection, whichever came last. All animal experiments were conducted in accordance with the Yale University Animal Care and Use Committee regulations.
Domain mapping.
The E protein ectodomain, DIII, and DI/DII were cloned into a yeast display vector, pYD1 (Invitrogen), as previously described (19). This expression vector displays proteins of interest as a fusion protein with the AGA2 gene of Saccharomyces cerevisiae. Briefly, the clones were then transformed into S. cerevisiae strain EBY 100. The transformed yeast cells were grown on minimal dextrose plates containing leucine. Single colonies were grown overnight in yeast nitrogen base-Casamino Acids medium containing 2% glucose, and display of the fusion protein was induced by the addition of 2% galactose at log phase. The expression of the fusion protein was monitored for 12 to 48 h postinduction to determine the optimal induction time for maximum display. Protein display was confirmed by staining with anti-Xpress antibody (Invitrogen).
Yeast cells expressing pYD1, the WNV ectodomain, WNV DIII, or WNV DI/DII were plated in 96-well plates and incubated for 30 min on ice with scFv-Fcs (1 μg/ml) conjugated to Alexa Fluor 647 (Invitrogen/Molecular Probes) at a 1:500 dilution. scFv-Fc conjugates were prepared according to the manufacturer's directions. Cells were washed three times with PBS-1% bovine serum albumin, and the cells were fixed in 1% paraformaldehyde and counted on a FACsCalibur (Becton Dickinson). Data were analyzed with Cell Quest software. Alternatively, unconjugated antibodies were incubated with yeast at a concentration of 50 μg/ml for 30 min on ice, followed by incubation with goat anti-human IgG-Alexa Fluor 647 (Invitrogen/Molecular Probes) at a concentration of 1:500 in 1 mg/ml bovine serum albumin in PBS for an additional 30 min. Cells were washed and fixed as described above.
RESULTS
Passive immunization with rabbit anti-rWNV-E IgG.
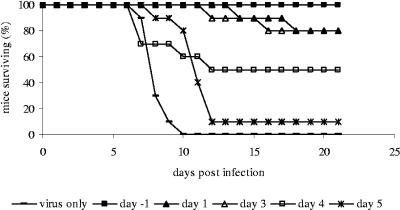
Studies were first performed with a well-characterized rabbit polyclonal rabbit anti-rWNV-E antibody to establish the dose and timing of antibody therapy in the murine model (30). As expected, passive transfer of rabbit anti-rWNV-E serum or the IgG fraction purified from the rabbit antiserum completely protected C57BL/6 mice from lethal infection with WNV when given prior to infection. In addition, a single dose of rabbit anti-rWNV-E IgG was active therapeutically up to 4 days post-viral challenge (Fig. 1). Antibodies given after day 5 were not able to rescue infected mice, and mice succumbed to infection (data not shown). Even though antibodies administered at days 1 and 3 after infection did not protect 100% of mice, there was a significant increase in the average survival time among mice administered antibody at day −1, 1, or 3 but not for mice administered antibody at day 4 or 5. Mice given antibody at day 4 or 5 (14.3 ± 7.2 or 11.0 ± 3.7 days, respectively) did not differ significantly in their average survival time from mice given virus only (7.3 ± 0.8 days).
FIG. 1.
Survival of mice passively immunized with rabbit anti-rWNV-E IgG. Groups of 10 mice were injected with a single dose of 500 μg of rabbit anti-rWNV-E IgG at the indicated times before or after infection with 100 PFU WNV. Data are representative of two experiments with similar results.
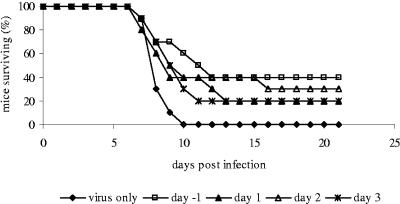
Although intact antibody molecules have the advantage of Fc-mediated functions, in some cases, the use of intact antibody molecules for immunoprophylaxis and therapy is limited. Intact antibody molecules are normally unable to cross the blood-brain barrier, and the Fc region is the source of cross-species neutralizing antibodies and potentially causes Fc-mediated antibody-dependent enhancement (ADE) of infection. To circumvent these complications, we also tested the efficacy of F(ab′)2 antibody fragments in in vivo protection assays. F(ab′)2 fragments provided only partial protection from a lethal dose of WNV (Fig. 2), suggesting that Fc-mediated effector functions are important for optimal protection against otherwise lethal WNV infection.
FIG. 2.
Survival of mice passively immunized with rabbit anti-rWNV-E F(ab′)2. Groups of 10 mice were injected with a single dose of 500 μg of anti-rWNV-E F(ab′)2 before or after intraperitoneal infection with 100 PFU WNV. The number of mice surviving was recorded daily. Data are representative of two experiments with similar results.
Identification of anti-rWNV-E scFvs.
scFv human monoclonal antibodies against the E protein were identified by phage display screening. Purified rWNV-E was used as an antigen to select antibodies from two nonimmune human scFv libraries. After four rounds of selection, a total of 384 clones were screened by ELISA for binding. Eleven unique anti-rWNV-E scFvs were then identified by DNA sequence analysis.
Amino acid sequences predicted by sequence analysis of the VH and VL of the 11 scFv genes are shown in Fig. 3. All of the VH sequences were in the VH1 gene family; VL sequences were in the VL1, VL2, VL3, and VL8 gene families. scFvs 10, 11, 15, 71, 73, 84, 85, and 95 had identical or nearly identical VH sequences, while scFvs 69, 79, and 94 had distinct VH sequences, particularly in CDR2 and CDR3, the primary domains involved in antigen binding. VL sequences were distinct for all 11 scFvs.
FIG. 3.
Amino acid sequences of anti-rWNV-E scFvs. Shown are the framework regions 1 to 4 (FW1 to FW4) and complementarity-determining regions 1 to 3 (CDR1 to CDR3) for VH and VL, as well as VH and VL gene family designations. The consensus amino acid sequence, encoded by more than 50% of the genes at a given position, is shown at the top. Dots in the consensus sequence are shown where <50% of the genes encode the same amino acid, and dots in each sequence represent the same amino acid as the consensus. Gaps are represented by dashes.
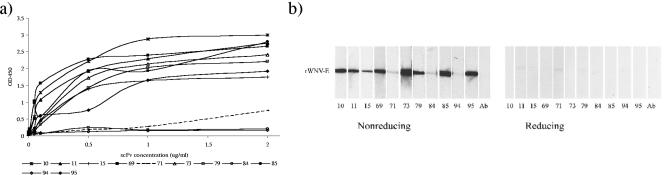
The 11 scFvs tagged with c-myc and His6 epitopes were expressed in E. coli and purified by immobilized metal affinity chromatography. scFv binding activity to rWNV-E was examined by both ELISA and Western blotting (Fig. 4). By ELISA, 8 of the 11 scFvs bound with high affinity to rWNV-E, while scFvs 71, 84, and 94 bound less avidly to rWNV-E (Fig. 4a). By Western blotting, we observed maximal binding to rWNV-E under nonreducing conditions. When rWNV-E was reduced by β-mercaptoethanol, only weak binding was observed. The complete epitopes recognized by the scFvs are likely conformation dependent and are unavailable for binding under reduced conditions (Fig. 4b). Secondary protein structure and conformational epitopes are thus critical for binding by the scFvs. When the scFvs were modified to include an Fc domain, the ELISA and Western blot binding profiles were similar (data not shown). Additionally, the scFvs recognized native E protein in Western blotting and immunofluorescence.
FIG. 4.
Binding of scFvs to rWNV-E. (a) Binding of scFvs to rWNV-E as measured by ELISA. ELISA plates were coated with rWNV-E (100 ng/well) overnight and incubated with serial dilutions of antibodies. Anti-His-HRP (1:4,000) was used as a secondary antibody, and the plates were developed and read at 450 nm (OD450). (b) rWNV-E (10 μg/gel) was run on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel under reducing (in the presence of 2-mercaptoethanol) or nonreducing conditions and blotted to nitrocellulose. Blots were incubated with scFvs (1 μg/ml), and binding was detected by incubation with anti-His-HRP (1:4,000). Lane Ab refers to control for background anti-His-HRP antibody binding to rWNV-E.
Affinity for rWNV-E.
Binding kinetic rates (Kon and Koff) and affinities of the scFvs and selected scFv-Fcs for rWNV-E were measured by surface plasmon resonance (Table 1). The scFvs ranged 20-fold in their affinity for the E protein, with the highest binders displaying nanomolar affinities for the E protein. In comparison to their original scFv, the Kd of the scFv-Fcs for rWNV-E was increased by approximately 2 orders of magnitude. This is likely a result of the increased avidity of the bivalent scFv-Fc for the E protein.
TABLE 1.
Kinetic rates and binding affinity of scFvs and selected scFv-Fcs for rWNV-E
| scFv or scFv-Fc | Kon (M−1 s−1) | Koff (s−1) | Ka (M−1) | Kd (M) |
|---|---|---|---|---|
| scFv | ||||
| 10 | 5.61 × 105 | 2.12 × 10−2 | 2.64 × 107 | 3.78 × 10−8 |
| 11 | 3.26 × 105 | 1.61 × 10−3 | 2.03 × 108 | 4.92 × 10−9 |
| 15 | 1.33 × 105 | 1.28 × 10−3 | 1.04 × 108 | 9.65 × 10−9 |
| 69 | 2.13 × 105 | 1.26 × 10−3 | 1.69 × 107 | 5.92 × 10−8 |
| 71 | 9.39 × 104 | 2.58 × 10−3 | 3.64 × 107 | 2.75 × 10−8 |
| 73 | 5.17 × 105 | 2.25 × 10−3 | 2.30 × 108 | 4.35 × 10−9 |
| 79 | 1.25 × 105 | 6.70 × 10−4 | 1.87 × 108 | 5.35 × 10−9 |
| 84 | 1.81 × 105 | 6.85 × 10−3 | 2.65 × 107 | 3.78 × 10−8 |
| 85 | 5.01 × 106 | 6.73 × 10−3 | 7.45 × 108 | 1.34 × 10−9 |
| 95 | 2.35 × 105 | 7.15 × 10−4 | 3.29 × 108 | 3.04 × 10−9 |
| scFv-Fc | ||||
| 79 | 2.62 × 104 | 3.58 × 10−7 | 7.34 × 1010 | 1.36 × 10−11 |
| 95 | 3.20 × 105 | 1.09 × 10−5 | 2.94 × 1010 | 3.40 × 10−11 |
Neutralization of WNV, SLEV, and DENV-2.
The rapid blood clearance time of monovalent scFvs and their small size may be a limiting factor for the use of these molecules in passive immunotherapy (27). Bivalent molecules containing the Fc region are often more effective at both virus neutralization and stimulation of Fc-mediated effector functions and have an increased serum half-life in vivo (21). To increase these functions, selected scFvs were converted to scFv-Fc fusions. The Fc expression vector used in these experiments, pcDNA 3.1 Hinge, contains the hinge, CH2, and CH3 domains of human IgG1 but lacks CH1.
Seven scFv-Fcs were assessed for neutralization of WNV in vitro using a standard Vero cell plaque assay. All of the seven scFv-Fcs tested neutralized WNV plaque formation by greater than 80%, at minimum concentrations ranging from 1.25 to 12.5 μg/ml (Table 2). Consistent with its lower affinity for rWNV-E, higher 84 scFv-Fc concentrations were required to reduce plaque formation. Neutralization by scFvs was 10- to 20-fold less effective than by corresponding scFv-Fc proteins (data not shown).
TABLE 2.
PRNT titers against WNV and DENV-2
| scFv-Fc | PRNT80 titer (μg/ml) against:
|
|
|---|---|---|
| WNV | DENV-2 | |
| 11 | 1.25 | 6.25 |
| 15 | 1.25 | 6.25 |
| 71 | 2.5 | NDa |
| 73 | 1.25 | ND |
| 79 | 5 | 6.25 |
| 84 | 12.5 | ND |
| 95 | 1.25 | 6.25 |
ND, not done.
We next assessed the scFv-Fcs for their ability to neutralize plaque formation by related flaviviruses. Several of the scFv-Fcs were tested in a neutralization assay with DENV-2, and one of the scFv-Fcs, scFv-Fc 79, was also tested for neutralization of SLEV. All of the scFv-Fcs tested (scFv-Fcs 11, 15, 79, and 95) resulted in greater than 80% neutralization of DENV-2 at a concentration of 6.25 μg/ml (Table 2). DENV-2 was not neutralized by the negative control antibody E53 or anti-OspB specific to the WNV E protein or Borrelia burdorferi OspB protein, respectively (not shown). scFv-Fc 79 also effectively neutralized SLEV, with 5 μg/ml resulting in >80% plaque neutralization (PRNT80) by PRNT assay.
In vivo protection by scFv and scFv-Fcs.
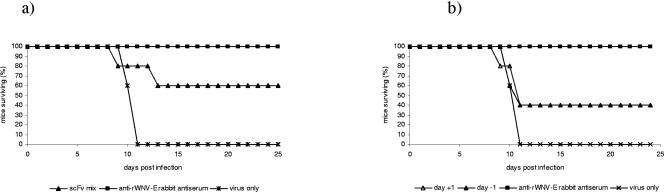
We next tested the ability of the scFvs and the scFv-Fc fusion proteins to protect mice from a lethal dose of WNV. A mixture of the scFvs (all except 10) provided partial protection against lethal WNV infection (Fig. 5a). Additionally, administration of 100 μg of a single scFv, 79, either 1 day before or 1 day after infection, provided only partial protection against viral challenge (Fig. 5b). To confirm the critical role of the Fc region in protection and to show that the bivalency of the scFv-Fc antibodies is not sufficient for protection, mice were immunized with bivalent F(ab′)2 fragments derived from scFv-Fc 79. scFv-Fc 79 F(ab′)2 was not protective in mice (data not shown), which is consistent with our previous studies showing that rabbit F(ab′)2 fragments were only partially protective (Fig. 2).
FIG. 5.
Survival of mice passively immunized with scFvs. (a) Groups of five mice were injected intraperitoneally with a mixture of 10 scFvs (100 μg each) 1 day prior to intraperitoneal inoculation with 100 PFU WNV. The number of mice surviving was recorded daily. Data are representative of two separate experiments with similar results. (b) Groups of five mice were injected subcutaneously with 100 μg of 79 scFv 79 either 1 day before or 1 day after intraperitoneal inoculation with 100 PFU WNV. The number of mice surviving was recorded daily. Rabbit anti-rWNV-E (100 μl) was given 1 day before infection. Data are representative of two separate experiments with similar results.
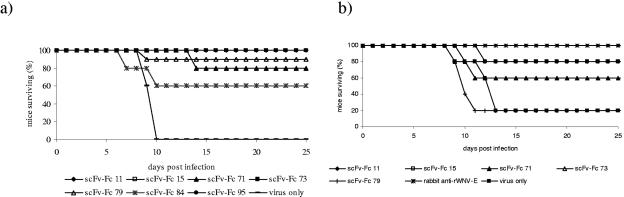
As expected from their high in vitro affinity for rWNV-E and the in vivo data with rabbit polyclonal anti-rWNV-E IgG, the scFv-Fcs were both protective and therapeutically active. Administration of 100 μg of any of the scFv-Fcs 1 day prior to infection with a lethal dose of WNV significantly increased survival (Fig. 6a). ScFv-Fcs 11, 15, 73, 85 (not shown), and 95 protected 100% of mice challenged, scFv-Fc 79 provided 90% protection, scFv-Fcs 71 and 94 (not shown) provided 80% protection, and scFv-Fc 84 provided 60% protection. The protective capacity of these antibodies correlated with their binding to rWNV-E in ELISA and Western blots (Fig. 4).
FIG. 6.
Survival of mice passively immunized with scFvs-Fcs. (a) Groups of 5 to 10 mice were injected subcutaneously with 100 μg of scFv-Fcs 1 day prior to intraperitoneal inoculation with 100 PFU WNV. The number of mice surviving was recorded daily. One hundred percent of mice treated with scFv-Fcs 11, 15, 73, and 95 survived. (b) Groups of 5 to 10 mice were injected subcutaneously with 100 μg of scFv-Fcs 1 and 4 days after intraperitoneal inoculation with 100 PFU WNV. The number of mice surviving was recorded daily. Eighty percent of mice treated with scFv-Fc 11 or 15 survived, and 60% of mice treated with scFv-Fc 71 or 73 survived.
We next examined the therapeutic activity of several of the scFv-Fcs. Both scFv-Fcs 11 and 15 were therapeutically active, with 80% of infected mice surviving when given two injections consisting of 100 μg of antibody at days 1 and 4 after infection (Fig. 6b). Further experiments showed that scFv-Fc 11 was more effective therapeutically than was scFv-Fc 15, with complete protection against a lethal challenge dose up to 3 days after infection by scFv-Fc 11. A dose of 250 μg of scFv-Fc 11 protected 100% of mice when given 1 day after infection, and a dose of 500 μg protected 100% of mice at day 3. Mice given 500 μg of scFv-Fc 11 at day 5 were partially protected from death, and 60% of the mice survived infection. Naïve mice had 100% mortality. A combination of antibodies 11 and 15 did not improve survival. In all WNV challenge experiments, mice administered antibodies showed no evidence of lasting morbidity at greater than 21 days postinfection, as evidenced by visual inspection for hind limb paralysis and daily weight measurements.
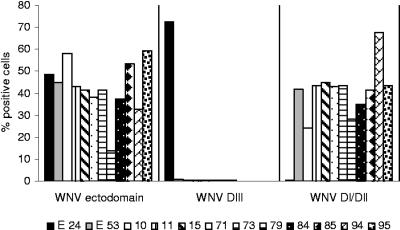
Domain binding assay: scFv-Fcs bind to DI/DII.
To determine which region of the WNV E protein is recognized by our panel of scFv-Fc antibodies, we used a yeast display system to express truncated WNV E proteins. Using this display method, we evaluated binding of the scFv-Fcs to either DI/DII or DIII of the WNV E protein. The control anti-WNV E MAbs E24 and E53 mapped to DIII and DI/DII, respectively, as shown previously (19). All of the scFvs in this study bound to the WNV E ectodomain. Interestingly, all of the scFVs in this study mapped to DI/DII, and none mapped to DIII. (Fig. 7).
FIG. 7.
Binding of scFv-Fcs to WNV E protein ectodomain, DI/DII, and DIII. The binding of scFv-Fcs to yeast displaying either the E protein ectodomain, DI/DII, or DII was measured by fluorescence-activated cell sorting. Yeast cells displaying the indicated fusions were incubated with scFv-Fcs, followed by incubation with the appropriate secondary antibody conjugated to Alexa Fluor 647, and the binding was measured by flow cytometry. The percentage of cells binding to each region was determined after subtracting background binding to control yeast cells expressing only the pYD1 vector. Mouse MAbs E24 and E53 served as positive controls for binding to DIII and DI/DII, respectively.
DISCUSSION
In the absence of a WNV vaccine for humans, passive immunization represents an important alternative strategy to prevent and treat WNV infections. In this study, we evaluated the protective and therapeutic capacity of human single-chain variable fragments obtained by screening two antibody phage display libraries against recombinant WNV E protein.
We found that passive administration of polyclonal rabbit antibodies against the E protein completely protected mice from lethal WNV infection. Previous studies have similarly protected mice from a lethal dose of WNV up to 5 days post-viral challenge; however, 100% survival was not recorded (7). Because virus infection of the brain occurs between days 3 and 4 postinfection, antibody continues to provide at least partial protection even after central nervous system infection has begun. This is significant as the timing of infection in humans is likely to be less clearly established than in an animal model and patients may present to their clinician after WNV has crossed the blood-brain barrier. Therefore, for an antibody to be effective as a therapeutic, it will ideally be effective even late in the course of infection.
Using a phage display screen, we identified a panel of 11 scFvs that bound the recombinant WNV E protein. Selected scFvs were converted into bivalent molecules with an IgG1 Fc region to increase their in vivo protective capacity. Fc-mediated activities are clearly important for efficacy of the antibody molecules, as survival of mice passively immunized with the Fc fusion proteins was increased, in some cases, to 100%. Similarly, when the Fc region was removed from polyclonal rabbit anti-E IgG, the F(ab′)2 fraction was only partially protective. Two of the scFv-Fc fusion proteins, 11 and 15, were found to be therapeutically active against otherwise lethal WNV infection. Although some previous studies identified anti-WNV antibodies that are protective in the absence of strong virus neutralization (23), we found a correlation between binding to the WNV E protein, virus neutralization, and in vivo protection.
The scFv-Fc fusion proteins have increased therapeutic and protective capacity as compared to their cognate scFvs. A bivalent molecule and/or addition of the Fc region is typically required for effective neutralization and in vivo efficacy by scFvs (16, 22). The small size (26 kDa) of the monovalent scFvs results in their rapid clearance, with a half-life as short as only 3.5 h (13), and is a limiting factor for their use in passive immunization. In contrast, the half-life of the scFv-Fc fusion proteins in vivo has been found to be increased to as long as 3 days (21). The comparatively short half-life of the scFvs may partially explain why the pooled scFvs were only partially protective against WNV infection. Additionally, it is likely that Fc-mediated activities in vivo contribute to the therapeutic efficacy of the Fc fusion proteins against otherwise lethal WNV infection (19).
Because many flaviviruses cocirculate in nature, an antibody that will protect against multiple viruses will have the highest utility as both a short-term prophylaxis and a treatment. All four of the scFv-Fcs tested provided in vitro protection against DENV-2. Only scFv-Fc 79 was tested in a neutralization assay with SLEV, and it effectively neutralized this related flavivirus. While the in vivo activity of these scFv-Fcs against flaviviruses other than WNV is not yet known, our in vitro data predict the possibility of effective cross-protection. Although there are theoretical concerns about the potential for ADE (29), we did not find any evidence of immune enhancement in cultivated human macrophages in vitro at saturating concentrations of antibody (L. H. Gould, unpublished data). Similarly, none of the antibodies enhanced infection in our WNV challenge assays. These results are consistent with those of other studies of antibodies and WNV infection (7), and the significance of ADE in vivo remains uncertain.
All of the scFv-Fcs characterized in this article positively influenced the outcome of an otherwise lethal WNV infection; however, there were distinct differences in the ability of these antibodies to protect mice, both before and after infection. As shown by the therapeutic experiments with scFv-Fc 11, an increased dose of antibody is needed to effectively treat mice later in the course of infection. The dose used in our experiments may be suboptimal for some of the antibodies. Further work is required to establish the most appropriate dosing and timing regimens. Future investigations, guided in part by detailed epitope mapping data, should also examine the potential for increased protection with combinations of two or more antibodies. Although the scFv-Fcs are clearly potent antiviral agents, they are not complete human monoclonal antibodies. Converting the scFv-Fcs to a full-length human IgG will potentially increase their efficacy by allowing for more flexible torsion around the hinge region.
All of the phage-selected antibodies produced in this study mapped to DI/DII of the WNV E protein. The majority of flavivirus antibodies characterized to date have been produced in mice, and it is apparent from this and another recent study (9) that human and other phage-displayed antibodies map to new, uncharacterized E protein epitopes. Further work to fine map these antibodies will help to further elucidate the regions of DI/DII that define these important protective epitopes.
The scFv-Fcs characterized in this study were produced by screening two antibody phage display libraries for molecules that bound the WNV E protein. Because of the nature of the creation of the libraries, these phage-displayed scFvs do not necessarily represent the same antibodies that would be found naturally occurring in an immunized or immune individual. The phage libraries were created with random recombination of VH and VL regions; therefore, the particular VH-VL combinations found in these antibodies are potentially entirely unique. Additionally, creation of antibodies by phage display eliminates the phenomenon of immunodominance of certain epitopes because there is no major histocompatibility restriction. Using a phage display screen is thus a way to identify novel and potent high-affinity antibodies to a target of interest in the absence of the constraints of the immune system.
The use of antibodies as a therapy for human flavivirus infections is promising, especially for elderly and immunocompromised patients with a deficient immune response. A monoclonal molecule such as the ones identified in this study may be an ideal therapy as it can be developed rapidly to a high titer, is free of blood-borne pathogens, and is specifically targeted to important protective epitopes. The in vivo protection and therapy provided by these molecules suggest that these antibodies can be further developed into a potent prophylaxis and therapy for WNV and related flavivirus infections. Human clinical trials are needed to establish a role for human monoclonal antibodies in the treatment and prevention of flavivirus infections.
Acknowledgments
We thank Deborah Beck for expert assistance with mouse experiments and Bonnie Hamid for assistance with PRNT assays.
This work was supported by grants from the NIH and CDC.
REFERENCES
- 1.Agrawal, A. G., and L. R. Petersen. 2003. Human immunoglobulin as a treatment for West Nile virus infection. J. Infect. Dis. 188:1-4. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, J. F., T. G. Andreadis, C. R. Vossbrinck, S. Tirrell, E. M. Wakem, R. A. French, A. E. Garmendia, and H. J. Van Kruiningen. 1999. Isolation of West Nile virus from mosquitoes, crows, and a Cooper's hawk in Connecticut. Science 286:2331-2333. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, J. F., and J. J. Rahal. 2002. Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro. Emerg. Infect. Dis. 8:107-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai, J., J. Sui, R. Y. Zhu, A. S. Tallarico, F. Gennari, D. Zhang, and W. A. Marasco. 2003. Inhibition of Tat-mediated transactivation and HIV-1 replication by human anti-hCyclinT1 intrabodies. J. Biol. Chem. 278:1433-1442. [DOI] [PubMed] [Google Scholar]
- 5.Camenga, D. L., N. Nathanson, and G. A. Cole. 1974. Cyclophosphamide-potentiated West Nile viral encephalitis: relative influence of cellular and humoral factors. J. Infect. Dis. 130:634-641. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, G. L., A. A. Marfin, R. S. Lanciotti, and D. J. Gubler. 2002. West Nile virus. Lancet Infect. Dis. 2:519-529. [DOI] [PubMed] [Google Scholar]
- 7.Engle, M. J., and M. S. Diamond. 2003. Antibody prophylaxis and therapy against West Nile virus infection in wild-type and immunodeficient mice. J. Virol. 77:12941-12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goncalvez, A. P., R. Men, C. Wernly, R. H. Purcell, and C. J. Lai. 2004. Chimpanzee Fab fragments and a derived humanized immunoglobulin G1 antibody that efficiently cross-neutralize dengue type 1 and type 2 viruses. J. Virol. 78:12910-12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goncalvez, A. P., R. H. Purcell, and C. J. Lai. 2004. Epitope determinants of a chimpanzee Fab antibody that efficiently cross-neutralizes dengue type 1 and type 2 viruses map to inside and in close proximity to fusion loop of the dengue type 2 virus envelope glycoprotein. J. Virol. 78:12919-12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gould, L. H., and E. Fikrig. 2004. West Nile virus: a growing concern? J. Clin. Investig. 113:1102-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haley, M., A. S. Retter, D. Fowler, J. Gea-Banacloche, and N. P. O'Grady. 2003. The role for intravenous immunoglobulin in the treatment of West Nile virus encephalitis. Clin. Infect. Dis. 37:e88-e90. [DOI] [PubMed] [Google Scholar]
- 12.Hamdan, A., P. Green, E. Mendelson, M. R. Kramer, S. Pitlik, and M. Weinberger. 2002. Possible benefit of intravenous immunoglobulin therapy in a lung transplant recipient with West Nile virus encephalitis. Transplant. Infect. Dis. 4:160-162. [DOI] [PubMed] [Google Scholar]
- 13.Huston, J. S., A. J. George, G. P. Adams, W. F. Stafford, F. Jamar, M. S. Tai, J. E. McCartney, H. Oppermann, B. T. Heelan, A. M. Peters, L. L. Houston, M. A. Bookman, E. J. Wolf, and L. M. Weiner. 1996. Single-chain Fv radioimmunotargeting. Q. J. Nucl. Med. 40:320-333. [PubMed] [Google Scholar]
- 14.Jordan, I., T. Briese, N. Fischer, J. Y. Lau, and W. I. Lipkin. 2000. Ribavirin inhibits West Nile virus replication and cytopathic effect in neural cells. J. Infect. Dis. 182:1214-1217. [DOI] [PubMed] [Google Scholar]
- 15.Kalil, A. C., M. P. Devetten, S. Singh, B. Lesiak, D. P. Poage, K. Bargenquast, P. Fayad, and A. G. Freifeld. 2005. Use of interferon-alpha in patients with West Nile encephalitis: report of 2 cases. Clin. Infect. Dis. 40:764-766. [DOI] [PubMed] [Google Scholar]
- 16.Lantto, J., J. M. Fletcher, and M. Ohlin. 2002. A divalent antibody format is required for neutralization of human cytomegalovirus via antigenic domain 2 on glycoprotein B. J. Gen. Virol. 83:2001-2005. [DOI] [PubMed] [Google Scholar]
- 17.Ledizet, M., K. Kar, H. G. Foellmer, T. Wang, S. L. Bushmich, J. F. Anderson, E. Fikrig, and R. A. Koski. 2005. A recombinant envelope protein vaccine against West Nile virus. Vaccine 23:3915-3924. [DOI] [PubMed] [Google Scholar]
- 18.Marfin, A. A., and D. J. Gubler. 2001. West Nile encephalitis: an emerging disease in the United States. Clin. Infect. Dis. 33:1713-1719. [DOI] [PubMed] [Google Scholar]
- 19.Oliphant, T., M. Engle, G. E. Nybakken, C. Doane, S. Johnson, L. Huang, S. Gorlatov, E. Mehlhop, A. Marri, K. M. Chung, G. D. Ebel, L. D. Kramer, D. H. Fremont, and M. S. Diamond. 2005. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 11:522-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen, L. R., and J. T. Roehrig. 2001. West Nile virus: a reemerging global pathogen. Emerg. Infect. Dis. 7:611-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powers, D. B., P. Amersdorfer, M. Poul, U. B. Nielsen, M. R. Shalaby, G. P. Adams, L. M. Weiner, and J. D. Marks. 2001. Expression of single-chain Fv-Fc fusions in Pichia pastoris. J. Immunol. Methods 251:123-135. [DOI] [PubMed] [Google Scholar]
- 22.Ray, K., M. J. Embleton, B. L. Jailkhani, M. K. Bhan, and R. Kumar. 2001. Selection of single chain variable fragments (scFv) against the glycoprotein antigen of the rabies virus from a human synthetic scFv phage display library and their fusion with the Fc region of human IgG1. Clin. Exp. Immunol. 125:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roehrig, J. T., L. A. Staudinger, A. R. Hunt, J. H. Mathews, and C. D. Blair. 2001. Antibody prophylaxis and therapy for flavivirus encephalitis infections. Ann. N. Y. Acad. Sci. 951:286-297. [DOI] [PubMed] [Google Scholar]
- 24.Sawyer, L. A. 2000. Antibodies for the prevention and treatment of viral diseases. Antivir. Res. 47:57-77. [DOI] [PubMed] [Google Scholar]
- 25.Sayao, A. L., O. Suchowersky, A. Al-Khathaami, B. Klassen, N. R. Katz, R. Sevick, P. Tilley, J. Fox, and D. Patry. 2004. Calgary experience with West Nile virus neurological syndrome during the late summer of 2003. Can. J. Neurol. Sci. 31:194-203. [DOI] [PubMed] [Google Scholar]
- 26.Shimoni, Z., M. J. Niven, S. Pitlick, and S. Bulvik. 2001. Treatment of West Nile virus encephalitis with intravenous immunoglobulin. Emerg. Infect. Dis. 7:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sui, J., W. Li, A. Murakami, A. Tamin, L. J. Matthews, S. K. Wong, M. J. Moore, A. S. Tallarico, M. Olurinde, H. Choe, L. J. Anderson, W. J. Bellini, M. Farzan, and W. A. Marasco. 2004. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. USA 101:2536-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tesh, R. B., J. Arroyo, A. P. Travassos da Rosa, H. Guzman, S. Y. Xiao, and T. P. Monath. 2002. Efficacy of killed virus vaccine, live attenuated chimeric virus vaccine, and passive immunization for prevention of West Nile virus encephalitis in hamster model. Emerg. Infect. Dis. 8:1392-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tirado, S. M., and K. J. Yoon. 2003. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 16:69-86. [DOI] [PubMed] [Google Scholar]
- 30.Wang, T., J. F. Anderson, L. A. Magnarelli, S. J. Wong, R. A. Koski, and E. Fikrig. 2001. Immunization of mice against West Nile virus with recombinant envelope protein. J. Immunol. 167:5273-5277. [DOI] [PubMed] [Google Scholar]
- 31.Weiss, D., D. Carr, J. Kellachan, C. Tan, M. Phillips, E. Bresnitz, and M. Layton. 2001. Clinical findings of West Nile virus infection in hospitalized patients, New York and New Jersey, 2000. Emerg. Infect. Dis. 7:654-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong, S. J., V. L. Demarest, R. H. Boyle, T. Wang, M. Ledizet, K. Kar, L. D. Kramer, E. Fikrig, and R. A. Koski. 2004. Detection of human anti-flavivirus antibodies with a West Nile virus recombinant antigen microsphere immunoassay. J. Clin. Microbiol. 42:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]