Abstract
The nucleoside kinase encoded by Kaposi's sarcoma-associated herpesvirus (KSHV) is a relatively inefficient enzyme with substrate specificity for thymidine alone, unlike alphaherpesvirus thymidine kinases (TKs). Similar to all gammaherpesvirus TKs, KSHV TK is composed of two distinct domains, a conserved C-terminal kinase and a novel and uncharacterized N terminus. Ectopic expression of KSHV TK in adherent cells induced striking morphological changes and anchorage independence although cells survived, a property shared with the related rhadinovirus TKs of rhesus monkey rhadinovirus and herpesvirus saimiri. To determine whether KSHV TK served alternate functions relevant to the rhadinovirus life cycle and to reveal the contribution of the N terminus, an enhanced green fluorescent protein-tagged fusion protein and serial mutants were generated for investigation of intracellular localization and cell biology. Analysis of truncation mutants showed that a proline-rich region located within the N terminus cooperated with the conserved C-terminal kinase to tether KSHV TK to a reticular network in the cytoplasm and to induce morphological change. Fusion of the KSHV N terminus to herpes simplex virus type 1 TK, a nucleus-localized enzyme, similarly resulted in cytoplasmic redistribution of the chimeric protein but did not alter cell shape or adhesion. Unlike other human herpesvirus TKs, KSHV TKs and related rhadinovirus TKs are constitutively tyrosine phosphorylated; a KSHV TK mutant that was hypophosphorylated failed to detach and grow in suspension. Loss of adhesion may enhance terminal differentiation, viral replication, and egress at the cellular level and at the organism level may facilitate detachment and distant migration of KSHV-replicating cells within body fluids—promoting oropharyngeal transmission and perhaps contributing to the multifocal lesions that characterize KS.
Several DNA viruses encode nucleic acid kinases that function in maintaining adequate nucleic acid pools to support viral DNA replication at times when host cell synthetic and salvage pathways are quiescent. In the herpesvirus family, both the alpha- and gammaherpesviruses encode deoxythymidine kinases. Although referred to simply as thymidine kinases (TKs), the alphaherpesvirus enzymes are in fact polynucleoside kinases that can phosphorylate all four nucleosides and related analogs. These highly active enzymes are required for efficient infection of primary epithelial cells (13) and reactivation of lytic replication from neuronal latency (7). In contrast, the gammaherpesvirus TKs are strict thymidine kinases that do not phosphorylate the other nucleosides and have greatly reduced enzymatic activity compared with the alphaherpesvirus TKs (16, 20, 21, 30, 46). It is not known whether these enzymes are required for lytic replication in terminally differentiated oral epithelial cells, following lytic reactivation from latently infected B lymphocytes or from endothelial tumor cells in the case of Kaposi's sarcoma-associated herpesvirus (KSHV).
The gammaherpesvirus TK proteins are structurally diverged from the alphaherpesviruses in that they contain a large N-terminal domain approximately two-thirds the size of the C-terminal domain that encodes the conserved enzyme. Secondary structure analysis suggests that the two domains are entirely independent and are separated by spacer residues (50). In the case of Epstein-Barr virus (EBV), it has been shown that the presence of the N-terminal domain in vitro does not significantly alter the ability of the C-terminal enzyme to phosphorylate thymidine (20). Although all gammaherpesvirus TKs contain N-terminal domains with high G, S, and P content (21), the similarity between these domains is otherwise modest (4, 15, 23, 30). Furthermore these N termini do not resemble other known proteins in current databases. Thus, the role of the N-terminal domain in relation to the function of the TK is unknown.
Kaposi's sarcoma-associated herpesvirus is a gammaherpesvirus of the rhadinovirus subfamily. The viral genome can be detected in Kaposi's sarcoma, primary effusion lymphoma, germinotrophic lymphoproliferative disease, and many cases of multicentric Castleman's disease and related malignancies (34). KSHV encodes a homolog of other gammaherpesvirus TKs (open reading frame 21) located at a position spanning bp 35383 to 37125 in the viral genome. Consistent with previous observations, KSHV TK shows selectivity for thymidine and, in particular, for the thymidine analog azidothymidine (21, 30). Nevertheless, the comparative activity of this enzyme even for thymidine is low (21, 30), and the virus encodes a functional thymidylate synthase (15), suggesting that activity of the KSHV TK alone may be inadequate to sustain virus replication.
To assess whether KSHV TK serves divergent functions relevant to the life cycle of rhadinoviruses, a series of enhanced green fluorescent protein (EGFP)-tagged fusion proteins were designed and generated to evaluate the cellular localization of the holoenzyme and its associated cell biology. Here, we demonstrate that expressed KSHV TK localizes to the cytoplasm and induces striking morphological changes at the cell surface. Amino acid residues within the proline rich N-terminal domain are essential for these alterations, although both the N and C termini collaborate to localize KSHV TK outside the nucleus and to alter the adhesive properties of the cell. Unlike the alphaherpesviruses and gamma-1 herpesviruses, we show that KSHV TK and related rhadinovirus TKs are constitutively tyrosine phosphorylated and propose that this phosphorylation may be required to elicit morphological change.
The alterations in cell shape and adhesion observed with individual expression of KHSV and related rhadinoviruses suggest that in addition to amplifying thymidine pools, these proteins modify the adhesive properties of the infected cell during virus replication and assembly. This may contribute to the process of viral egress, shedding of infected cells into saliva, and/or release of virus-replicating endothelial cells into blood or lymph for delivery to distant sites, consistent with the multifocal nature of Kaposi's sarcoma.
MATERIALS AND METHODS
Expression constructs.
All virus-derived gene constructs were amplified with Pfu polymerase (Stratagene) and confirmed by sequencing. The mammalian expression clones listed below were generated by amplifying either full- or partial-length genes using both the forward (F) and reverse (R) primers stated and cloned into pEGFP-C2, pEGFP-C3, or pEGFP-N2 (Clontech) by using the restriction sites indicated below by underlining. The numbers in parentheses indicate amino acid residues. The primers were as follows (restriction sites follow the sequence, in parentheses): for pEGFPC2-KSHV TK (1 to 580), F/MG165 (5′TGCTCGAGCATGGCAGAAGGCGGTTTTGGAGC3′, XhoI) and R/MG166 (5′GGAATTCAGGCTAGACCCTGCATGTCTCC3′, EcoRI); for pEGFPC2-KSHV TK (1 to 253), F/MG165 and R/MG262 (5′ CCGGAA TTCCTAAACATTCCTGTAGTCCACG 3′, EcoRI); pEGFPC2-KSHV TK (1 to 531), F/MG165 and R/MG261 (5′ CCGGAATTCCTAATCCGCGTCGGCTACG 3′, EcoRI); pEGFPC3-KSHV TK (254 to 531), F/MG260 (5′CCGCTCGAGTATTTGCTTTACTTAGAGG3′, XhoI) and R/MG261; pEGFPC3-KSHV TK (254 to 580), F/MG260 and R/MG166; pEGFPC2-KSHV TK (21 to 580), F/MG335 (5′CCGCTCGAGCACTAGGGGAGGCAGGTGG3′, XhoI) and R/MG166; (42 to 580), F/MG336 (5′CCGCTCGAGCAGCACGGATAT GGACGACCTCC3′, XhoI) and R/MG166; (61 to 580), F/MG337(5′CCGCTCGAGCACCTCGTACATATACGACG3′, XhoI) and R/MG166; (81 to 580), F/MG338 (5′CCGCTCGAGCGACAACTCCCTCTACGC3′, XhoI) and R/MG166; (101 to 580), F/MG350 (5′CCGCTCGAGCCCTCCAAATCACCCACCTCC3′, XhoI) and R/MG166; (141 to 580), F/MG351 (5′CCGCTCGAGCTTAAAAAC ATCTACCAAGG3′, XhoI) and R/MG166; (175 to 580), F/MG352 (5′CCGCTCGAGCAAATCTGCCATAGG3′, XhoI) and R/MG166; (207 to 580), F/MG353 (5′CCGCTCGAGCCTCATTAGAACGCCTGTGACC3′, XhoI) and R/MG166. For pEGFPN2-EBV TK, F/MG155 (5′TGCTCGAGACCATGGCTGGATTTCCAGGAAAGG3′, XhoI) and R/MG156 (5′GGAATTCGTCCCGATTTCCCCTCTCAAAATCAG3′, EcoRI); for pEGFPC2-HVS (for herpesvirus saimiri) TK, F/MG357 (5′CCGCTCGAGCATGACAGGAAGAGGACAGC3′, XhoI) and R/MG358 (5′CGGAATTCTCATTGAGAGTTAAATGTGC3′, EcoRI); for pEGFPC2-RRV (for rhesus monkey rhadinovirus) TK, F/MG342 (5′CCGCTCGAGCATGGCCGAAGGAGGGTCTGG3′, XhoI) and R/MG343 (5′GGGGTACCTTAATTGGCTGCATTGCTTTCC3′, KpnI); for pEGFPC2-MHV68 (for murine gammaherpesvirus 68) TK, F/MG354 (5′CCGCTCGAGCATGGCTTCTGGAGGTAAAAACAACC3′, XhoI) and R/MG355 (5′CGG AATTCCTACTGAGGGTCTCCACC3′, EcoRI) and herpes simplex virus type 1 (HSV-1) TK, F/MG163 (5′TGCTCGAGCATGGCTTCGTACCCCTGCCATCAACACG3′, XhoI); and R/MG164 (5′GGAATTCTCAGTGAGCCTCCC CCATCTCC3′, EcoRI).
To generate pEGFPC2-KSHV TKΔATP, KSHV TK was amplified using MG306 (F, 5′TGCTCGAGCACGCTGGTCAACGCCGTGTGC5′, XhoI) and MG166 primers and cloned into pEGFPC2, producing pEGFPC2-KSHVTK_N terminus-ΔATP. The N-terminal domain of KSHV TK was amplified using MG165 and MG305 (F, 5′TGCTCGAGATAACATTCCTGTAGTCCACG3′; XhoI) primers, and the resultant fragment was subcloned into pEGFPC2- KSHVTK_N terminus-ΔATP, to replace the N-terminal domain using the restriction sites stated below. The pEGFPC2-KSHV/HSV1 TK expression clone was generated by amplifying AA1 to 253 of KSHV TK (MG165 and MG305) and cloning the amplified fragment into the XhoI restriction site of pEGFPC2-HSV1 TK, placing the KSHV TK N-terminal domain between EGFP and HSV-1 TK. Nontagged RRV TK and MHV-68 TK clones were generously provided by Ronald Desrosiers (Harvard University, Boston, MA) and Samuel Speck (Emory University, Atlanta, GA), respectively.
Cell culture and transfections.
143B TK−, a human rhabdomyosarcoma cell line that lacks human TK-1 expression (ATCC), and 293T cells, human embryonic kidney cells immortalized by sheared adenovirus and transfected with simian virus 40 T antigen (ATCC) were grown in Dulbeco's modified Eagle's medium supplemented with 10% heat-inactivated calf serum, 100-U/ml penicillin, and 100-μg/ml streptomycin (BioWhittaker). 143B TK- and 293T cells were transfected with Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer's instructions. In brief, cells were plated on glass coverslips (at ∼105 cells/cm2) in 12-well plates 24 h before being transfected with 0.5 μg DNA. For kinase inhibition experiments, cells were incubated with 1 μM Gleevec (a gift of Glen Bubley, Beth Israel Deaconess Medical Center, Boston, MA), 800 nM herbimycin A (Cell Biology Products), or 30 μM genistein (Sigma-Aldrich) at the time of transfection. Primary human keratinocytes were grown in Complete K-SFM (keratinocyte serum-free medium supplemented with 30-μg/ml bovine pituitary extract, 0.2-ng/ml epidermal growth factor, and 0.3 mM CaCl2) (Invitrogen) as previously described (43). Cells were plated at 5 × 104 in a six-well culture dish 1 or 2 days prior to transient transfection with FuGene (Invitrogen).
Antibodies and cell staining and imaging.
At 12 to 24 h posttransfection, cells were counterstained with Hoechst 33342 (Molecular Probes) and fixed in 3.7% paraformaldehyde-bovine serum albumin (BSA) for 20 min. Fixed cells were permeabilized in phosphate-buffered saline (PBS)-0.1% Triton X-100 for 15 min at room temperature (RT), extensively washed with PBS, and then blocked with 3% BSA-1× PBS for 1 h. For localization studies, cells were incubated with the primary antibodies anti-α-tubulin (Sigma-Aldrich T5168), anti-β-tubulin (Santa Cruz sc-5274), anti-vimentin (Santa Cruz sc-6260) or anti-phosphotyrosine PY99 (Santa Cruz sc-7020) at a 1:1,000 dilution in PBS for 1 h at RT. After being extensively washed (with PBS), cells were incubated with either goat anti-mouse phycoerythrin (Biosource International AMI4407) or goat anti-rabbit phycoerythrin (Biosource International ALI4407) conjugate at a 1:1,000 dilution in PBS for 30 min at RT. The details required for actin staining and cell mounting were described previously (18). Immunofluorescence images were taken under an 100× oil objective on a Nikon E600 microscope with a SPOT2 digital camera with SPOTcam software, version 3.5.5. (Digital Instruments, Inc). Z-stacks were obtained with a Leica confocal microscope at ×63 magnification using oil immersion.
Immunoblotting.
Cell lysates were prepared from transfected adherent cells as previously described (18). Briefly, cells were collected by the addition of preboiled lysis buffer (50 mM Tris-1% sodium dodecyl sulfate [SDS]-0.5% β-mercaptoethanol), sonicated, and boiled for 5 min; protein concentration was quantified. SDS gels (5 to 12% gradient; Bio-Rad) were run with 15 μg total cell lysate and transferred to nitrocellulose (Osmonics) by electroblotting. For GFP, β-tubulin, and tyrosine phosphorylation (pTyr) detection, blots were incubated with either anti-GFP (Santa Cruz/sc-8334), anti-β-tubulin (see above), or anti-pTyr (see above) antibodies at a 1:400 dilution in PBS-5% BSA (1 h); extensively washed; then incubated with either the secondary antibody, goat anti-rabbit horseradish peroxidase (Sigma-Aldrich A5420) for GFP and pTyr, or goat anti-mouse horseradish peroxidase (Sigma-Aldrich A2554) for β-tubulin (diluted 1:3,000 in PBS-5% BSA) for 30 min at RT; washed; and developed with chemiluminescent substrate (ECL; Amersham). For antibody stripping, membranes were incubated with stripping buffer (containing 53.6% of 0.5 M Tris-HCl [pH 6.8], 42.9% of 20% SDS, and 3.5% β-mercaptoethanol) at 50°C for 45 min and then extensively washed with PBS. Stripped blots were then reprobed by standard immunoblot procedures.
RESULTS
KSHV TK localizes to the cytoplasm and alters cellular morphology.
Unlike HSV-1 TK and EBV TK, ectopic expression of KSHV TK in 143B TK- cells was observed to cause cell rounding and detachment over time in tissue culture (21). These changes were initially assumed to represent the limited capacity of KSHV TK to substitute for human TK-1 (deficient in 143B TK- cells) upon hypoxanthine-aminopterin-thymidine (HAT) selection leading to an increased rate of apoptotic cell death. However, further studies revealed that the transfected cells most often survived in suspension, indicating these morphological alterations could not be attributed to cell death per se.
The presence of a large uncharacterized N-terminal domain and the diminished nucleoside kinase activity of KSHV TK suggested that this protein might provide functions distinct from thymidine salvage. Therefore, to determine the basis for morphological changes and to assess whether they resulted from inadequate thymidine nucleotide pools in HAT-selected 143B cells or represented an independent function of KSHV TK expression, a fusion construct was generated linking EGFP to the N terminus of KSHV TK (see Materials and Methods). The fusion protein permitted visualization and confirmation of intracellular TK expression in the absence of a suitable antibody source.
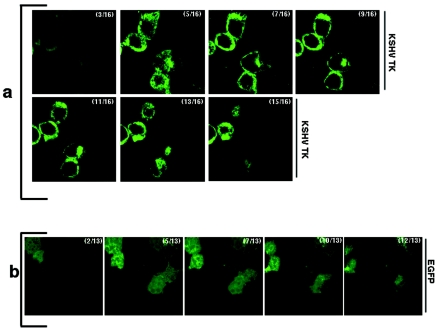
When EGFP-KSHV TK was transiently expressed in 143B TK- cells in the absence of HAT selection or when it was expressed in different adherent and TK-1 plus cell lines (293, HeLa, and CHO), the striking changes in morphology and frequent detachment of cells noted during TK rescue experiments persisted. Whereas transfection of 293 cells with EGFP alone resulted in minimal detachment of fluorescing cells 48 h posttransfection (7.4% ± 0.9% EGFP-positive cells, of which 67.6% ± 2.7% excluded trypan blue staining), EGFP-KSHV TK-expressing 293 cells became rounded and detached at these same time points. The montage of images shown in Fig. 1a and b represent Z-stack views of 293 cells obtained by confocal microscopy. The images highlight the cellular rounding induced by KSHV TK expression (Fig. 1a), in contrast to EGFP-bearing cells, which remained elongated and displayed intact cellular junctions (Fig. 1b). In three duplicate experiments 12 h posttransfection, 15.8% ± 1.6% of the transfected 293 cells expressing EGFP-KSHV TK detached, of which 78.7% ± 2.1% excluded trypan blue. At later time points (24 and 48 h), 49% ± 3% and 74.4% ± 2.1% of the KSHV TK-expressing cells detached, of which 74.4% ± 1.6% and 76.4% ± 4.5% excluded trypan blue, respectively. Notably, the suspended cells continued to survive in culture when analyzed over a 2-month period (not shown). Interestingly, whereas 293 cell transfectants very rapidly rounded and detached, 143B TK- cells and certain of the other cell lines remained adherent for a longer period (∼24 to 48 h), possibly reflecting the increased efficiency of 293 transfection or unknown differences in the temporal response of these cell lines.
FIG. 1.
EGFP-KSHV TK expression alters cell morphology. A montage of images obtained from 293 cells expressing either EGFP-KSHV TK (a) or EGFP alone (b) demonstrates the rounding and loss of cellular junctions in the KSHV TK-bearing cells compared with the EGFP-bearing control cells. Numbers indicate the corresponding Z-stack of a series collected at 0.5 μm, where 0 = the top of the cell.
Upon expression, EGFP-KSHV TK could be clearly seen to decorate an intricate network of cytoplasmic filaments or fibers (Fig. 2 a1 to a10). In some images, KSHV TK appeared to accumulate at the plasma membrane (Fig. 2a1), at leading edge structures (Fig. 2a5) and proximate to microtubule organizing centers during the cell cycle (Fig. 2a6). As noted above, these cells continued to express EGFP-KSHV TK and to survive with no evidence of nuclear fragmentation (Fig. 2a1 to 3, 5, and 6). In contrast, and as previously reported (12) EGFP-HSV-1 TK localized to the nucleus (Fig. 2a11), whereas EGFP alone was dispersed throughout adherent cells (Fig. 2a13). The localization of KSHV TK was the same in both adhered and suspended cells (Fig. 1 and 2a) and was maintained whether expressed in normal primary keratinocytes (Fig. 2a7 to 10) in immortalized 293T cells, or in different tumor cell lines of epithelial and lymphoid origin, including induced BCBL-1, a KSHV-infected lymphoma cell line (not shown). Cytoplasmic expression was also observed when a human heteroserum sample from a patient with classic KS was used to indirectly detect wild-type KSHV TK in 143B TK- cells (data not shown) and when TK was expressed as a C-terminal EGFP fusion protein (not shown), confirming the physiologic behavior of EGFP-KSHV TK.
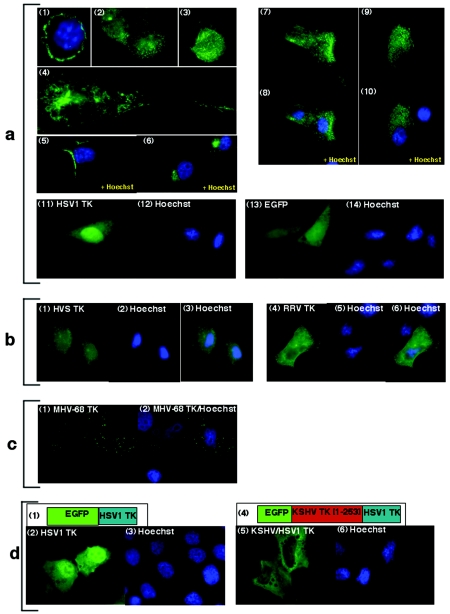
FIG. 2.
EGFP-KSHV TK and related gamma-2 herpesvirus TKs demonstrate a similar pattern of intracellular localization. (a) EGFP-KSHV TK localizes to the cytoplasm: 293 cells (panels 1 to 3), 143B TK- cells (panels 4 to 6), and primary keratinocytes (panels 7 to 10), whereas EGFP-HSV1 TK expressed in 143B TK- cells (panel 11) localizes to the nucleus and EGFP alone (panel 13) localizes diffusely. (b) EGFP-HVS TK (panel 1) and EGFP-RRV TK (panel 3), as well as EGFP-MHV-68 TK (c, panels 1 to 2), all localize to cytoplasmic compartments. (d) EGFP-HSV1 TK (panel 2) localizes to the nucleus; however, the chimera EGFP-KSHV/HSV1 TK (panel 5) localizes to the cytoplasm. Schematic diagrams detailing the structure of EGFP-HSV1 TK and EGFP-KSHV/HSV1 TK are shown in panels 1 and 4, respectively. Cells counterstained with Hoechst for nuclear discrimination are shown in panels 1, 5, 6, 8, 10, 12, and 14 (a), 2 and 4 (b), 2 (c), and 3 and 6 (d) (blue staining).
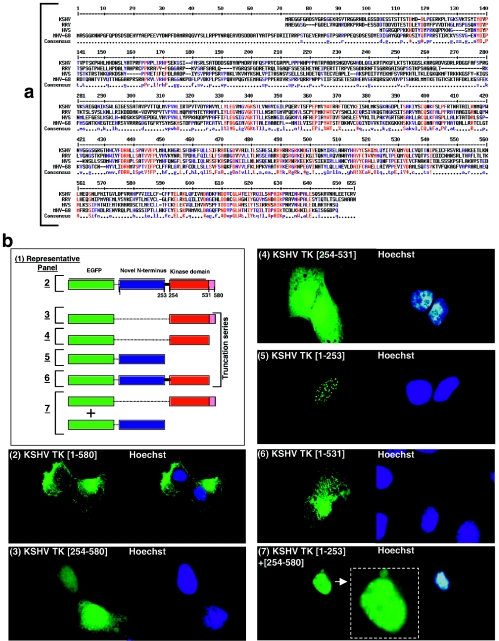
FIG.3.
Comparative alignment of the amino acid sequences of the N- and C-terminal domains of wild-type KSHV TK and related rhadinoviruses; generation and analysis of serial truncation mutants. (a) KSHV, RRV, HVS, and MHV-68 TKs were aligned using MultiAlign (9). (b) 143B TK- cells expressing EGFP-KSHV TK (WT) (panel 2) or truncation clones KSHV TK (254 to 580) (panel 3), KSHV TK (254 to 531) (panel 4), KSHV TK (1 to 253) (panel 5), KSHV TK (1 to 531) (panel 6), or a combination of both KSHV TK (1 to 253) plus KSHV TK (254 to 580) (panel 7). Cells counterstained with Hoechst are shown in the adjacent right-hand panels (blue staining).
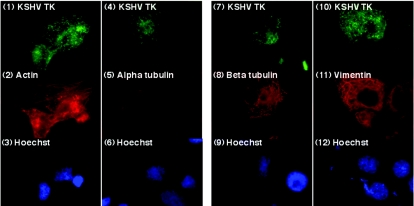
FIG. 5.
KSHV TK localizes to a reticular network in the cytoplasm that is devoid of major cytoskeletal proteins. 143B TK- cells expressing KSHV TK (panels 1, 4, 7, and 10) costained for actin (panel 2), α-tubulin (panel 5), β-tubulin (panel 8), and vimentin (panel 11). Cells counterstained with Hoechst stain are shown in panels 3, 6, 9, and 12.
FIG. 6.
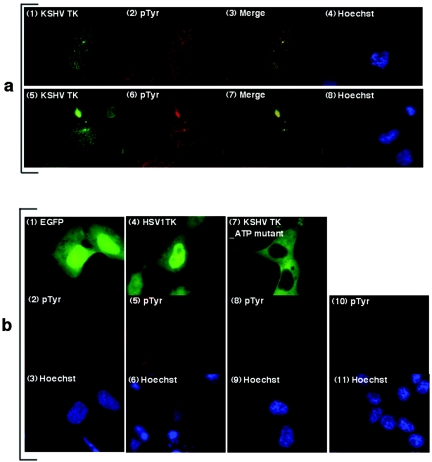
Expressed KSHV TK colocalizes with a protein(s) stained by an anti-pTyr antibody. (a) 143B TK- cells expressing EGFP-KSHV TK (panels 1 and 5) were costained for tyrosine phosphorylation (anti-pTyr) (panels 2 and 6), and the images were merged (yellow) (panels 3 and 7). (b) 143B TK- cells expressing EGFP (panel 1), EGFP-HSV1 TK (panel 4), EGFP-KSHV TKΔATP (panel 7), or mock transfected were costained with anti-pTyr as shown in panels 2, 5, 8, and 10, respectively. Cells counterstained with Hoechst are shown in panels 4 and 8 (a) and panels 3, 6, 9, and 11 (b) (blue staining).
Importantly, although synthetic and salvage pathway enzymes providing thymidine nucleotides were adequate in these different transfected cell populations, EGFP-KSHV TK-expressing cells were still observed to round up, lose contact inhibition, and detach from tissue culture plates, similar to 143B TK- cells expressing wild-type KSHV TK under selection pressure. Therefore, the observed morphological changes occurred independently of the effects of KSHV TK on intracellular thymidine pools.
The KSHV TK-related rhadinovirus TKs are functionally conserved.
The rhadinovirus (gamma-2 herpesvirus) TKs expressed by herpesvirus saimiri (HVS) and RRV are most closely related to KSHV TK (32). Although their N termini can be aligned, there is considerable divergence between amino acid residues of the individual proteins (Fig. 3a). Therefore, to determine whether the related TKs behaved similarly, independent clones were generated in which each TK was fused at the N terminus to EGFP, as described for EGFP-KSHV TK. When expressed, both proteins demonstrated a pattern of localization similar to that of KSHV TK (Fig. 2b1 to 3 and 4 to 6) and caused cellular swelling, albeit at a reduced level compared with the KSHV homolog. The TK of MHV-68, a more distantly related gammaherpesvirus TK, was likewise cloned and assessed. Although MHV-68 TK also localized to the cytoplasm, the pattern of distribution was distinct (Fig. 2c1 to 2), and MHV-68 TK expression did not alter the morphology or change the adhesive properties of the infected cell (data not shown).
FIG. 4.
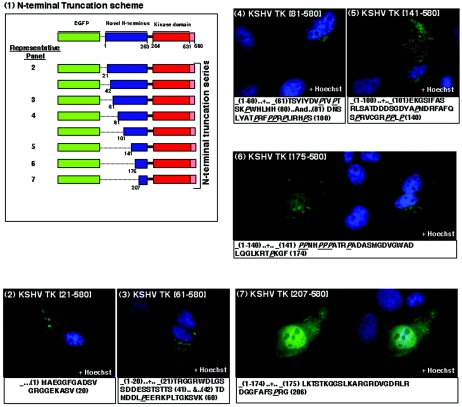
The N-terminal domain of KSHV TK is required for both protein localization and morphological change. A schematic diagram of N-terminal KSHV TK truncation clones is shown in panel 1. 143B TK- cells expressing the KSHV TK truncations: KSHV TK (21 to 580) (panel 2), KSHV TK (61 to 580) (panel 3), KSHV TK (81 to 580) (panel 4), KSHV TK (141 to 580) (panel 5), KSHV TK (175 to 580) (panel 6), and KSHV TK (207 to 580) (panel 7). Details of the sequence deletions are displayed under each of the representative panels. Cells counterstained with Hoechst stain are shown in panels 2 to 5; on the right, images of panels 6 to 7 (blue staining) are shown.
Metamorphosis of HSV1 TK into a gammaherpesvirus TK causes nuclear to cytoplasmic redistribution, but does not alter cell morphology.
To evaluate the role of the N terminus of KSHV TK and its interaction with the C-terminal domain of KSHV TK in protein localization and function and to determine which features of this interaction might be conserved with other herpesvirus kinase domains, a chimera was generated, effectively transforming an alpha- into a gammaherpesvirus TK. The EGFP-chimera comprised the N terminus of KSHV TK (amino acids [aa]1 to 253), followed by the entire HSV1 TK protein (Fig. 2d4). When expressed, the KSHV/HSV1 TK fusion protein exclusively localized to the cytoplasm (Fig. 2d5 and 6) in contrast to the nuclear localization of EGFP-HSV1 TK (Fig. 2d2 and 3). Although addition of the N-terminal domain of KSHV TK effectively relocalized HSV1 TK from the nucleus to the cytoplasm of the cell (Fig. 2d5 and 6), it did not localize to the same reticular network as EGFP-KSHV TK or induce morphological change.
KSHV TK N- and C-terminal domains independently distribute to the nucleus, although the holoenzyme resides in the cytoplasm.
To investigate the contribution of both the N- and C-terminal domains in relation to KSHV TK function and to define the amino acid residues responsible for overall protein localization, a series of truncation clones bearing EGFP at the N terminus were generated and studied (Fig. 3b1). First, the entire C-terminal region of the enzyme was analyzed to clarify its role. Two fusion proteins, one encompassing the entire C-terminal domain (aa 254 to 580) and one containing only the conserved kinase region (aa 254 to 531) were engineered by PCR and expressed in cells (Fig. 3b3 and 4, respectively). In contrast to the full-length protein (Fig. 3b2), as expected, both of the C-terminal fusion proteins localized to the nucleus (Fig. 3b3 and 4) with minor spread to the cytoplasm in a pattern virtually identical to that of HSV1 TK (Fig. 2a11). A nearly full-length mutant (aa 1 to 531) that lacked only the penultimate 49 aa of the protein still remained associated with network-like structures, similar to the wild-type protein, and also caused the morphological changes seen with wild-type KSHV TK expression (Fig. 3b6).
Surprisingly, when a fusion protein that contained only the unique N-terminal domain (aa 1 to 253) of the enzyme was expressed, this fusion protein was also identified in the nucleus where it colocalized with nuclear speckles that had a doughnut-shaped appearance, suggestive of nuclear bodies (Fig. 3b5). Notably, coordinate expression of the individual N-terminal (aa 1 to 253) and C-terminal domains (aa 254 to 580) did not reconstitute either the cellular distribution or morphological changes associated with expression of the wild-type protein. Although the respective domains could be clearly visualized in the same cell, they did not associate with one another (Fig. 3b7). Additionally, when a nontagged C-terminal domain was expressed together with the EGFP-tagged N terminus, the precise pattern of nuclear localization was maintained (not shown). Thus, the linker sequences between the N- and C-terminal domains of KSHV TK appeared to be a prerequisite for amino acid interactions facilitating cytoplasmic distribution.
N-terminal domain amino acids 141 to 175 are critical for both TK localization and induction of morphological change.
The observation that the N terminus of KSHV TK mediated distribution of the holoenzyme to the cytoplasm, yet when expressed independently failed to localize as the wild-type protein, suggested that a complex interplay between the N and the C termini might be required to produce the conformation necessary for this localization. To precisely determine which residues in the N terminus were important for subcellular distribution and which were responsible for induction of morphological change, sequential deletion mutants of the full-length protein were generated and expressed (Fig. 4, panel 1). Whereas the first 141 aa of KSHV TK proved dispensable for protein localization and alteration of cell shape (Fig. 4, panels 2 to 5), removal of amino acids 142 to 175, beginning with a stretch of polyproline residues, again began to alter protein distribution and abolish the induced changes in cell morphology. A fusion protein EGFP-KSHV TK (aa 175 to 580) lost its network-like association and relocalized diffusely within the cytoplasm (Fig. 4, panel 6), further truncation of the protein removing aa 176 to 206 caused redistribution to the nucleus (Fig. 4, panel 7), similar to mutants GFP (aa 254 to 580 and 254 to 531) and HSV1 TK. These results suggested that a polyproline-rich stretch of amino acids was important for the subcellular distribution and morphological changes elicited by expression of KSHV TK.
KSHV TK does not colocalize with microfilament, microtubule, or intermediate filament networks.
Visualization of EGFP- KSHV TK showed that the fusion protein was associated with a filamentous network extending from the plasma membrane to the nuclear envelope and that disruption of a stretch of polyproline residues in the N terminus altered both protein localization and the ability to induce morphological change. Similar proline-rich sequences in diverse proteins mediate association with the cytoskeleton that can occur either through direct or indirect interaction (22). This suggested that KSHV TK might also directly interact with a major cytoskeletal element or might indirectly interact with a cytoskeleton-associated protein. When the three major protein families comprising filamentous structures within the cell were investigated by coordinate staining of cells transiently expressing EGFP-KSHV TK, none of the distinct cytoskeletal proteins actin (microfilaments), α- and β-tubulin (microtubules), or vimentin (intermediate filaments) (Fig. 5) colocalized with KSHV TK. To further assess whether KSHV TK was indirectly associated with the cytoskeleton through other associated proteins, the colocalization experiments were repeated with reagents that disrupted cytoskeletal structures and released associated proteins. Addition of nocodazole induced microtubule depolarization, as highlighted by β-tubulin staining; however, the cellular localization of expressed EGFP-KSHV TK remained unaffected (data available upon request). Similarly, although addition of cytochalasin B induced microfilament depolymerization as shown by actin staining, the distribution of EGFP-KSHV TK (though modestly altered by collapse of the microfilament network and cell shrinkage) remained unchanged (data available upon request). Furthermore, no association between KSHV TK and the Golgi network or peroxisomes could be documented (data not shown). The composition of the TK-associated reticular network therefore remains unknown.
KSHV TK and related rhadinovirus TKs are constitutively tyrosine phosphorylated.
Cell survival under conditions that result in the loss of normal adhesive contacts may accompany processes such as immortalization or terminal differentiation. These changes are frequently associated with activation of cytoskeleton-associated proteins by nonreceptor tyrosine kinases such as Src and may be manifested by global alterations in cellular tyrosine phosphorylation (24). Therefore, to determine whether KSHV TK expression altered cellular pTyr levels, 143B TK- cells transiently expressing EGFP-KSHV TK were fixed and stained with an anti-pTyr specific antibody (PY99) and were compared to cells expressing EGFP-HSV1 TK, EGFP alone, or mock transfectants. Interestingly, only cells expressing EGFP-KSHV TK (Fig. 6a1 and 5; green) demonstrated significant pTyr staining (Fig. 6a2 and 6; red). Unexpectedly, however, the intracellular pattern of EGFP-KSHV TK and pTyr staining precisely overlapped (Fig. 6a3 and 7; yellow), and this result was consistent whether primary keratinocytes or established cell lines expressing KSHV TK were used (data not shown). In contrast, control transfectants expressing EGFP or EGFP-HSV1 TK (and mock transfected) showed only limited pTyr staining (Fig. 6b1 to 6 and 10 to 11).
FIG.7.
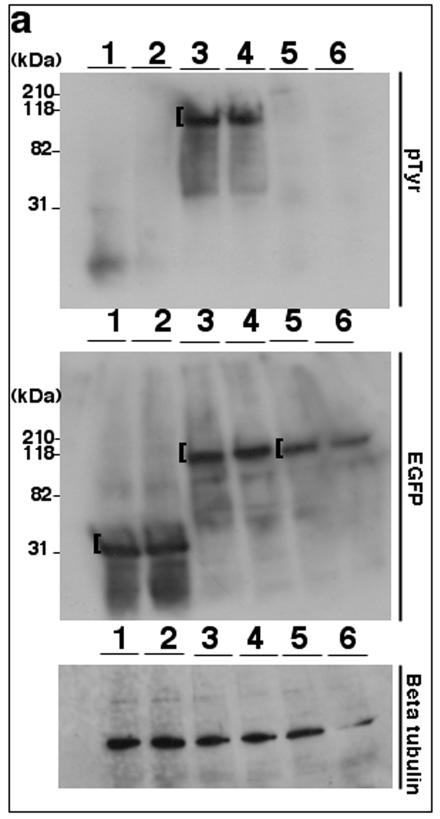
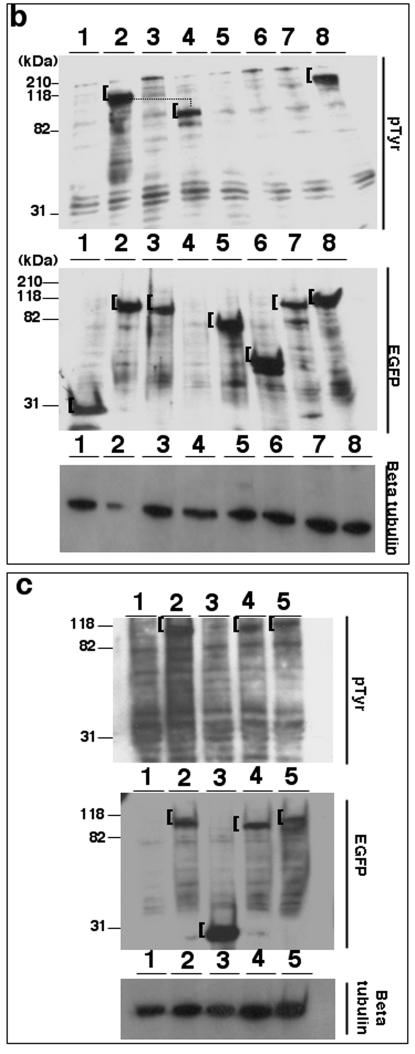
Tyrosine phosphorylation of TK is conserved among related rhadinoviruses. (a) Lysates from 293T cells (15 μg/lane) expressing EGFP (lanes 1 and 2), EGFP-KSHV TK (lanes 3 and 4), or EGFP-KSHV TKΔATP (lanes 5 and 6) were stained using either an anti-pTyr (top) or an anti-GFP antibody (middle). (b) Equivalent lysates expressing EGFP (lane 1), EGFP-KSHV TK (lane 2), EGFP-KSHV TKΔATP (lane 3), nontagged KSHV TK (lane 4), EGFP-HSV1 TK (lane 5), EGFP-vaccinia virus TK (lane 6), EGFP-EBV TK (lane 7), or EGFP-MHV-68 TK (lane 8) were stained using an anti-pTyr (top) or an anti-GFP monoclonal antibody (middle). (c) Lysates prepared from 293T cells (15 μg) alone (lane 1) or expressing EGFP-KSHV TK (lane 2), EGFP (lane 3), EGFP-HVS TK (lane 4), or EGFP-RRV TK (lane 5) were stained using anti-pTyr (top) or an anti GFP antibody (middle). Protein loading (control) blots were developed using an antibody to β-tubulin and are shown on the bottom of panels a to c. A bracket ([) indicates detected expressed fusion proteins.
Although the imaging data clearly demonstrated that expressed KSHV TK up-regulated and colocalized with cellular pTyr, it did not in fact reveal whether KSHV TK was itself phosphorylated or whether its expression had altered the phosphorylation of the cellular protein(s) that fortuitously colocalized with EGFP-KSHV TK. To resolve this question, cell lysates were generated from cells expressing EGFP-KSHV TK compared with EGFP alone and analyzed by immunoblotting using both anti-GFP- and anti-pTyr-specific antibodies. Although both EGFP and EGFP-KSHV TK could be detected when probing with anti-GFP was carried out (Fig. 7a, middle), only EGFP-KSHV TK was observed when a duplicate blot was probed with anti-pTyr (Fig. 7a, top, lanes 3 and 4). To assure that the protein detected by anti-pTyr was not a pTyr-modified cellular protein of the same molecular mass as the fusion protein (∼100 kDa), cell lysates were next generated from cells expressing nontagged KSHV TK and coordinately analyzed by immunoblotting with the anti-pTyr-specific and anti-GFP-specific monoclonal antibodies. Anti-GFP detected both the control EGFP and the EGFP-tagged kinase, but not the untagged enzyme (Fig. 7b, middle, lane 4), whereas anti-pTyr readily detected nontagged KSHV TK at the expected molecular mass (Fig. 7b, top, lane 4). Notably, no additional band(s) was present at ∼100 kDa, the estimated mass of EGFP-KSHV TK (Fig. 7b, top, lane 2).
Tyrosine phosphorylation of viral TKs has not been reported. To assess whether other herpesvirus TKs were similarly tyrosine phosphorylated, cells expressing a panel of EGFP-tagged viral TKs of different origin were analyzed for pTyr by immunoblot analysis. When probed for GFP, all of the expressed fusion proteins were present at the correct molecular mass as shown in Fig. 7b. However, HSV-1 TK (Fig. 7b, lane 5), EBV TK (lane 7), and the poxvirus enzyme TK (vaccinia virus TK) (lane 6, control) were not detected by anti-pTyr. Thus, pTyr was present only on TKs encoding KSHV TK (tagged and nontagged), as well as MHV-68 TK (Fig. 7b, top, lanes 2, 4, and 8).
To establish whether HVS and RRV TKs, the most closely related viruses, were similarly tyrosine phosphorylated, cell lysates were generated from 293T cells expressing EGFP-HVS TK, EGFP-RRV TK, EGFP-KSHV TK (positive control), or EGFP (irrelevant control). Of note, all of these expressed fusion proteins could also be detected when probed for both GFP and pTyr at the correct apparent molecular mass (Fig. 7c, lane 2 [KSHV TK], lane 3 [EGFP alone], lane 4 [HVS TK], and lane 5 [RRV TK], top and middle).
To gain insight into the mechanism and the function of tyrosine phosphorylation, three well-characterized tyrosine kinase inhibitors were evaluated to determine whether they could directly modify KSHV TK biology. Blockade of tyrosine phosphorylation by different inhibitors can alter cytoskeletal signaling in a cell type-dependent fashion and cause changes in adhesiveness and in cell shape. Cells expressing EGFP-KSHV TK or EGFP were incubated with various concentrations of either (i) Gleevec (ST1571; imatinib mesylate) (1), (ii) genistein (27), (iii) herbimycin A (35), or (iv) a combination of the latter two compounds and stained for pTyr. Although Gleevac caused some pTyr-stained proteins in both EGFP and EGFP-KSHV TK-expressing cells to accumulate into patch-like intracytoplasmic structures, EGFP-KSHV TK did not sort with these aggregates. Moreover, none of the three kinase inhibitors significantly altered pTyr staining of the enzyme, nor did they modify expression, localization, or the ability of EGFP-KSHV TK to induce morphological change (data available upon request).
While additional inhibitors are being evaluated, these observations raised the possibility that KSHV TK might be phosphorylated by an unknown or insensitive kinase or might be autophosphorylated. In preliminary experiments, an internal deletion mutant lacking the conserved herpesvirus TK ATP binding domain (256-L/V/I-Y/F-I/L-D/E-G-X-X-G-X-G-K-266) (16, 20, 21, 30, 46), as well as two upstream amino acids, Y-254 and L-255 (aa 254 to 266), was tested. Interestingly, the mutant protein was well expressed as a GFP fusion and still localized to the cytoplasm (Fig. 6b7), although it did not associate with the reticular network. Strikingly, the mutant was not detectably tyrosine phosphorylated when assessed by both immunoblotting (Fig. 7a, lane 5 versus lanes 3 to 4 containing wild-type KSHV TK) and direct cell staining (Fig. 6b8 compared with Fig. 6a2 and 6 containing wild-type KSHV TK). Moreover, the mutant did not induce morphological changes (Fig. 6b7). Tyrosine-257 is highly conserved among alphaherpesviruses and therefore is unlikely to be a site of constitutive tyrosine phosphorylation; tyrosine-254 is absent in HVS, which is constitutively phosphorylated. These findings suggest that rather than deletion of critical tyrosine residues, the inability of KSHV TK to autophosphorylate another tyrosine residue(s) may account for the observed changes in the cell biology of KSHV TK. Additional experiments will clearly be needed to establish the significance of these observations.
DISCUSSION
Disease caused by herpesvirus infection is common, particularly in the immunocompromised host, although the different subfamilies produce illness by distinct mechanisms. The pathological consequences of alphaherpesvirus infection result from uncontrolled lytic replication. Thus, nucleoside analogs that are specifically phosphorylated by viral TKs and incorporated into DNA by viral polymerases are highly effective as antiviral agents (10). Once phosphorylated by viral TK, the purine analog ganciclovir is also efficiently incorporated into cellular DNA, assuring apoptosis of cells expressing alphaherpesvirus TKs (41).
Although the gammaherpesviruses also synthesize TKs, because illness predominantly results from uncontrolled proliferation of cells that are latently infected, lytic cycle enzymes are not regarded as viable therapeutic targets. Nevertheless, KSHV DNA in plasma, DNA that is believed to derive from replicating virions, predicts the onset of latent disease (14). This suggests that uncontrolled lytic replication expands the pool of latently infected cells and in so doing increases the risk of development of a proliferative disorder. Evidence that both KS and multicentric Castleman's disease lesions contain subpopulations of virus-replicating cells that participate in the initiation and maintenance of lesions (6), particularly during early tumorigenesis, also suggests that limiting virus replication might contribute to therapy. In recent years, several investigators have proposed that in vivo induction of gammaherpesvirus replication in latently infected tumors with antiproliferative agents, even if abortive, may generate sufficient viral TK to permit accumulation and bystander uptake of cytotoxic nucleic acid analogs, causing specific killing of virus-infected tumor cells (20, 21, 29, 33, 40, 45, 48). In murine models of alphaherpesvirus and gammaherpesvirus infection (8), virions lacking TK are compromised in their ability to replicate after primary infection and/or to reactivate from latency, suggesting that even noncompetitive small molecule inhibitors of the herpesviral TKs might prove useful for preventing or managing disease.
The potential utility of KSHV TK as a therapeutic target prompted evaluation of the enzyme by several laboratories. Similar to other gammaherpesvirus TKs, KSHV TK was identified as an early gene product (38) with narrow substrate specificity, as the purified enzyme phosphorylated only thymidine and thymidine analogs (21, 30). In addition, kinetic analyses revealed that KSHV TK was less active than other human herpesvirus TKs and that a functional thymidylate synthase (15) was also synthesized by the virus, providing an additional source of TTP. Surprisingly and in contrast to other human herpesviruses, when the holoenzyme was expressed in adherent cells, striking morphological changes including swelling, rounding, and loss of major adhesive contacts were observed. Nevertheless, nuclear staining revealed that the cells were alive and in fact continued to survive in suspension culture. These observations raised speculation that the role of this gamma-2 herpesvirus enzyme was incompletely understood.
The gammaherpesvirus TKs are composed of novel and uncharacterized N termini with limited similarity to one another except for abundant proline, glycine, and serine residues. To uncover the role of this domain relative to the overall functions of KSHV TK, the enzyme was cloned and expressed as an N-terminal fusion with EGFP providing direct visual evidence of its intracellular location. Whether expressed in different cell lines or in primary human cells, KSHV TK consistently localized to a reticulum-like network in the cytoplasm and strikingly altered cell shape and adhesion. The induced morphological changes were indeed independent of TK function, as the majority of the cell lines examined contained adequate thymidine pools. Although the network-like cytoplasmic localization and ability to alter cell shape suggested that KSHV TK associated with the cytoskeleton, no direct association with microfilaments, microtubules, intermediate filaments, or other cytoskeleton-associated proteins could be demonstrated.
To gain further insight into the mechanisms by which these changes were achieved and to determine whether and how the unique N terminus contributed to enzyme localization, a chimera between the N terminus of KSHV TK and the well-characterized nuclear enzyme HSV-1 TK was synthesized. When expressed, the KSHV/HSV-1 chimera relocalized nuclear HSV-1 TK to the cytoplasm, though no modification of cell shape was detected. Thus, the N terminus effectively altered the intracellular localization of the conserved kinase, but it was not sufficient to alter cell morphology in the absence of the KSHV TK C terminus, emphasizing the functional divergence of the two viral kinase domains.
As the structural basis for altered morphology remained unresolved, a series of N- and C-terminal truncation clones were analyzed to investigate whether and how each domain contributed to cytoplasmic localization and morphological change. Surprisingly, when independently expressed or when expressed within the same cell, both the N- and C-terminal (kinase) domains demonstrated distinct patterns of nuclear localization, indicating that either a physical linker or specific amino acids between the N and C termini were required to duplicate the behavior of the holoenzyme. Previous analyses predicted that the linker region was unstructured (50) and likely functioned as a simple tether between two independent domains. However, motif analyses (e.g., ELM and ProSite) performed to help evaluate current findings identified several peptide motifs including a predicted immunoreceptor tyrosine-based inhibition motif(248-VDYRNV-253) (25) in the linker region, raising the possibility that the linker may also be of functional importance.
Within the N-terminal domain of KSHV TK the region between aa 68 to 151 is proline rich (∼25%) and contains three distinct proline clusters (aa 68 to 75, 88 to 99, and 137 to 151). Interestingly, these proline-rich regions, as well as upstream flanking amino acids, are moderately conserved among related rhadinoviruses. Domains comprised of proline-rich motifs (PRMs) are frequent in cellular (22, 26) and some viral (2, 39, 49) adaptor proteins involved in the formation of rapidly exchanging protein complexes; many of these proteins function as signal transducers in pathways mediating cytoskeletal reorganization. Distinct PRM motifs have been previously described (22) that preferentially interact either with profilin or with proteins containing SH3, WW, and EVH1 domains. The N terminus of KSHV TK contains seven of these motifs; although N-terminal mutants that removed the first two proline-rich clusters did not clearly alter cytoplasmic localization, deletions extending through amino acids 141 to 174 that disrupted the third and final proline-rich stretch resulted in both enzyme relocalization and loss of ability to alter cell shape. While additional mutants are necessary to define the precise contribution of proline residues to KSHV TK biology, both the presence of multiple N-terminal PRMs and the changes observed when these prolines are absent from the enzyme suggest that KSHV TK may interact with intracellular proteins containing SH3, WW, or EVH1 domains that could in turn regulate inside-out signal transduction, enabling cytoskeletal reorganization and detachment of KSHV TK-expressing cells from the extracellular matrix (5, 31, 37).
The morphological changes induced by KSHV TK expression were reminiscent of changes that can accompany epithelial cell immortalization and/or differentiation. These processes are marked by major shifts in protein expression patterns and protein tyrosine phosphorylation (24). Identification of the phosphorylated targets has often provided clues about functional mechanisms (28, 47). When an anti-phosphotyrosine antibody was used to identify cellular proteins modified after expression of KSHV TK, few cellular proteins were identified. However, surprisingly, most of the anti-phosphotyrosine fluorescence colocalized with the enzyme itself. Further biochemical analysis revealed that KSHV TK was the major phosphotyrosine-bearing protein in these cells and that this property was conserved among the related gamma-2 herpesviruses (RRV, HVS, and also MHV-68). Thus, the rhadinovirus TKs were constitutively tyrosine phosphorylated in contrast to other known viral TKs, including those of HSV-1, vaccinia virus, and even gamma-1 herpesvirus EBV, which also differed markedly in its overall biology (unpublished data). Studies with a TK mutant deleted for the ATP binding site and two flanking tyrosines further suggest that the enzyme may be autophosphorylated and that tyrosine phosphorylation could be required to elicit morphological change.
The current studies demonstrate that KSHV TK is a tyrosine-phosphorylated cytoplasmic protein that, in addition to phosphorylating thymidine, can cause dramatic alteration of cell shape and adhesion. The recent discovery that human papillomavirus type 8 E2 protein produces similar changes in cellular morphology when expressed in epithelial cells may provide new insights into KSHV TK functions as well (36). Notably, the papillomavirus E2 proteins are transcription factors that effectively antagonize the ability of E6 and E7 to block senescence (19), although the precise mechanisms are unknown. Recent studies showed that when expressed alone in cell lines or primary keratinocytes, certain E2 proteins directly downregulate β4-integrin (and to some extent β1-integrin) expression (36). In vivo, decreased β4-integrin expression is associated with terminal differentiation of stratified epithelia and is manifest by a loss of epithelial cell adhesion, altered cell morphology, and egress from basal to suprabasal layers (11, 17), suggesting that E2 causes senescence by a similar mechanism. Interestingly, as with both human papillomavirus and EBV, physiologic rhadinovirus replication is likely tied to terminal differentiation in oral keratinocytes.
In light of the ability of E2 to antagonize the functions of the E6 and E7 oncoproteins, we were intrigued by recent reports that the latent membrane protein 2A (LMP2A) and LMP2B proteins also reside in the cytoplasm of infected epithelial cells and that when individually expressed, each protein can mediate adhesion and in particular cell spreading. LMP2 shares a conserved location on the viral genome with K15 of KSHV; several lines of evidence indicate that the proteins may be structural and functional orthologs (3, 44). Remarkably, a comparison between KSHV TK and K15, LMP2A/LMP2B (and even LMP1) showed that in each case the paired proteins could be continuously aligned with minimal gaps and with multiple interspersed regions of conserved sequence. Though highly speculative, these findings raise the intriguing possibility that KSHV TK may compete with or antagonize certain K15 protein interactions and, similar to E2's antagonism of E6/E7, may cause alterations of cell morphology that promote senescence.
How might the observed behavior of KSHV TK contribute to the biology of the virus? As with the other human herpesviruses, oral epithelium is the probable physiologic site of KSHV lytic replication. In addition to supplying TMP, loss of adhesion despite cell survival could stimulate terminal keratinocyte differentiation, leading to amplification of virus production and facilitating virion egress by increasing the plasma membrane surface available for budding. The release of virus-replicating epithelial cells into saliva could further increase the efficiency of virus transmission, as there is evidence that contact with virus-replicating cells may be the most effective mode of KSHV transfer (42). The release of virus-replicating endothelial cells into plasma or lymph could facilitate coordinate delivery of large numbers of virions to distant sites, an intriguing possibility distinct from metastasis and consistent with the multifocal nature of KS.
Acknowledgments
We thank Jim Rheinwald (Harvard Skin Disease Research Center, Brigham and Women's Hospital, Boston, MA) for generous access to microscopic equipment.
This work was supported by an AHA grant-in-aid, by a K24 award (NCI), and by grant ROI DE12186.
REFERENCES
- 1.Bechtel, J. T., R. C. Winant, and D. Ganem. 2005. Host and viral proteins in the virion of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:4952-4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouamr, F., J. A. Melillo, M. Q. Wang, K. Nagashima, M. de Los Santos, A. Rein, and S. P. Goff. 2003. PPPYVEPTAP motif is the late domain of human T-cell leukemia virus type 1 Gag and mediates its functional interaction with cellular proteins Nedd4 and Tsg101. J. Virol. 77:11882-11895. (Author's correction, 78: 4383, 2004.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann, M. M., M. Glenn, L. Rainbow, A. Kieser, C. Henke-Gendo, and T. F. Schulz. 2003. Activation of mitogen-activated protein kinase and NF-κB pathways by a Kaposi's sarcoma-associated herpesvirus K15 membrane protein. J. Virol. 77:9346-9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull, T. M., C. D. Cool, A. E. Serls, P. R. Rai, J. Parr, J. M. Neid, M. W. Geraci, T. B. Campbell, N. F. Voelkel, and D. B. Badesch. 2003. Primary pulmonary hypertension, Castleman's disease and human herpesvirus-8. Eur. Respir. J. 22:403-407. [DOI] [PubMed] [Google Scholar]
- 5.Caron, E. 2003. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J. Cell Sci. 116:435-440. [DOI] [PubMed] [Google Scholar]
- 6.Cesarman, E. 2003. Kaposi's sarcoma-associated herpesvirus—the high cost of viral survival. N. Engl. J. Med. 349:1107-1109. [DOI] [PubMed] [Google Scholar]
- 7.Coen, D. M., M. Kosz-Vnenchak, J. G. Jacobson, D. A. Leib, C. L. Bogard, P. A. Schaffer, K. L. Tyler, and D. M. Knipe. 1989. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. USA 86:4736-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman, H. M., B. de Lima, V. Morton, and P. G. Stevenson. 2003. Murine gammaherpesvirus 68 lacking thymidine kinase shows severe attenuation of lytic cycle replication in vivo but still establishes latency. J. Virol. 77:2410-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crumpacker, C. S., II. 1989. Molecular targets of antiviral therapy. N. Engl. J. Med. 321:163-172. [DOI] [PubMed] [Google Scholar]
- 11.Danen, E. H., and A. Sonnenberg. 2003. Integrins in regulation of tissue development and function. J. Pathol. 200:471-480. [DOI] [PubMed] [Google Scholar]
- 12.Degreve, B., M. Johansson, E. De Clercq, A. Karlsson, and J. Balzarini. 1998. Differential intracellular compartmentalization of herpetic thymidine kinases (TKs) in TK gene-transfected tumor cells: molecular characterization of the nuclear localization signal of herpes simplex virus type 1 TK. J. Virol. 72:9535-9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efstathiou, S., S. Kemp, G. Darby, and A. C. Minson. 1989. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J. Gen. Virol. 70:869-879. [DOI] [PubMed] [Google Scholar]
- 14.Engels, E. A., R. J. Biggar, V. A. Marshall, M. A. Walters, C. J. Gamache, D. Whitby, and J. J. Goedert. 2003. Detection and quantification of Kaposi's sarcoma-associated herpesvirus to predict AIDS-associated Kaposi's sarcoma. AIDS 17:1847-1851. [DOI] [PubMed] [Google Scholar]
- 15.Gaspar, G., E. De Clercq, and J. Neyts. 2002. Gammaherpesviruses encode functional dihydrofolate reductase activity. Biochem. Biophys. Res. Commun. 297:756-759. [DOI] [PubMed] [Google Scholar]
- 16.Gentry, G. A. 1992. Viral thymidine kinases and their relatives. Pharmacol. Ther. 54:319-355. [DOI] [PubMed] [Google Scholar]
- 17.Geuijen, C. A., and A. Sonnenberg. 2002. Dynamics of the α6β4 integrin in keratinocytes. Mol. Biol. Cell 13:3845-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill, M. B., J. Roecklein-Canfield, D. R. Sage, M. Zambela-Soediono, N. Longtine, M. Uknis, and J. D. Fingeroth. 2004. EBV attachment stimulates FHOS/FHOD1 redistribution and co-aggregation with CD21: formin interactions with the cytoplasmic domain of human CD21. J. Cell Sci. 117:2709-2720. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin, E. C., E. Yang, C. J. Lee, H. W. Lee, D. DiMaio, and E. S. Hwang. 2000. Rapid induction of senescence in human cervical carcinoma cells. Proc. Natl. Acad. Sci. USA 97:10978-10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustafson, E. A., A. C. Chillemi, D. R. Sage, and J. D. Fingeroth. 1998. The Epstein-Barr virus thymidine kinase does not phosphorylate ganciclovir or acyclovir and demonstrates a narrow substrate specificity compared to the herpes simplex virus type 1 thymidine kinase. Antimicrob. Agents Chemother. 42:2923-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustafson, E. A., R. F. Schinazi, and J. D. Fingeroth. 2000. Human herpesvirus 8 open reading frame 21 is a thymidine and thymidylate kinase of narrow substrate specificity that efficiently phosphorylates zidovudine but not ganciclovir. J. Virol. 74:684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt, M. R., and A. Koffer. 2001. Cell motility: proline-rich proteins promote protrusions. Trends Cell Biol. 11:38-46. [DOI] [PubMed] [Google Scholar]
- 23.Holton, R. H., and G. A. Gentry. 1996. The Epstein-Barr virus genome encodes deoxythymidine kinase activity in a nested internal open reading frame. Intervirology 39:270-274. [DOI] [PubMed] [Google Scholar]
- 24.Irish, J. M., R. Hovland, P. O. Krutzik, O. D. Perez, O. Bruserud, B. T. Gjertsen, and G. P. Nolan. 2004. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell 118:217-228. [DOI] [PubMed] [Google Scholar]
- 25.Isnardi, I., R. Lesourne, P. Bruhns, W. H. Fridman, J. C. Cambier, and M. Daeron. 2004. Two distinct tyrosine-based motifs enable the inhibitory receptor FcγRIIB to cooperatively recruit the inositol phosphatases SHIP1/2 and the adapters Grb2/Grap. J. Biol. Chem. 279:51931-51938. [DOI] [PubMed] [Google Scholar]
- 26.Kay, B. K., M. P. Williamson, and M. Sudol. 2000. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 14:231-241. [PubMed] [Google Scholar]
- 27.Kim, H., T. G. Peterson, and S. Barnes. 1998. Mechanisms of action of the soy isoflavone genistein: emerging role for its effects via transforming growth factor beta signaling pathways. Am. J. Clin. Nutr. 68(Suppl. 6):1418S-1425S. [DOI] [PubMed] [Google Scholar]
- 28.Kim, S. Y., N. Chudapongse, S. M. Lee, M. C. Levin, J. T. Oh, H. J. Park, and I. K. Ho. 2005. Proteomic analysis of phosphotyrosyl proteins in morphine-dependent rat brains. Brain Res. Mol. Brain Res. 133:58-70. [DOI] [PubMed] [Google Scholar]
- 29.Lee, R. K., J. P. Cai, V. Deyev, P. S. Gill, L. Cabral, C. Wood, R. P. Agarwal, W. Xia, L. H. Boise, E. Podack, and W. J. Harrington, Jr. 1999. Azidothymidine and interferon-alpha induce apoptosis in herpesvirus-associated lymphomas. Cancer Res. 59:5514-5520. [PubMed] [Google Scholar]
- 30.Lock, M. J., N. Thorley, J. Teo, and V. C. Emery. 2002. Azidodeoxythymidine and didehydrodeoxythymidine as inhibitors and substrates of the human herpesvirus 8 thymidine kinase. J. Antimicrob. Chemother. 49:359-366. [DOI] [PubMed] [Google Scholar]
- 31.Longhurst, C. M., and L. K. Jennings. 1998. Integrin-mediated signal transduction. Cell. Mol. Life Sci. 54:514-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGeoch, D. J., and A. J. Davison. 1999. The descent of human herpesvirus 8. Semin. Cancer Biol. 9:201-209. [DOI] [PubMed] [Google Scholar]
- 33.Mentzer, S. J., J. Fingeroth, J. J. Reilly, S. P. Perrine, and D. V. Faller. 1998. Arginine butyrate-induced susceptibility to ganciclovir in an Epstein-Barr-virus-associated lymphoma. Blood Cells Mol. Dis. 24:114-123. [DOI] [PubMed] [Google Scholar]
- 34.Moore, P. S., and Y. Chang. 2001. Molecular virology of Kaposi's sarcoma-associated herpesvirus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:499-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogino, S., K. Tsuruma, T. Uehara, and Y. Nomura. 2004. Herbimycin A abrogates nuclear factor-κB activation by interacting preferentially with the IκB kinase beta subunit. Mol. Pharmacol. 65:1344-1351. [DOI] [PubMed] [Google Scholar]
- 36.Oldak, M., H. Smola, M. Aumailley, F. Rivero, H. Pfister, and S. Smola-Hess. 2004. The human papillomavirus type 8 E2 protein suppresses β4-integrin expression in primary human keratinocytes. J. Virol. 78:10738-10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patcha, V., J. Wigren, M. E. Winberg, B. Rasmusson, J. Li, and E. Sarndahl. 2004. Differential inside-out activation of beta2-integrins by leukotriene B4 and fMLP in human neutrophils. Exp. Cell Res. 300:308-319. [DOI] [PubMed] [Google Scholar]
- 38.Paulose-Murphy, M., N. K. Ha, C. Xiang, Y. Chen, L. Gillim, R. Yarchoan, P. Meltzer, M. Bittner, J. Trent, and S. Zeichner. 2001. Transcription program of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 75:4843-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plemenitas, A., X. Lu, M. Geyer, P. Veranic, M. N. Simon, and B. M. Peterlin. 1999. Activation of Ste20 by Nef from human immunodeficiency virus induces cytoskeletal rearrangements and downstream effector functions in Saccharomyces cerevisiae. Virology 258:271-281. [DOI] [PubMed] [Google Scholar]
- 40.Roychowdhury, S., R. Peng, R. A. Baiocchi, D. Bhatt, S. Vourganti, J. Grecula, N. Gupta, C. F. Eisenbeis, G. J. Nuovo, W. Yang, P. Schmalbrock, A. Ferketich, M. Moeschberger, P. Porcu, R. F. Barth, and M. A. Caligiuri. 2003. Experimental treatment of Epstein-Barr virus-associated primary central nervous system lymphoma. Cancer Res. 63:965-971. [PubMed] [Google Scholar]
- 41.Rubsam, L. Z., B. L. Davidson, and D. S. Shewach. 1998. Superior cytotoxicity with ganciclovir compared with acyclovir and 1-beta-D-arabinofuranosylthymine in herpes simplex virus-thymidine kinase-expressing cells: a novel paradigm for cell killing. Cancer Res. 58:3873-3882. [PubMed] [Google Scholar]
- 42.Sakurada, S., H. Katano, T. Sata, H. Ohkuni, T. Watanabe, and S. Mori. 2001. Effective human herpesvirus 8 infection of human umbilical vein endothelial cells by cell-mediated transmission. J. Virol. 75:7717-7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schon, M., J. Benwood, T. O'Connell-Willstaedt, and J. G. Rheinwald. 1999. Human sweat gland myoepithelial cells express a unique set of cytokeratins and reveal the potential for alternative epithelial and mesenchymal differentiation states in culture. J. Cell Sci. 112:1925-1936. [DOI] [PubMed] [Google Scholar]
- 44.Sharp, T. V., H. W. Wang, A. Koumi, D. Hollyman, Y. Endo, H. Ye, M. Q. Du, and C. Boshoff. 2002. K15 protein of Kaposi's sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. J. Virol. 76:802-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song, M. J., H. Deng, and R. Sun. 2003. Comparative study of regulation of RTA-responsive genes in Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 77:9451-9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tung, P. P., and W. C. Summers. 1994. Substrate specificity of Epstein-Barr virus thymidine kinase. Antimicrob. Agents Chemother. 38:2175-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Unwin, R. D., D. W. Sternberg, Y. Lu, A. Pierce, D. G. Gilliland, and A. D. Whetton. 2005. Global effects of BCR/ABL and TEL/PDGFRβ expression on the proteome and phosphoproteome: identification of the Rho pathway as a target of BCR/ABL. J. Biol. Chem. 280:6316-6326. [DOI] [PubMed] [Google Scholar]
- 48.Westphal, E. M., W. Blackstock, W. Feng, B. Israel, and S. C. Kenney. 2000. Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: a potential method for treating EBV-positive malignancies. Cancer Res. 60:5781-5788. [PubMed] [Google Scholar]
- 49.Yasuda, J., M. Nakao, Y. Kawaoka, and H. Shida. 2003. Nedd4 regulates egress of Ebola virus-like particles from host cells. J. Virol. 77:9987-9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu, L., J. V. White, and T. F. Smith. 1998. A homology identification method that combines protein sequence and structure information. Protein Sci. 7:2499-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]