Abstract
Linezolid is a new oxazolidinone with activity against gram-positive cocci. We determined the in vivo activity of linezolid against four strains of Staphylococcus aureus (two methicillin-susceptible S. aureus [MSSA] strains and two methicillin-resistant S. aureus strains) and one penicillin-susceptible Streptococcus pneumoniae (PSSP) strain, two penicillin-intermediate S. pneumoniae strains, and five penicillin-resistant S. pneumoniae strains. The mice had 106.3 to 107.7 CFU/thigh before therapy and were then treated for 24 h with 5 to 1,280 mg of linezolid/kg divided into 1, 2, 4, 8, or 16 doses. The killing activities after 4 h of therapy ranged from 2.4 to 5.0 log10 CFU/thigh against S. pneumoniae and 1.35 to 2.2 log10 CFU/thigh against S. aureus. Increasing doses produced minimal concentration-dependent killing; doses of 20 and 80 mg/kg produced no in vivo postantibiotic effects (PAEs) with PSSP and modest PAEs (3.4 and 3.2 h) with MSSA. Pharmacokinetic studies at doses of 20 and 80 mg/kg by high-pressure liquid chromatography analysis exhibited peak dose values of 0.68 and 0.71 and elimination half-lives of 1.02 and 1.00 h. Linezolid MICs ranged from 0.5 to 1.0 μg/ml for S. pneumoniae and from 1.0 to 4.0 μg/ml for S. aureus. A sigmoid dose-response model was used to estimate the dose required to achieve a net bacteriostatic effect over 24 h. Static doses against S. pneumoniae ranged from 22.2 to 97.1 mg/kg/24 h and from 133 to 167 mg/kg/24 h for S. aureus. The 24-h area under the concentration-time curve (AUC)/MIC ratio was the major parameter determining the efficacy of linezolid against PSSP (R2 = 82% for AUC/MIC versus 57% for T>MIC and 59% for the peak level in serum/MIC [peak/MIC]). It was difficult to determine the most relevant pharmacokinetic/pharmacodynamic parameter with S. aureus, although the outcomes correlated slightly better with the 24-h AUC/MIC ratio (R2 = 75%) than with the other parameters (T>MIC R2 = 75% and peak/MIC R2 = 65%). The 24-h AUC/MIC ratio required for a bacteriostatic effect with linezolid varied from 22 to 97 (mean = 48) for pneumococci and from 39 to 167 (mean = 83) for staphylococci. Based upon a pharmacokinetic goal of a 24-h AUC/MIC of 50 to 100, a dosage regimen of 600 mg given either intravenously or orally twice daily would achieve success against organisms with MICs as high as 2 to 4 μg/ml.
The number of infections due to resistant gram-positive bacteria, including penicillin-resistant Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus faecium, continues to increase (8, 10, 12, 17). The development of effective antimicrobial agents to treat these infections is an area of intense research. Oxazolidinone antimicrobial agents represent a promising new class of compounds which act by inhibiting protein synthesis and have demonstrated potency against these emerging pathogens (13, 16; R. N. Jones, M. A. Pfaller, M. E. Erwin, and M. L. Beach, Abstr. 37th Infect. Dis. Soc. Am. Annu. Meet., abstr. 97, 1999).
Linezolid is a new oxazolidinone that has demonstrated both in vitro activity and in vivo efficacy in animal models and clinical trials (2, 13, 15; Jones et al., Abstr. 37th Infect. Dis. Soc. Am. Annu. Meet.). The goals of our studies were (i) to characterize the in vivo time course of antimicrobial activity of linezolid against susceptible and resistant S. pneumoniae and S. aureus strains and (ii) to determine the PK/PD parameter and the magnitude of the PK/PD parameter predictive of efficacy.
(Part of this work was presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., September 1998.)
MATERIALS AND METHODS
Bacteria, media, and antibiotic.
Eight strains of S. pneumoniae (one penicillin-susceptible, two penicillin-intermediate, and five penicillin-resistant S. pneumoniae strains) and four strains of S. aureus (two methicillin-susceptible and two methicillin-resistant S. aureus strains) were used for these experiments. S. aureus and S. pneumoniae organisms were grown, subcultured, and quantified in Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.), on Mueller-Hinton agar (Difco Laboratories, Detroit, Mich.), and on sheep blood agar plates (Remel, Milwaukee, Wis.). Linezolid was supplied by Pharmacia & Upjohn Company, Kalamazoo, Mich.
In vitro susceptibility studies.
The MICs of linezolid, penicillin, and methicillin for the various isolates were determined by using standard National Committee for Clinical Laboratory Standards microdilution methods.
Murine infection model.
Six-week-old, specific-pathogen-free, female ICR/Swiss mice weighing 23 to 27 g (Harlan Sprague-Dawley, Madison, Wis.) were used for all studies. All animal studies were approved by the Animal Research Committee of the William S. Middleton Memorial VA Hospital. Mice were rendered neutropenic (neutrophils at <100/mm3) by injecting cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, Ind.) intraperitoneally 4 days (150 mg/kg) and 1 day (100 mg/kg) before experimental infection. Previous studies have shown that this regimen produces neutropenia in this model for 5 days (1). Broth cultures of freshly plated bacteria were grown to logarithmic phase overnight to an absorbance of 0.3 at 580 nm (Spectronic 88; Bausch and Lomb, Rochester, N.Y.). After a 1:10 dilution into fresh Mueller-Hinton broth, bacterial counts of the inoculum ranged from 106 to 107 CFU/ml. Thigh infections with each of the isolates were produced by injection of 0.1 ml of inoculum into the thighs of the mice at 2 h before therapy with linezolid.
Drug pharmacokinetics.
Single-dose serum pharmacokinetic studies were performed in thigh-infected mice given subcutaneous doses of linezolid (20 and 80 mg/kg). For each of the doses examined, four groups of three mice were sampled by intracardiac puncture at 30- to 60-min intervals. Samples were allowed to coagulate at room temperature for 60 min. Samples were then centrifuged for 5 min at 10,000 × g, and the serum was removed and stored at −70°C. Linezolid concentrations in serum were determined by high-pressure liquid chromatography analysis. Linezolid was extracted on a solid-phase cartridge and separated on a reversed-phase column with 20% (vol/vol) acetonitrile in water as the mobile phase. The chromatographic peak-height ratios or area ratios versus an internal standard based on UV absorbance at 251 nm were used for quantitative analysis (14). The lower limit of quantitation was 0.02 μg/ml. The intraday variation was <6%. Pharmacokinetic constants, including elimination half-life, area under the concentration-time curve (AUC), and peak level were calculated by using a one-compartment model with zero-order absorption and first-order elimination.
Treatment protocols. (i) In vivo PAE.
At 2 h after infection with either S. pneumoniae ATCC 10813 or S. aureus ATCC 6538p, mice were treated with single subcutaneous doses of linezolid (20 or 80 mg/kg). Groups of two linezolid-treated mice and saline-treated control mice were sacrificed at each sampling time interval ranging from 2 to 6 h. Control growth was determined at four sampling times over 24 h. The treated groups were sampled four times over 24 h. The thighs were removed at each time point and were processed immediately for CFU determination. The time after administration of the two doses studied that the levels of linezolid in the serum remain above the MIC for the organisms was calculated from the pharmacokinetic data. Both total drug and free drug (30% protein binding) concentrations were considered (K. Chiba, K. L. Feenstra, J. G. Slatter, et al., 38th Intersci. Conf. Antimicrob. Agents Chemother. [ICAAC], abstr. A123, 1998; T. A. Sisson, G. L. Jungbluth, and N. K. Hopkins, 39th ICAAC, abstr. 1194, 1999). The postantibiotic effect (PAE) was calculated by subtracting the time it took for organisms to increase 1 log in the thighs of saline-treated animals from the time it took organisms to grow the same amount in linezolid-treated animals after levels in serum fell below the MIC of the infecting organism (4): PAE = T − C, where C is the time needed for 1 log10 control growth and T is the time needed for 1 log10 treatment growth after levels have fallen below MIC.
(ii) Pharmacodynamic parameter determination.
Neutropenic mice were infected with either a standard strain of penicillin-susceptible S. pneumoniae ATCC 10813 or methicillin-susceptible S. aureus ATCC 6538p. Groups of two mice were treated for 24 h with 16 different linezolid dosing regimens by using fourfold increasing total doses. Doses were fractionated into 2, 4, 8, or 16 doses to vary the PK/PD parameters and limit the interdependence among parameters with dose escalation. Total dose increases of linezolid varied 512-fold (range, 2.5 to 1,280 mg/kg/24 h). Drug doses were administered subcutaneously in 0.2-ml volumes. The mice were sacrificed after 24 h of therapy, the thighs removed and processed for CFU determination. Saline treated control mice were sacrificed just before treatment and after 24 h.
(iii) Pharmacodynamic parameter magnitude studies.
Similar dosing studies by using 4-fold increasing total linezolid doses administered every 6 h were utilized to treat animal infected with eight strains of S. pneumoniae (one penicillin-susceptible, two penicillin-intermediate, and five penicillin-resistant S. pneumoniae strains) and four strains of S. aureus (two methicillin-susceptible S. aureus strains and two methicillin-resistant S. aureus strains) (Table 1). The linezolid MICs of these isolates varied by only eightfold. Penicillin and methicillin MICs, however, varied by 1,000- and 16-fold, respectively. The total daily dose of linezolid varied from 5 to 640 mg/kg.
TABLE 1.
In vitro susceptibility of linezolid against susceptible and resistant S. pneumoniae and S. aureus strains
| Organism and strain | MIC/MBC (mg/liter)
|
|
|---|---|---|
| Penicillin-methicillin | Linezolid | |
| S. pneumoniae | ||
| ATCC 10813 | 0.008/0.008 | 1.0/2.0 |
| CDC141 | 0.25/0.25 | 1.0/1.0 |
| CDC1396 | 0.5/0.5 | 1.0/2.0 |
| CDC1325 | 2.0/2.0 | 0.5/1.0 |
| CDC1293 | 2.0/2.0 | 1.0/1.0 |
| CDC145 | 4.0/4.0 | 1.0/2.0 |
| CDC146 | 4.0/4.0 | 1.0/2.0 |
| CDC673 | 8.0/8.0 | 1.0/1.0 |
| S. aureus | ||
| 6538p | 1.0/1.0 | 2.0/>4.0 |
| 25923 | 2.0/2.0 | 4.0/>8.0 |
| 33591 | >16/<16 | 1.0/>2.0 |
| MRSAa | >16/>16 | 2.0/>8.0 |
Methicillin-resistant S. aureus.
Data analysis.
The results of these studies were analyzed by using the sigmoid dose effect model. The model is derived from the Hill equation: log10 SD = {log10[E/(Emax − E)]/N } + log ED50, where SD is the static dose or the 24-h dose level necessary to reduce organism counts to the level at the start of the experiment, E is the control growth or in this case the log change in CFU per thigh in untreated controls after the 24-h period of study, Emax is the maximum effect, ED50 is the dose required to achieve 50% of Emax, and N is the slope of the dose effect curve. The indices—Emax, ED50, and N—were calculated by using nonlinear least-squares regression. The correlation between efficacy and each of the three pharmacokinetic-pharmacodynamic parameters (T>MIC, AUC/MIC, and peak level in serum/MIC [peak/MIC]) studied was determined by nonlinear least-squares multivariate regression (Sigma Stat; Jandel Scientific Software, San Rafael, Calif.). The coefficient of determination or R2 was used to estimate the variance that could be due to regression with each of the pharmacodynamic parameters.
To allow a more meaningful comparison of the potency of linezolid against a variety of organisms, we calculated the 24-h static dose or the dose required to achieve no net growth over 24 h.
RESULTS
In vitro susceptibility testing.
The MICs of linezolid, penicillin, and methicillin for the 12 study strains are shown in Table 1. Penicillin MICs against the eight S. pneumoniae strains varied by 1,000-fold (range, 0.008 to 8.0 μg/ml). Methicillin MICs against the four strains of S. aureus varied by >16-fold. Penicillin and methicillin resistance did not influence linezolid MICs, which ranged from only 0.5 to 1.0 μg/ml against S. pneumoniae and from 1.0 to 4.0 μg/ml against S. aureus.
Pharmacokinetics.
The time course of levels of linezolid in serum in infected neutropenic mice after subcutaneous doses of 20 and 80 mg/kg are shown in Fig. 1. The elimination half-life was similar with each dose (1.02 and 1.00 h, respectively). The peak dose values for the escalating single doses were 0.68 and 0.71, respectively.
FIG. 1.
Linezolid concentrations in serum after administration of single doses of 20 and 80 mg/kg in neutropenic infected mice. Symbols: □, data obtained with the 20-mg/kg dose; ○, data obtained with the 80-mg/kg dose. Each symbol represents the geometric mean ± standard deviation of the levels in the sera of three mice. t1/2, serum elimination half-live in hours; Cmax, peak level in serum; AUC0−∞, serum AUC.
In vivo time kill and PAE.
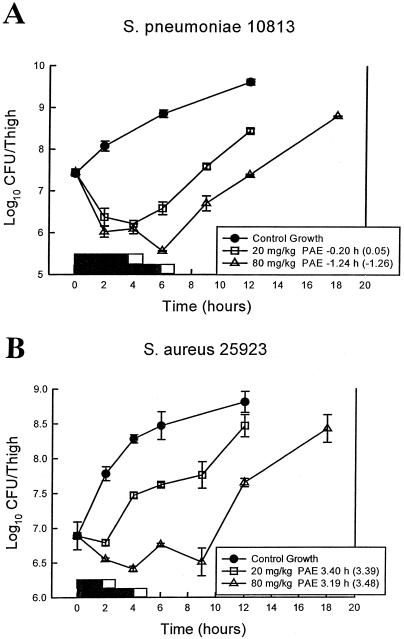
After thigh inoculation of 107.4 CFU of S. pneumoniae/ml and 106.3 CFU of S. aureus/ml, the growth of organisms in the thighs of saline-treated mice increased 2.2 ± 0.07 and 1.9 ± 0.15 log10 CFU/thigh, respectively. Growth of 1 log10 CFU/thigh in saline-treated animals occurred within 4.9 and 3.9 h in S. pneumoniae- and S. aureus-infected animals, respectively. No drug carryover was observed in either treatment group. Based upon the serum pharmacokinetic determinations, serum linezolid levels after the single doses of 20 and 80 mg/kg would remain above the MIC of S. pneumoniae ATCC 10813 (MIC, 1.0 μg/ml) for 4.4 h (free drug, 3.9 h) and 6.5 h (free drug, 6.0 h), respectively. The time above the MIC of these treatments against S. aureus ATCC 6538p (MIC 4.0 μg/ml) would be 2.4 h (free drug, 1.9) and 4.5 h (free drug, 4.0 h). These linezolid doses produced modest reductions in colony counts of S. pneumoniae (1.1 ± 0.12 and 1.9 ± 0.07 log10 CFU/thigh). Both doses produced only a static effect against S. aureus, with thigh count reductions of 0.1 ± 0.07 and 0.5 ± 0.03 log10 CFU/thigh, respectively. The growth curves of both S. pneumoniae and S. aureus for the control groups and treated mice are shown graphically in Fig. 2. Against S. pneumoniae both doses produced no PAEs even when the free-drug levels were considered. A study with S. aureus demonstrated modest PAEs of 3.2 to 3.4 h with both linezolid doses. Consideration of free-drug levels in the staphylococcal experiment also had a minimal impact on this duration.
FIG. 2.
(A) In vivo PAE of linezolid after administration of single doses of 20 and 80 mg/kg against S. pneumoniae ATCC 10813. Symbols: □, data obtained with the 20-mg/kg dose; ▵, data obtained with the 80-mg/kg dose. Each symbol represents the mean ± the standard deviation for two mice. The solid bars represent the time that free-drug levels remained above the MIC. The open bars represent the time that total drug levels remained above the MIC. (B) In vivo PAE of linezolid after administration of single doses of 20 and 80 mg/kg against S. aureus ATCC 6538p. Symbols: □, data obtained with the 20-mg/kg dose; ▵, data obtained with the 80-mg/kg dose. Each symbol represents the mean ± the standard deviation for two mice. The solid bars represent the time that free-drug levels remained above the MIC. The open bars represent the time that total drug levels remained above the MIC.
Pharmacodynamic parameter determination.
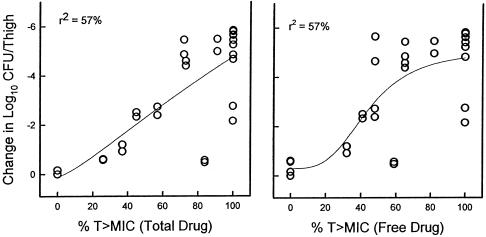
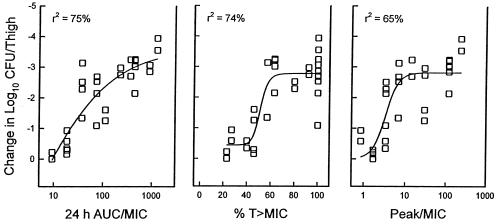
At the start of therapy mice had 7.49 ± 0.05 and 6.29 ± 0.02 log10 CFU of S. pneumoniae ATCC 10813 and S. aureus ATCC 6538p/thigh, respectively. After 24 h the organisms grew 1.21 ± 0.13 and 1.39 ± 0.26 log10 CFU/thigh, respectively, in saline-treated control mice. Escalating doses of linezolid resulted in significant killing of S. pneumoniae with the highest doses studied (4.21 ± 0.12 log10 CFU/thigh). Maximal killing of S. aureus, however, was only 2.7 ± 0.07 log10 CFU/thigh. The relationships between microbiologic effect against S. pneumoniae ATCC 10813 and each of the pharmacodynamic parameters, i.e., the percent time above the MIC, the AUC/MIC, and the peak/MIC, are shown in Fig. 3. The strongest relationship was seen when results were correlated with the 24-h AUC/MIC, with an R2 value of 82%. Correlation with the other parameters was not nearly as strong (T>MIC R2 = 57% and peak/MIC R2 = 59%). Consideration of free-drug levels for the T>MIC analysis did not change this relationship (R2 = 57%), as shown in Fig. 4. Similar analysis of a study with S. aureus was difficult due to the lack of significant microbiologic effect (maximal organism reduction with the highest doses studied was <3 log10). This narrow outcome range did not provide sufficient difference between effective and ineffective dosing regimens to discriminate among the parameters (Fig. 5). However, regression of the data with the 24-h AUC/MIC ratio did have a relatively high coefficient of determination (R2 = 75%). Consideration of free-drug levels also had no appreciable effect on the time above MIC relationship (R2 = 74% versus 75%).
FIG. 3.
Relationships between the percentage of the dosing interval that levels in serum remained above the MIC for S. pneumoniae ATCC 10813, the 24-h AUC/MIC, and the peak/MIC and the log10 number of CFU/thigh after 24 h of therapy. Each symbol represents the data for two mice. The lines represent the best fit line. R2 is the coefficient of determination.
FIG. 4.
Relationship between between the percentage of the dosing interval that both total and free-drug levels in serum remained above the MIC for S. pneumoniae ATCC 10813. Each symbol represents the data for two mice. The lines represent the best fit line. R2 is the coefficient of determination.
FIG. 5.
Relationships between the percentage of the dosing interval that levels in serum remained above the MIC for S. aureus ATCC 6538p, the 24-h AUC/MIC, and the peak/MIC and the log10 number of CFU/thigh after 24 h of therapy. Each symbol represents the data for two mice. The lines represent the best-fit line. R2 is the coefficient of determination.
24-Hour static dose determination.
The growth curves of the eight pneumococcal and four staphylococcal strains in control animals were similar. At the start of therapy mice had between 6.17 ± 0.23 and 7.64 ± 0.02 log10 CFU/thigh. The organisms grew to 1.54 ± 0.16 to 2.67 ± 0.18 log10 CFU/thigh in saline-treated control mice. Maximal organism reduction in S. pneumoniae-infected mice was 4.24 ± 0.83 log10 CFU/thigh (range, 2.38 to 5.24). A smaller degree of killing was observed in animals infected with S. aureus (1.69 ± 0.35; range, 1.36 to 2.19). Table 2 shows the 24-h total dose required to achieve a static effect and corresponding 24-h AUC/MIC ratio for the 6-h regimens against the various pneumococci and staphylococci. Against S. pneumoniae, static doses ranged from 18 to 72 mg/kg/day, and the corresponding 24-h AUC/MIC ratios ranged from 22 to 97 mg/kg/day. Against the four S. aureus strains, the 24-h static doses ranged from 95 to 119 mg/kg/day and the 24-h AUC/MIC from 59 to 167 mg/kg/day.
TABLE 2.
Bacteriostatic doses for linezolid against S. pneumoniae and S. aureus
| Organism and strain | MIC (mg/liter) | Mean static dose ± SE (mg/kg/24 h) | 24-h AUC (mg · h/liter) | 24-h AUC/ MICa |
|---|---|---|---|---|
| S. pneumoniae | ||||
| ATCC 10813 | 1.0 | 23.0 ± 0.02 | 22.2 | 22.2 |
| CDC141 | 1.0 | 67.2 ± 0.09 | 89.3 | 89.3 |
| CDC1325 | 0.5 | 17.8 ± 4.9 | 16.5 | 33.0 |
| CDC1396 | 1.0 | 37.6 ± 4.7 | 42.0 | 42.0 |
| CDC673 | 1.0 | 24.5 ± 0.05 | 24.1 | 24.1 |
| CDC145 | 1.0 | 33.4 ± 4.6 | 36.0 | 36.0 |
| CDC1293 | 1.0 | 38.0 ± 0.80 | 42.6 | 42.6 |
| CDC146 | 1.0 | 71.7 ± 21 | 97.1 | 97.1 |
| S. aureus | ||||
| 6538p | 2.0 | 95.2 ± 7.2 | 133.3 | 66.6 |
| 33591 | 1.0 | 119 ± 17.9 | 167.0 | 167.0 |
| MRSAb | 2.0 | 84.4 ± 15.0 | 118.0 | 59.1 |
| 25923 | 4.0 | 111 ± 14.7 | 155.0 | 38.9 |
For S. pneumoniae strains, the mean ± the standard deviation was 48.3 ± 28.8. For S. aureus strains, the mean ± the standard deviation was 82.9 ± 57.3.
Methicillin-resistant S. aureus.
DISCUSSION
Escalating resistance of gram-positive bacteria to antimicrobial agents has severely limited therapeutic options for many nosocomial and community-acquired infections (8, 10, 12, 17). The timely development of potent antimicrobial agents and effective administration of these compounds has become a pressing priority. The use of animal infection models to understand the pharmacodynamic activity of antimicrobial agents is one approach that has proven helpful for (i) the design of effective dosing regimens in humans and (ii) the development of appropriate in vitro susceptibility breakpoints (1, 3, 6, 7, 11, 18).
Antimicrobial pharmacodynamic studies characterize the time course of antibiotic effect (3). The time course of antimicrobial activity can be determined by two characteristics: (i) the effect of increasing drug concentrations on the extent of organism killing and (ii) the presence or absence of antimicrobial effects that persist after the levels in serum have fallen below the MIC (3). For example, demonstration of the concentration-dependent killing and prolonged PAE with the aminoglycoside class has provided the basis for once-daily dosing of these drugs (6). This regimen optimizes the concentration-dependent parameters, the peak level/MIC and the AUC/MIC, which have been shown to predict efficacy, limit toxicity, and reduce the development of organism resistance. On the other hand, β-lactam antibiotics exhibit minimal concentration-dependent killing and produce minimal PAEs (11, 18). Thus, the goal of a dosing regimen would be to optimize the duration of antibiotic exposure. Several experimental models have proven that the duration of time serum levels exceed the MIC is the pharmacodynamic parameter predicting the efficacy of β-lactams (11, 18).
Because the target of antimicrobial agents is located in the pathogen and not in the host, pharmacodynamic parameters can correct for differences in pharmacokinetics among animal species. Thus, the magnitude of pharmacodynamic parameters for a variety of antimicrobial classes should be similar in different animal species, including humans (1, 3, 5, 7). For example, efficacy of β-lactams in animal models and in upper-respiratory-tract infection clinical trials is achieved when the magnitude of the time above MIC reaches 40 to 50% (W. A. Craig, S. Ebert, and Y. Wantanabe, 33rd ICAAC, abstr. 86, p. 135, 1993).
The present study characterized the in vivo time course antimicrobial activity of the new oxazolidinone, linezolid. Similar to the findings of other studies, we observed here a narrow range of in vitro susceptibility among the organisms examined (13, Jones et al., Abstr. 37th Infect. Dis. Soc. Am. Annu. Meet.). Penicillin resistance in S. pneumoniae and methicillin resistance in S. aureus had no impact upon the in vitro and in vivo potency of linezolid. Similar to the β-lactam and macrolide classes, linezolid antimicrobial activity was not enhanced by escalating drug concentrations. Furthermore, linezolid produced only minimal to modest PAEs. Given these pharmacodynamic characteristics one would predict the duration of time serum levels exceed the MIC would be the pharmacodynamic parameter that most strongly correlated with efficacy of linezolid. However, the 24-h AUC/MIC ratio best correlated with outcomes against S. pneumoniae and was also important in describing the in vivo activity against S. aureus.
Analysis of experiments with several S. pneumoniae demonstrated a 24-h AUC/MIC ratio (48.3 ± 29) is necessary for efficacy with linezolid. Linezolid was less potent in vivo against S. aureus. The 24-h AUC/MIC ratios required to produce a net static effect against S. aureus (82.9 ± 57) were 1.7-fold greater than those observed against pneumococci. This microbiologic outcome in this infection model has correlated with clinical and microbiologic efficacy in humans (7). Based upon a pharmacodynamic goal of achieving a 24-h AUC/MIC ratio of 50 to 80, the linezolid regimen of 600 mg given twice daily in humans would be successful against organisms with MICs of up to 2 to 4 μg/ml (9).
REFERENCES
- 1.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chien, J. W., M. L. Kucia, and R. A. Salata. 2000. Use of linezolid, an oxazolidinone, in the treatment of multidrug-resistant gram-positive bacterial infections. Clin. Infect. Dis. 30:146-151. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A. 1998. Pharmacokinetics and pharmacodynamics of antibiotics in mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 4.Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 5.Craig, W. 1993. Relevance of animal models for clinical treatment. Eur. J. Clin. Microbiol. Infect. Dis. 12(Suppl. 1):55-57. [DOI] [PubMed] [Google Scholar]
- 6.Craig, W. A., J. Redington, and S. C. Ebert. 1991. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J. Antimicrob. Chemother. 27(Suppl. C):29-40. [DOI] [PubMed] [Google Scholar]
- 7.Craig, W. A., and D. R. Andes. 1996. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr. Infect. Dis. J. 15:255-259. [DOI] [PubMed] [Google Scholar]
- 8.Doern, G., M. Pfaller, K. Kuugler, et al. 1998. Prevalence of antimicrobial resistance among respiratory tract isolates of Streptococcus pneumoniae in North America: 1997 results from the SENTRY antimicrobial surveillance program. Clin. Infect. Dis. 27:764-770. [DOI] [PubMed] [Google Scholar]
- 9.Gee, T., R. Ellis, G., Marshall, J. Andrews, et al. 2001. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob. Agents Chemother. 45:1843-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herold, B. C., L. C. Immergluck, M. C. Maranan, et al. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 279:593-598. [DOI] [PubMed] [Google Scholar]
- 11.Leggett, J. E., B. Fantin, S. Ebert, K. Totsuka, B. Vogelman, W. Calame, H. Mattie, and W. A. Craig. 1989. Comparative antibiotic dose-effect relationships at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 159:281-292. [DOI] [PubMed] [Google Scholar]
- 12.Murray, B. E. 2000. Vancomycin-resistant enterococcal infections. New Engl. J. Med. 342:710-721. [DOI] [PubMed] [Google Scholar]
- 13.Noskin, G. A., F. Siddiqui, V. Storsor, D. Hacek, and L. R. Peterson. 1999. In vitro activities of linezolid against important gram-positive bacterial pathogens including vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 43:2059-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng, G. W., R. P. Stryd, S. Murata, M. Igarashi, K. Chiba, H. Aoyama, M. Aoyama, and T. Zenki. 1999. Determination of linezolid in plasma by reversed-phase high-performance liquid chromatography. J. Pharm. Biomed. Anal. 20:65-73. [DOI] [PubMed] [Google Scholar]
- 15.Schulin, T., C. Thauvin-Eliopoulos, R. C. Moellering, and G. M. Eliopoulos. 1999. Activities of the oxazolidinones linezolid and eperezolid in experimental intro-abdominal abscess due to Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 43:2873-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swaney, S. M., H. Aoki, M. C. Ganoza, and D. L. Shinabarger. 1998. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob. Agents Chemother. 42:3251-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson, R. L., I. Cabezudo, and R. P. Wenzel. 1982. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann. Intern. Med. 97:309-317. [DOI] [PubMed] [Google Scholar]
- 18.Vogelman, B., S. Gudmundsson, J. Leggett, J. Turnidge, S. Ebert, and W. A. Craig. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J. Infect. Dis. 158:831-847. [DOI] [PubMed] [Google Scholar]