Abstract
The pharmacokinetics of gatifloxacin were assessed in serum and in skin blister fluid (SBF), as was the pharmacodynamic activity in SBF. Five hours after a single dose of gatifloxacin, SBF killed 2.5 logs of Streptococcus pneumoniae and 1.5 log of Staphylococcus aureus during a 2-h incubation ex vivo.
Gatifloxacin is active against gram-positive and gram-negative organisms, including anaerobes (5, 17); Mycoplasma, Chlamydia, and Legionella (14, 16); and mycobacteria (9). Like other quinolones, gatifloxacin penetrates well into leukocytes, which can deliver active drug to sites of infection and play an important role in the treatment of intracellular pathogens (21).
We analyzed the pharmacokinetic parameters of gatifloxacin in serum and skin blister fluid (SBF), and the pharmacodynamic activity in SBF. Time-kill curves were obtained by inoculation of 3 × 106 CFU/ml for studies with a clinical isolate of Streptococcus pneumoniae and by inoculation of 1.5 × 106 CFU/ml for studies with a methicillin-susceptible laboratory strain of Staphylococcus aureus. The MICs and minimal bactericidal concentrations (MBCs) of gatifloxacin for both test strains were established by a standard macrodilution assay in Mueller-Hinton broth (MHB; Becton Dickinson), with a final inoculum of approximately 5 × 105 CFU/ml and incubated at 37°C for 24 h (12). Gatifloxacin was obtained as crystalline powder for in vitro testing from Grünenthal GmbH, Aachen, Germany. For the volunteer study, we used film-coated tablets containing 400 mg of gatifloxacin as an active ingredient obtained from the same manufacturer.
Approval for this study was obtained from the Ethics Committee of the University Hospitals. Twenty healthy Caucasian volunteers participated in the study. All subjects completed the study, and no adverse events were reported. Skin blisters were induced by applying eight plasters (1 by 1 cm) impregnated with 0.2% cantharidin ointment (Adler Pharmacy, Alf an der Mosel, Germany) to the forearm of each volunteer, as previously described (7). Fourteen hours later, the plasters were removed, and SBF was sampled by puncture of the blisters. The pharmacokinetic parameters were calculated from the serum and SBF samples of all 20 volunteers. The pharmacodynamic activity of SBF from the first and second groups of 10 subjects was determined against S. pneumoniae and S. aureus, respectively. One aliquot of the SBF was analyzed without centrifugation (i.e., containing leukocytes), a second was centrifuged (12,000 × g for 3 min at 4°C) before incubation with the test strain, and a third was stocked after centrifugation for the pharmacokinetic measurements.
Eight milliliters of venous blood was collected through an indwelling catheter for determination of concentrations of gatifloxacin in serum at the following time points in relation to the time of drug administration: before (0 h); 15, 30, and 60 min after; and 2, 3, 5, 7, 9, and 24 h after drug intake. Blood samples were collected in plain tubes (Vacutainers), immediately cooled on ice, and then centrifuged at 12,000 × g for 3 min at 4°C. SBF samples were collected in Eppendorf tubes for the determination of gatifloxacin levels at the same time points as the blood samples, with the exception of the 15- and 30-min points. Serum and centrifuged SBF samples were stored in plastic tubes at −20°C until assayed. Gatifloxacin levels in serum and SBF were determined by a validated agar plate diffusion microbiological assay with Escherichia coli (V6311/65) as a test strain (Hoechst Marion Roussel, Frankfurt am Main, Germany) (19). The standard curve was performed in Hanks' balanced salt solution containing 40% of decomplemented pooled serum. The curve was linear between 0.08 and 20 μg/ml. The limit of sensitivity of the assay was 0.05 μg/ml in both serum and SBF. The mean intra- and interassay coefficients of variation were <5%. By using the same standards for serum and SBF, this agar plate diffusion assay compares the unbound fraction of gatifloxacin in both matrices, which is essential in a pharmacodynamic analysis. Serum and SBF concentration-time data were analyzed with TopFit software (18). The Akaike information criteria were used to discriminate among candidate models and a two-compartment open distribution model with first-order absorption was selected for the serum data. SBF concentration-time data were analyzed separately by a one-compartment distribution model with first-order absorption. The area under the concentration-time curve from time zero to 24 h (AUC0-24) was determined by the log-linear trapezoidal method. The AUC from time zero to infinity (AUC0-∞) was calculated as the integral over the analytical function that describes the concentration profile. The apparent terminal elimination rate constant (Ke) was estimated by curve fitting with use of a nonlinear least-square regression analysis with reciprocal (1/y) weighting. The degree of penetration into the inflammatory exudate was determined from the ratio of the AUC0-∞ of the SBF to that of the serum. Apparent oral clearance (CL/F) was determined by dividing the dose by the AUC0-∞. The apparent volume of distribution (Varea/F) was calculated by dividing the CL/F by Ke.
The phagocytic bactericidal assay was miniaturized to a final volume of 100 μl, as previously described (7). A medium containing 40% pooled normal human serum, 40% phosphate-buffered saline, and 20% MHB supported growth without autolysis of S. pneumoniae for at least 6 h. Each test tube contained 90 μl of medium or SBF and 10 μl of bacterial inoculum (3 × 105 S. pneumoniae CFU or 1.5 × 105 S. aureus CFU). At each time point, four different mixtures were incubated in Eppendorf tubes as follows: (i) 90 μl of medium plus 10 μl of bacterial inoculum as growth control; (ii) 90 μl of medium with twice the MICs of gatifloxacin for S. pneumoniae and S. aureus (0.5 and 0.25 μg/ml, respectively) plus 10 μl of bacterial inoculum as drug control; (iii) 90 μl of uncentrifuged (complete) SBF plus 10 μl of bacterial inoculum as a time-kill curve with leukocytes; and (iv) 90 μl of centrifuged SBF plus 10 μl of bacterial inoculum as a time-kill curve without leukocytes. These four mixtures were incubated at a 35° angle on a rotator (250 rpm) at 37°C. Before and 2 and 6 h after inoculation, tubes were vortexed, and 10-μl aliquots were sampled for quantitative culture after appropriate dilution in sterile water. Mean values and standard deviations (SD) are given for the demographic and pharmacokinetic data. The killing of test strains in SBF was compared by the Wilcoxon signed-rank test. P values of <0.05 were considered statistically significant.
The MIC and MBC of gatifloxacin for S. pneumoniae were 0.25 and 0.5 μg/ml, and the MIC and MBC for S. aureus were 0.125 and 0.125 μg/ml, respectively. Twenty healthy subjects ranging in age from 21 to 50 years were enrolled (mean ± SD, 36.4 ± 8.7 years). Their weights ranged from 54 to 90 kg (mean ± SD, 73.3 ± 10.2 kg), and their heights ranged from 161 to 188 cm (mean ± SD, 172 ± 9.8 cm). The volume per inflammatory blister ranged from 90 to 250 μl. The median number of leukocytes counted in SBF increased during the experiment from 2.0 × 106/ml (range, 0.5 × 106 to 17.0 × 106/ml) before drug intake up to 11.6 × 106/ml (range, 2.3 × 106 to 75.0 × 106/ml) 5 h after drug intake. More than 95% of the cells were polymorphonuclear leukocytes (PMN). The final cell concentration incubated ex vivo in the phagocytic bactericidal assay was 90% of that measured in SBF.
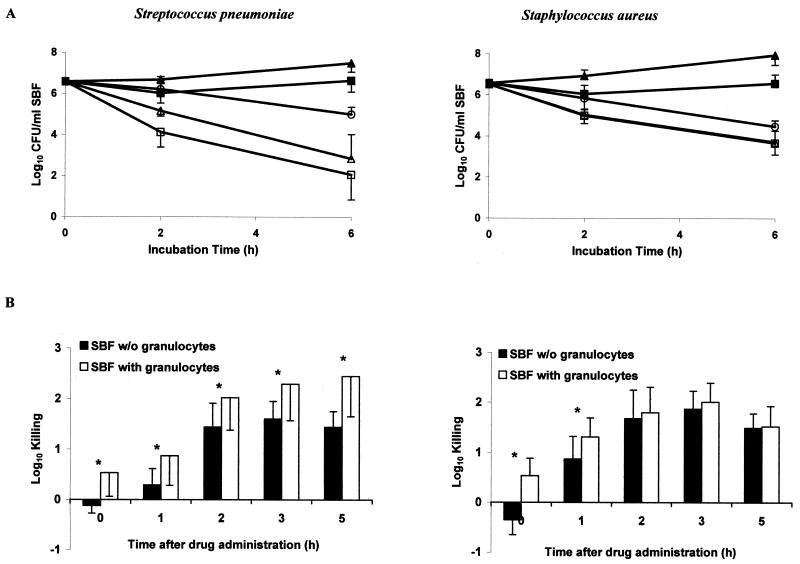
The mean pharmacokinetic parameters of unbound gatifloxacin in serum and in SBF from 20 healthy volunteers after the administration of a single 400-mg oral dose of gatifloxacin are summarized in Table 1. In SBF, only 66% of the mean maximum concentration of drug in serum (Cmax) was reached; however, the drug penetration into the SBF was 107% ± 19%. The mean elimination half-life (t1/2) was slightly longer in serum than in SBF (9.4 versus 7.8 h). The difference did not reach statistical significance. Figure 1A shows time-kill studies of the test strains before and 5 h after intake of a 400-mg tablet of gatifloxacin. Each point corresponds to the mean value of all 10 volunteers in each study group. Incubation of bacteria with centrifuged SBF samples (containing no leukocytes), obtained before drug administration resulted in growth, whereas in uncentrifuged SBF bacterial counts remained stable. Medium containing twice the MIC of gatifloxacin showed 0.4- and 1.6-log killing of S. pneumoniae after 2 and 6 h of incubation, respectively, and 0.7- and 2.1-log killing of S. aureus after 2 and 6 h of incubation, respectively. Centrifuged SBF drawn 5 h after drug administration showed 1.4- and 3.8-log killing of S. pneumoniae and 1.5- and 2.8-log killing of S. aureus after 2 and 6 h of incubation, respectively. Killing of S. pneumoniae but not of S. aureus was significantly improved in the presence of PMN (P < 0.05). Figure 1B shows the 2-h killing of test strains by uncentrifuged and centrifuged SBF harvested at different sampling time points after drug administration. It indicates the respective role of the antimicrobial drug and the PMN in SBF. The killing of S. pneumoniae by gatifloxacin was significantly better in the presence of PMN at each time-point (P < 0.05). In contrast, the killing of S. aureus by gatifloxacin was improved in the presence of PMN only at early time points.
TABLE 1.
Pharmacokinetic parameters of gatifloxacin in serum and SBF samples in 20 healthy volunteers following administration of a single 400-mg oral dose of gatifloxacina
| Sample type | Cmax (μg/ml)b | Tmax (h)c | t1/2 (h)d | AUC0-24 (μg · h/ml) | AUC0-∞ (μg · h/ml) | Lag time (h) | CL/F (ml/min)e | Varea/F (liter)f |
|---|---|---|---|---|---|---|---|---|
| Serum | 3.97 ± 1.25 | 1.30 ± 0.50 | 9.41 ± 1.85 | 29.53 ± 8.50 | 35.46 ± 9.62 | 0.37 ± 0.21 | 194.2 ± 51.7 | 141.3 ± 46.4 |
| SBF | 2.63 ± 0.83 | 4.29 ± 2.06 | 7.84 ± 2.46 | 31.69 ± 9.05 | 37.93 ± 10.93 | 0.80 ± 0.36 | NAg | NAg |
Values are means ± SD.
Cmax, peak concentration of the drug.
Tmax, time to reach peak concentration of the drug.
t1/2, elimination half-life.
CL/F, apparent oral clearance.
Varea/F, apparent volume of distribution.
NA, not applicable.
FIG. 1.
(A) Median time-kill curves of S. pneumoniae and S. aureus following incubation with centrifuged (▴) and uncentrifuged SBF (▪) sampled before drug administration and following incubation with centrifuged (▵) and uncentrifuged SBF (□) sampled 5 h after drug intake. In addition, kill curves with medium containing twice the MIC of gatifloxacin (○) are shown. Error bars indicate SD. (B) Killing of S. pneumoniae and S. aureus 2 h after incubation with centrifuged (solid bars) and uncentrifuged (open bars) SBF. Asterisks indicate statistically significant differences (P < 0.05). Error bars indicate SD.
Our pharmacokinetic results were in good agreement with those of previous studies (10, 11, 15, 20). The MICs at which 90% of the isolates tested are inhibited (MIC90s) of gatifloxacin for S. pneumoniae (0.5 μg/ml) (6) and methicillin-susceptible S. aureus (0.1 to 0.5 μg/ml) (1) are in the range of those for our test strains. AUC0-24/MIC ratios of 50 to 125 have been reported to predict a good outcome with quinolones in animal models and in humans (3). The AUC0-24/MIC ratios in SBF were 127 for S. pneumoniae and 254 for S. aureus. The Cmax/MIC ratios were 10.5 for S. pneumoniae and 21.0 for S. aureus.
Skin blisters induced by cantharidin provoke an exudate. The percent penetration of gatifloxacin into this inflammatory fluid was 107% ± 19%. This is similar to those of ciprofloxacin (103%) (2), temafloxacin (105%) (13), sparfloxacin (117%) (8), and levofloxacin (124%) (19). The fraction of the drug available for antimicrobial killing is determined by the drug concentration and protein binding at the site of infection. SBF contains two-thirds of the serum protein level, with identical distributions of the different types of proteins (22). Gatifloxacin is only moderately bound by serum proteins (20%) (4). With the microbiological assay, which is based on the diffusion principle, only the free drug fraction is measured. Thus, in our experiments, only the microbiologically active drug fraction is measured.
In conclusion, we observed a potent bactericidal effect of gatifloxacin against the test strains investigated and a complete equilibration of the drug between serum and artificial inflammatory exudate. The killing of S. pneumoniae by gatifloxacin was significantly better in PMN-containing SBF samples than in centrifuged SBF samples without PMN. In contrast, the additive effect of PMN with gatifloxacin in the killing of S. aureus was observed only at the first sampling time point (1 h), when the phagocytic bactericidal activity was more important than antibiotic activity.
(These data were presented at the 41th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 16 to 19 December 2001.)
Acknowledgments
We thank Grünenthal GmbH (Aachen, Germany) for their educational grant.
REFERENCES
- 1.Blondeau, J. M. 1999. A review of the comparative in-vitro activities of 12 antimicrobial agents, with a focus on five new respiratory quinolones. J. Antimicrob. Chemother. 43(Suppl. B):1-11. [DOI] [PubMed] [Google Scholar]
- 2.Catchpole, C., J. M. Andrews, J. Woodcock, and R. Wise. 1994. The comparative pharmacokinetics and tissue penetration of single-dose ciprofloxacin 400 mg i.v. and 750 mg p.o. J. Antimicrob. Chemother. 33:103-110. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 4.Gajjar, D. A., F. P. LaCreta, H. D. Uderman, G. D. Kollia, G. Duncan, M. J. Birkhofer, and D. M. Grasela. 2000. A dose-escalation study of the safety, tolerability, and pharmacokinetics of intravenous gatifloxacin in healthy adult men. Pharmacotherapy 20:49S-58S. [DOI] [PubMed] [Google Scholar]
- 5.Grasela, D. M.2000. Clinical pharmacology of gatifloxacin, a new fluoroquinolone. Clin. Infect. Dis. 31(Suppl. 2):S51-S58. [DOI] [PubMed] [Google Scholar]
- 6.Hoellman, D. B., G. Lin, M. R. Jacobs, and P. C. Appelbaum. 1999. Anti-pneumococcal activity of gatifloxacin compared with other quinolone and non-quinolone agents. J. Antimicrob. Chemother. 43:645-649. [DOI] [PubMed] [Google Scholar]
- 7.Hoogkamer, J. F. W., W. H. Hesse, S. Sansano, and W. Zimmerli. 1993. Pharmacodynamic activity of a cephalosporin, Ro 40-6890, in human skin blister fluid: antibiotic activity in concert with host defense mechanisms. Antimicrob. Agents Chemother. 37:2622-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson, J. H., M. A. Cooper, J. M. Andrews, and R. Wise. 1992. Pharmacokinetics and inflammatory fluid penetration of sparfloxacin. Antimicrob. Agents Chemother. 36:2444-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawakami, K., K. Namba, M. Tanaka, N. Matsuhashi, K. Sato, and M. Takemura. 2000. Antimycobacterial activities of novel levofloxacin analogues. Antimicrob. Agents Chemother. 44:2126-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lober, S., S. Ziege, M. Rau, G. Schreiber, A. Mignot, P. Koeppe, and H. Lode. 1999. Pharmacokinetics of gatifloxacin and interaction with an antacid containing aluminum and magnesium. Antimicrob. Agents Chemother. 43:1067-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naber, C. K., M. Steghafner, M. Kinzig-Schippers, C. Sauber, F. Sörgel, H.-J. Stahlberg, and K. G. Naber. 2001. Concentrations of gatifloxacin in plasma and urine and penetration into prostatic and seminal fluid, ejaculate, and sperm cells after single oral administrations of 400 milligrams to volunteers. Antimicrob. Agents Chemother. 45:293-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard. NCCLS document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Nye, K., Y. G. Shi, J. M. Andrews, J. P. Ashby, and R. Wise. 1989. The in-vitro activity, pharmacokinetics and tissue penetration of temafloxacin. J. Antimicrob. Chemother. 24:415-424. [DOI] [PubMed] [Google Scholar]
- 14.Pendland, S. L., K. J. Losnedahl, and C. A. Schriever. 1999. In-vitro activity of gatifloxacin, a novel fluoroquinolone, compared with that of ciprofloxacin against Legionella spp. J. Antimicrob. Chemother. 44:295-297. [DOI] [PubMed] [Google Scholar]
- 15.Perry, C. M., J. A. Barman Balfour, and H. M. Lamb. 1999. Gatifloxacin. Drugs 58:683-696. [DOI] [PubMed] [Google Scholar]
- 16.Roblin, P. M., and M. R. Hammerschlag. 1999. In-vitro activity of gatifloxacin against Chlamydia trachomatis and Chlamydia pneumoniae. J. Antimicrob. Chemother. 44:549-551. [DOI] [PubMed] [Google Scholar]
- 17.Schaumann, R., G. Ackermann, B. Pless, M. C. Claros, and A. C. Rodloff. 1999. In vitro activities of gatifloxacin, two other quinolones, and five nonquinolone antimicrobials against obligately anaerobic bacteria. Antimicrob. Agents Chemother. 43:2783-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanswell, P., and J. Koup. 1993. TopFit: a PC-based pharmacokinetic/pharmacodynamic data analysis program. Int. J. Clin. Pharmacol. Ther. Toxicol. 31:514-520. [PubMed] [Google Scholar]
- 19.Trampuz, A., M. Wenk, Z. Rajacic, and W. Zimmerli. 2000. Pharmacokinetics and pharmacodynamics of levofloxacin against Streptococcus pneumoniae and Staphylococcus aureus in human skin blister fluid. Antimicrob. Agents Chemother. 44:1352-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wise, R., J. M. Andrews, J. P. Ashby, and J. Marshall. 1999. A study to determine the pharmacokinetics and inflammatory fluid penetration of gatifloxacin following a single oral dose. J. Antimicrob. Chemother. 44:701-704. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto, T., H. Kusajima, M. Hosaka, H. Fukuda, Y. Oomori, and H. Shinoda. 1996. Uptake and intracellular activity of AM-1155 in phagocytic cells. Antimicrob. Agents Chemother. 40:2756-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmerli, W., S. Sansano, and B. Wittke. 1996. Pharmacokinetics of cefetamet in plasma and skin blister fluid. Antimicrob. Agents Chemother. 40:102-104. [DOI] [PMC free article] [PubMed] [Google Scholar]