Abstract
Neutrophils are effectors of the innate immune response to adenovirus vectors. Following the systemic administration of Cy2-labeled AdLuc in mice, flow cytometry and PCR analysis of liver leukocytes revealed that 25% of recruited neutrophils interacted with adenovirus vectors. In vitro, flow cytometry of human neutrophils incubated with Cy2-labeled AdLuc also demonstrated a significant interaction with adenovirus vectors. Fluorescence and electron microscopy confirmed vector internalization by neutrophils. The AdLuc-neutrophil interaction reduced vector transduction efficiency by more than 50% in coincubation assays in epithelium-derived cells. Adenovirus vector uptake by neutrophils occurred independently of coxsackievirus adenovirus receptor (CAR) and capsid RGD motifs, since neutrophils do not express CAR and uptake of the RGD-deleted vector AdL.PB* was similar to that of AdLuc. Furthermore, both AdLuc and AdL.PB* activated neutrophils and induced similar degrees of L-selectin shedding. Neutrophil uptake of AdLuc was dependent on the presence of complement and antibodies, since the interaction between AdLuc and neutrophils was significantly reduced when they were incubated in immunoglobulin G-depleted or heat-inactivated human serum. Blocking of complement receptor 1 (CD35) but not complement receptor 3 (CD11b/CD18) significantly reduced neutrophil uptake of AdLuc. Blocking of FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16) individually or together also reduced neutrophil uptake of AdLuc, although less than blocking of CD35 alone. Combined CR1 and Fc receptor blockade synergistically inhibited neutrophil-AdLuc interactions close to baseline. These results demonstrate opsonin-dependent adenovirus vector interactions with neutrophils and their corresponding receptors.
The use of adenovirus vectors (Ad vectors) for gene therapy is a promising strategy to treat numerous medical conditions. Ad vectors have several useful characteristics, such as their ease to produce in the laboratory, their ability to carry large amounts of DNA, and broad cell tropism (30). Unfortunately, the clinical application of adenovirus-mediated gene therapy is hampered by the immune response against these vectors (31). While much progress has been made in understanding and limiting the adaptive immune response against Ad vectors, the preceding innate immune response remains a significant barrier to the safe and effective application of these agents in humans (15).
To study the innate immune response to Ad vectors, we have developed a model of acute liver inflammation in mice (24). We have previously shown that the intravenous administration of Ad vectors results in acute liver inflammation and injury that is mediated in large part by the rapid recruitment of neutrophils (24). Although these studies confirm that neutrophils are a major effector of the innate immune response to Ad vectors, a direct interaction has not been shown. Our studies and others examining the innate immune response in vivo demonstrate little difference in the inflammatory potential of coxsackievirus adenovirus receptor (CAR) and integrin-ablated tropism-modified Ad vectors that represent the traditional paradigm of adenovirus-cellular interactions (1, 20, 22). The finding of RGD-dependent cellular interactions uncovered in the absence of liver resident macrophages (Kupffer cells) suggests that adenovirus-leukocyte interactions occur independent of the traditional paradigm involving CAR and integrins (20). Recently, several studies have demonstrated opsonization of Ad vectors in vivo that play a significant role in vector tropism (29, 37). The adenovirus capsid binds to and activates proteins in the classical and alternative complement pathways, including C3 and C4BP (5, 14, 29). In addition, since the majority of people are immunized against the common adenovirus serotypes, antibody-dependent cellular interactions will play a significant role in viral tropism and the host response to Ad vectors in humans (23, 33). In this regard, antibody-Fc receptor binding substantially enhances Ad vector-dendritic cell interactions (23).
Within minutes following infection or injury, neutrophils begin the process of exiting the systemic circulation and migrating into the site of inflammation (4, 27). While in the tissue, activated neutrophils engulf pathogens, foreign material, and damaged host cells in order to remove the offending stimuli, promote wound healing, and resume homeostasis (35). Much of the neutrophil interaction with foreign microbes is facilitated by the recognition of opsonized material. Neutrophils, via the complement receptors CR1 (CD35), CR3 (CD11b/CD18, αMβ2), and CR4 (CD11c/CD18, αXβ2), recognize antigen-bound complement components such as C3B or C4B (12, 17). Neutrophils also express Fc receptors FcγRIIA (CD32) and FcγRIIIB (CD16), and can be induced to express FcγRI (CD64), to recognize antigen-antibody complexes, especially in immunized hosts (11, 17). The known biology of neutrophil function suggests that these phagocytes might interact with Ad vectors in an opsonin-dependent manner.
In this study, we characterized the interaction between Ad vectors and neutrophils. We show for the first time that neutrophils internalize Ad vectors in a complement- and immunoglobulin G (IgG)-dependent manner. Furthermore, we show specifically that Ad vector-neutrophil interactions occur via the complement receptor CR1 and the Fc receptors FcγRI, FcγRII, and FcγRIII. These results shed new light on the relationship between Ad vectors and the innate immune system and introduce a novel paradigm regarding adenovirus-leukocyte interactions.
MATERIALS AND METHODS
Antibodies.
The following antibodies were used as described below: phycoerythrin (PE)-anti-Ly-6G (rat IgG2a, clone 1A8; BD Biosciences), anti-CD11b (αM integrin, mouse IgG1, clone ICRF44; Calbiochem), anti-CD18 (β2 integrin, mouse IgG2a, clone IB4; Calbiochem), anti-CD35 (CR1, mouse IgG1, clone J3D3; Beckman Coulter), anti-CD16 (FcγRIII, mouse IgG1, clone 3G8; BD Biosciences); PE-anti-CD62L (L-selectin, mouse IgG2a, clone SK11; BD Biosciences), anti-CD32 (FcγRII, mouse IgG1, clone AT10; Serotec), PE-rat IgG2a isotype control (BD Biosciences), mouse IgG2a isotype control (Chemicon), and mouse IgG1 and PE-mouse IgG2a isotype controls (Cedarlane, Hornby, Ontario, Canada).
Adenovirus vectors.
Type 5, E1-deleted, E3-defective Ad vectors expressing the luciferase transgene under the control of the cytomegalovirus promoter, with either wild-type capsid (AdLuc) or RGD-deleted capsid (AdL.PB*) (32), were amplified in human embryonic kidney 293 cells and purified on cesium chloride gradients as previously described (2). Vectors were dialyzed against sucrose vehicle buffer (10 mM Tris [pH 7.4], 2 mM MgCl2, 150 mM NaCl, and 3% sucrose in sterile H2O). Ad vectors were labeled with a green fluorescent cyanine dye (Cy2), using an antibody labeling kit (FluoroLink; Amersham Biosciences) as previously described (18). Labeled vectors were tested in transduction assays in vitro and compared to unlabeled vectors to ensure that the labeling procedure did not affect vector characteristics. Adenovirus particle titer was determined by measuring the optical density at 260 nm and expressed as particles per cell. Vectors were screened for replication-competent adenovirus by plaque assay on HeLa cells and PCR and remained at consistently less than 1:1010 particles. Low-endotoxin H2O, buffers, and tissue culture reagents were used for vector production and experiments. Ad vectors were routinely tested for the presence of endotoxin by using the Limulus amebocyte lysate assay kit (BioWhittaker Molecular Applications). All vectors contained less than 0.1 endotoxin unit/ml.
Liver leukocyte isolation.
Male C57BL/6 mice, weighing 25 to 30 g, were housed under single-barrier conditions (Charles River). Animal studies were performed in accordance with the Animal Care Committee guidelines at the University of Calgary. Mice were injected via the femoral vein with either saline-sucrose vehicle control (50:50 sucrose vehicle/0.9% saline infusion) or Cy2-AdLuc (2.5 × 1011 particles) in a 100-μl total volume. At 3 h, mice were completely anesthetized using intraperitoneal ketamine hydrochloride and xylazine hydrochloride. The carotid artery was cannulated and the circulation flushed with 20 ml of heparinized saline. The liver was then quickly excised, rinsed in fresh saline, and minced in a petri dish containing 10 ml digestive medium (0.002% DNase I, grade I from bovine pancreas; Roche) and collagenase (0.05%, type II from Clostridium histolyticum; Sigma) in Hanks' balanced salt solution without calcium or magnesium. The tissue suspension was incubated at 37°C for 30 min and then passed through a 40-μm-pore cell strainer and washed with fresh sterile phosphate-buffered saline (PBS) without calcium or magnesium (pH 7.4). The cell suspension was centrifuged for 5 min at 300 × g and 20°C. After removal of the supernatant, residual red blood cells were removed by hypotonic lysis with cold 0.2% and 1.6% NaCl solutions. The cell suspension was again centrifuged for 5 min at 300 × g and 20°C. The cell pellet was then resuspended in 10 ml 35% Percoll (Sigma) and centrifuged for 10 min at 360 × g and 20°C. After removal of the supernatant, the leukocyte pellet was washed in 5 ml PBS, resuspended in 1 ml PBS, and analyzed as described below.
Human neutrophil isolation.
Blood was drawn from healthy volunteers by standard venipuncture and immediately mixed with heparin (10 units/ml). The heparinized blood was then mixed with a half volume of dextran (6% in saline), and erythrocytes were allowed to sediment for 60 min at room temperature. Residual erythrocytes were removed by hypotonic lysis with cold 0.2% and 1.6% NaCl solutions, and granulocytes were isolated by density gradient centrifugation on Lymphoprep 1077 (Axis-Shield, Oslo, Norway). Final cell suspensions were typically >95% neutrophils. For all experiments, isolated neutrophils were resuspended in Hanks' balanced salt solution (with calcium and magnesium) at a final concentration of 2 × 106 cells/ml. Unless otherwise specified, neutrophil-Ad vector incubations were performed in 20% human serum.
Human serum.
Serum was collected from 10 healthy donors, pooled, and filtered through a sterile 0.45-μm mesh; this is referred to as human serum or complete human serum. For selected experiments, human serum was pretreated as follows. For depletion of gamma globulin, serum aliquots were treated with an equal volume of protein G-coated Sepharose beads (GE Healthcare) as previously described (6). For total inhibition of the complement system, serum was heat inactivated for 30 min at 56°C as described previously (16, 28). In order to inhibit the classical complement pathway, serum was treated with 10−2 M EGTA and 10−2 M MgCl2 as previously described (5, 8, 28). For inhibition of the alternative complement pathway, serum was partially heat inactivated for 15 min at 50°C as described previously (13, 28).
Flow cytometry.
Flow cytometric analysis of isolated liver leukocytes and human neutrophils was carried out using FACScan instrumentation and CellQuest Pro software (BD Biosciences). To determine the differential proportions of liver leukocyte populations and neutrophil interactions with fluorescent Ad vectors, leukocytes were fixed in 2% formalin in PBS prior to analysis. For L-selectin and Ly-6G expression, leukocytes were incubated with 10 μg/ml PE-anti-CD62L, PE-anti-Ly-6G, or an isotype control for 30 min at 4°C. Cells were washed free of unbound antibody and fixed in 2% formalin in PBS before flow cytometric analysis.
Electron and fluorescence microscopy.
Freshly isolated neutrophils were incubated with Ad vectors for 20 min at 37°C as described above. Cells were then fixed with 2% formalin in PBS and pelleted for transmission electron microscopy. The pellets were postfixed with 2.5% glutaraldehyde in PBS for 2 h, rinsed twice in PBS for 30 min, treated with 1% OsO4 for 1 h, dehydrated in a graded ethanol series, and embedded in Spurr's resin. Cells were then stained with uranyl acetate and lead citrate before being cut into ultrathin sections (60 to 80 nm) with a diamond knife and viewed using a Hitachi H-7000 transmission electron microscope. Alternatively, cells were prepared for fluorescence microscopy as follows. Cells were incubated with fluorescent Cy2-AdLuc particles for 60 min as described above, followed by washing and fixation with 2% formalin in PBS. Cell nuclei were stained with 4′,6-diamidino-2-phenyindole (DAPI) (Sigma-Aldrich) at a final concentration of 2 μg/ml for 15 min at room temperature. Cells were then washed in PBS and transferred to a glass slide by using a Shandon cytospin apparatus (Histotronix, Atlantic, IA). Cells were then visualized using an Olympus IX70 inverted epifluorescence microscope. Excitation/emission filter cubes of 490/528 nm and 360/457 nm were used to detect virus particles and cell nuclei, respectively.
DNA isolation and PCR.
To obtain total DNA from isolated leukocytes, cells were lysed in 400 μl lysis buffer (1% sodium dodecyl sulfate, 50 mM Tris-HCl [pH 8], 100 mM EDTA, 200 μg/ml proteinase K) and incubated at 56°C for 2 h. The protein content was precipitated by addition of 125 μl saturated (5 M) NaCl, followed by DNA precipitation from the supernatant by addition of ice-cold isopropanol. The DNA pellet was resuspended in 100 μl water and extracted with phenol-chloroform-isoamyl alcohol (25:24:1). DNA was precipitated from the aqueous layer using 0.25 M sodium acetate (pH 5.2) and 100% ethanol. After a final wash in ice-cold 70% ethanol, DNA was resuspended in water.
For PCR, 200 ng of total DNA was added to a reaction mix containing (final concentrations) 500 nM of each primer, 0.1 U of Taq polymerase (QIAGEN), 200 μM of each deoxynucleoside triphosphate, and 1× reaction buffer in a final volume of 50 μl. Primer sequences for the adenovirus 5 fiber gene were 5′-CGCCGCACCTCTAATGGTCG-3′ (sense) and 5′-CCTGGACCAGTTGCTACGGTC-3′ (antisense), with an expected amplification product of 332 bp. Primers directed against GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were used as a positive control. The reactions were performed with denaturing at 95°C (45 s), annealing at 55°C (45 s), and extension at 72°C (1 min) for a total of 20 cycles.
Luciferase transduction assays.
To determine neutrophil transduction, cells were incubated with AdLuc (1 × 104 particles/cell) in human serum for 60 min, washed, and incubated in RPMI medium for 6 and 24 h at 37°C. For luciferase activity, cells were lysed with 400 μl reporter lysis buffer (Promega). The cell lysate was then briefly vortexed and centrifuged at 16,000 × g for 10 min at 4°C. Ten microliters of supernatant was added to 50 μl of luciferase assay reagent (Promega) and luciferase activity measured using a Monolight 3010 luminometer (BD Biosciences).
To determine neutrophil effects on AdLuc transduction of target cells, AdLuc (1 × 104 particles/cell) was added to 1-ml neutrophil suspensions in the presence of human serum and incubated for 60 min at 37°C. Control tubes contained equivalent amounts of adenovirus in human serum or fetal bovine serum, but without neutrophils. Samples were then centrifuged at 300 × g for 5 min, and 50 μl of the supernatant plus 2 ml fresh cell culture medium (Dulbecco's modified Eagle's medium plus 1% fetal bovine serum and 1% penicillin/streptomycin) was then placed in 60- by 15-mm tissue culture plates containing 90% confluent murine epithelium-derived (REC) cells (∼1.5 × 106 cells) (24) and incubated overnight at 37°C before luciferase activity was measured. For coincubation studies, 1.5 × 106 REC cells were transduced with AdLuc (1 × 104 particles/cell) in the presence of human serum and neutrophils in increasing ratios to REC cells (25:1, 5:1, and 1:1). The coincubation was allowed to proceed for 60 min, and then the REC cells were washed and incubated in fresh medium (Dulbecco's modified Eagle's medium plus 1% fetal bovine serum and 1% penicillin/streptomycin) for 6 h before luciferase activity was measured.
Neutrophil receptor blockade.
For neutrophil blocking experiments, freshly isolated neutrophils were pretreated with 30 μg/ml of anti-CD11b, anti-CD18, anti-CD35, anti-CD16, anti-CD32, mouse IgG2a, and/or mouse IgG1 for 10 min at 37°C. Cells were then incubated with fluorescent Ad vectors and analyzed as described above.
Statistics.
Statistical analyses were performed using GraphPad Instat version 3.01. Results were analyzed for statistical variance using an unpaired Student t test or one-way analysis of variance followed by pairwise comparison using either a Tukey HSD test or a Dunett's multiple-comparison test as appropriate. Results were considered significant when the P value was <0.05.
RESULTS
Neutrophils interact with adenovirus vectors.
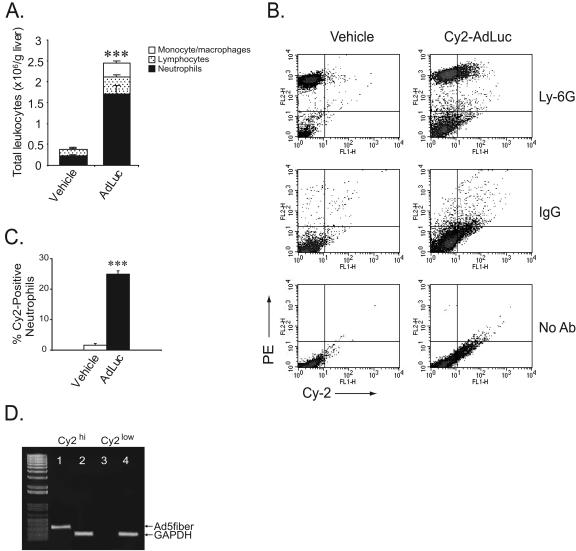
Ad vectors activate the innate immune system and induce the rapid recruitment of neutrophils to the liver following intravenous administration (19, 24). To determine if neutrophils could directly interact with Ad vectors in vivo, mice were administered 2.5 × 1011 particles of the Cy2-labeled first-generation Ad vector AdLuc (Cy2-AdLuc) intravenously, and leukocytes were isolated from the inflamed livers at 3 h. The differential proportion of leukocyte subsets was first assessed by flow cytometry analysis of light scatter properties characteristic of neutrophils, lymphocytes, and monocytes/macrophages. At 3 h, livers from adenovirus-treated mice contained fivefold more leukocytes than those of vehicle-treated mice (Fig. 1A). Neutrophils were the predominant leukocyte cell type, representing approximately 70% of total liver leukocytes, while the remaining 30% of the leukocytes recruited to the liver were comprised of lymphocytes and monocytes/macrophages. Differential analysis by flow cytometry was corroborated with microscopic analysis of cytospin preparations.
FIG. 1.
Neutrophil-adenovirus interaction in vivo. (A) Total liver leukocytes at 3 h following systemic administration of AdLuc or vehicle control. Each bar is subdivided to show the differential recruitment of leukocyte subsets. For neutrophils with AdLuc versus vehicle control, P < 0.005 (***); n = 6 to 14. Error bars indicate standard deviations. (B) Flow cytometry analysis of liver leukocytes from mice 3 h following intravenous administration of Cy2-AdLuc or vehicle control. Leukocytes were analyzed alone (lower panels) or stained with PE-conjugated isotype control rat IgG2a (middle panels) or anti-Ly-6G antibody (upper panels). The fluorescence histogram shows a subpopulation of Cy2-high, Ly-6G-positive liver leukocytes in Cy2-AdLuc-treated mice. (C) Percentage of Cy2-high cells within the Ly-6G-positive neutrophil population. For AdLuc versus vehicle control, P < 0.005 (***); n = 6. (D) PCR analysis of viral DNA in Cy2-high and Cy-2-low liver leukocyte fractions. Lane 1, Cy2-high, Ad5 fiber. Lane 2, Cy2-high, GAPDH. Lane 3, Cy2-low, Ad5 fiber. Lane 4, Cy2-low, GAPDH.
Next, liver-recruited leukocytes were analyzed by flow cytometry for Cy2 fluorescence. Following the administration of Cy2-AdLuc, isolated leukocytes contained a subpopulation of Cy2-high cells (Fig. 1B). Further analysis showed that these fluorescent cells had light scatter properties consistent with neutrophils. To confirm that the Cy2-positive leukocytes were neutrophils, dual-fluorescence flow cytometry was performed using PE-conjugated neutrophil-specific anti-Ly-6G or isotype control antibodies (Fig. 1B). The majority of Cy2-high cells (>80%) also stained positive for Ly-6G, confirming that this cell population was primarily neutrophils. Furthermore, Cy2-high leukocytes represented 25% of the total number of neutrophils recruited to the liver during the innate immune response to Ad vectors (Fig. 1C). To confirm that neutrophils were directly interacting with adenovirus particles and to eliminate the possibility that these observations were due to nonspecific neutrophil labeling, we performed PCR analysis for the adenovirus fiber gene on total DNA from Cy2-high and Cy2-low sorted leukocytes. Cy2-high leukocytes were strongly positive for the adenovirus fiber gene compared to Cy2-low cells, which contained very low levels of adenovirus DNA (Fig. 1D). These results show that neutrophils recruited to the liver during the innate immune response take up Ad vectors in vivo.
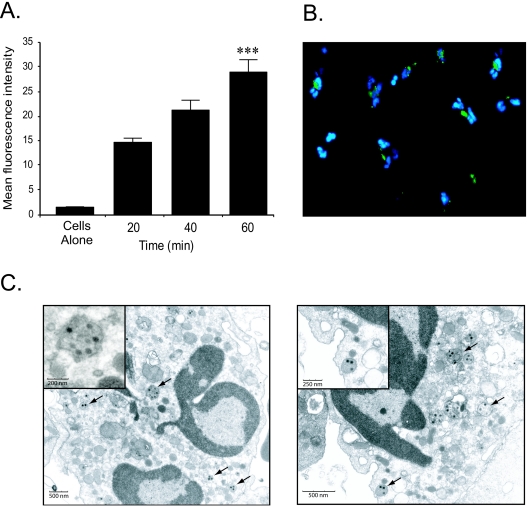
To assess the mechanism of neutrophil-Ad vector interaction, in vitro studies were next performed. Purified human neutrophils were incubated with 1 × 104 particles/cell of Cy2-AdLuc in the presence of human serum. Cells were analyzed by flow cytometry at 20, 40, and 60 min. Compared to cells alone, neutrophil fluorescence increased significantly at all time points over the 60-min experimental period (Fig. 2A). These results suggested that neutrophils could internalize adenovirus particles. To confirm that the increased neutrophil fluorescence was not due to extracellular binding of Cy2- AdLuc, external fluorescence was quenched using trypan blue. There was no significant reduction in neutrophil fluorescence as determined by flow cytometry, consistent with vector internalization (data not shown). To further ensure that Ad vectors were internalized, fluorescence microscopy and electron microscopy were performed. At 60 min following the incubation with Cy2-AdLuc, microscopy revealed focal cellular Cy2 fluorescence around nuclei in more than 50% of neutrophils, consistent with internalized vector (Fig. 2B). Finally, neutrophils were incubated with unlabeled AdLuc and analyzed by electron microscopy at 20 min. Adenovirus particles were clearly identified within phagosomes, confirming vector internalization (Fig. 2C). Furthermore, the uptake of unlabeled AdLuc rules out the Cy2 labeling procedure as a confounding variable in neutrophil-adenovirus interactions.
FIG. 2.
Neutrophil-adenovirus interaction in vitro. (A) Neutrophils were incubated with Cy2-AdLuc vectors for 20 to 60 min and mean neutrophil fluorescence analyzed by flow cytometry. For 60 min versus cells alone, P < 0.005 (***); n = 8 to 10. Error bars indicate standard deviations. (B) Fluorescence microscopy of neutrophils incubated with Cy2-AdLuc at 60 min (Cy2, green; DAPI, blue). Magnification, ×60. (C) Electron microscopy of neutrophils incubated with AdLuc at 20 min.
Neutrophils reduce adenovirus vector transduction of target cells.
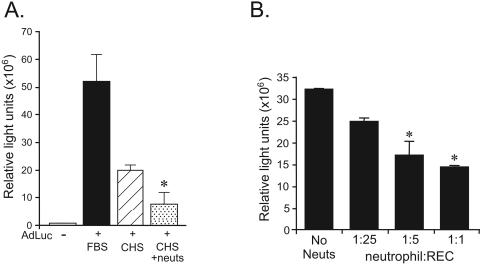
To assess the impact of neutrophil-adenovirus interaction, cellular transduction experiments were performed. First, to determine if neutrophils were transduced by internalized Ad vectors, cells were incubated with AdLuc (1 × 104 particles/cell) and analyzed for luciferase transgene expression. At 6 and 24 h, neutrophils expressed no luciferase, similar to the case for control cells, demonstrating that neutrophils internalize but are not transduced by an Ad vector carrying a cytomegalovirus-regulated transgene (data not shown). Next, we investigated the ability of neutrophils to interfere with Ad vector transduction of target cells. AdLuc (1 × 104 particles/cell) was incubated in human serum in the presence of purified neutrophils. At 60 min, cells were centrifuged and the supernatant was applied to epithelium-derived REC cells (24) as described in Materials and Methods. AdLuc incubated in human serum or fetal bovine serum in the absence of neutrophils was used in control REC transductions. At 24 h, luciferase activity was determined. Compared to cells transduced with AdLuc in fetal bovine serum, the presence of complete human serum reduced luciferase activity by 70%, consistent with the presence of neutralizing adenovirus antibodies and the prevalence of antiadenoviral immunity in the general population. The preincubation of AdLuc in human serum with neutrophils reduced REC cell transduction and luciferase activity by an additional 50% compared to AdLuc incubated in human serum alone (Fig. 3A). To investigate further the impact of neutrophil-Ad vector interactions on vector bioavailability, a coincubation study was performed. REC cells (1.5 × 106 cells) were coincubated with an increasing number of neutrophils (neutrophil/REC ratios of 25:1, 5:1, and 1:1) and transduced with AdLuc (1 × 104 particles/REC cell) for 60 min. REC cells were washed, and luciferase activity was determined at 6 h. Increasing neutrophil/REC coincubation ratios correlated inversely with AdLuc transduction, with a significant reduction in REC cell luciferase activity compared to that of REC cells transduced in the absence of neutrophils (Fig. 3B). These data demonstrate that neutrophil uptake of Ad vectors can affect vector bioavailability and target cell transduction.
FIG. 3.
Effect of neutrophils on AdLuc transduction of target cells. (A) Luciferase activity at 24 h in REC cells transduced with vehicle (−), AdLuc pretreated with fetal bovine serum (FBS), AdLuc pretreated with complete human serum (CHS), or AdLuc pretreated with complete human serum and neutrophils (CHS + neuts). For CHS versus CHS + neuts, P < 0.05 (*); n = 3. (B) Luciferase activity at 6 h following AdLuc transduction in REC cells coincubated with an increasing number of neutrophils (neutrophil/REC ratio, 25:1, 5:1, or 1:1). For no neutrophils versus coincubation ratio of 5:1 or 1:1, P < 0.05; n = 3. Error bars indicate standard deviations.
Adenovirus-neutrophil interactions occur independently of CAR and capsid RGD.
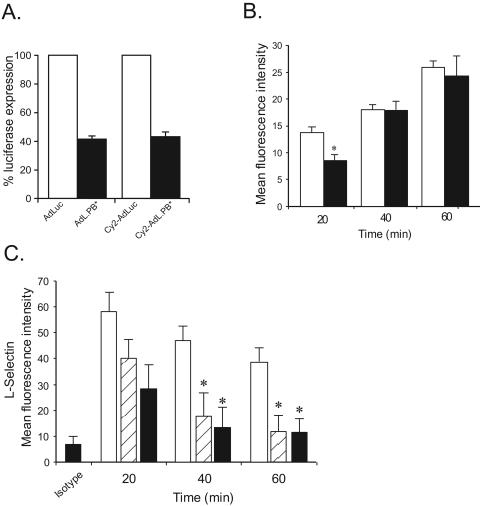
Ad vectors use the CAR and cell surface integrins to bind and internalize in nonhematopoietic cells (22). To determine if adenovirus-neutrophil interactions employed similar mechanisms, studies examining CAR- and RGD (arginine-glycine-aspartate)-dependent internalization were undertaken. CAR is generally not expressed on leukocytes (3, 23) and was also not detectable in neutrophils by reverse transcription-PCR, making it unlikely that Ad vectors used CAR to interact with neutrophils (data not shown). Conversely, the penton base RGD motif mediates adenovirus interaction with dendritic cells (23). To determine the role of capsid RGD motifs, neutrophils were incubated with Cy2-labeled AdL.PB* (1 × 104 particles/cell), a tropism-modified Ad vector that is deleted of its penton RGD motifs (32). The Cy2 labeling procedure did not affect the tropism of AdLuc and AdL.PB*, since REC cells transduced with Cy2-AdL.PB* expressed two- to threefold less luciferase at 6 h than did Cy2-AdLuc, consistent with the transduction differential seen using unlabeled vectors (Fig. 4A). At 20 min, Cy2-AdL.PB* uptake in neutrophils was slightly but significantly lower than Cy2-AdLuc uptake as determined by flow cytometry, suggesting a possible role for the capsid RGD domain in early Ad vector-neutrophil interaction. However, by 40 and 60 min, neutrophil uptake of both vectors was similar, confirming that capsid RGD motifs play a minor role in Ad vector-neutrophil interactions (Fig. 4B).
FIG. 4.
Role of capsid RGD in neutrophil-adenovirus interaction. (A) Transduction efficiency of Cy2-labeled and unlabeled AdL.PB* compared to AdLuc in REC cells. (B) Neutrophils were incubated with Cy2-AdLuc (open bars) or Cy2-AdL.PB* (closed bars) and fluorescence analyzed at 20 to 60 min. For AdLuc versus AdL.PB* at 20 min, P < 0.05 (*); n = 3. (C) Neutrophil L-selectin expression in response to Ad vectors. Neutrophils were incubated with vehicle (open bars), unlabeled AdLuc (hatched bars), or AdL.PB* (closed bars). L-selectin expression was determined by flow cytometry at 20 to 60 min For AdLuc or AdL.PB* versus vehicle, P < 0.05 (*); n = 5. Error bars indicate standard deviations.
Ad vectors induce liver inflammation that is dependent on neutrophil recruitment and the expression of chemokines such as MIP-2 (19, 24). The proteolytic cleavage of L-selectin is a reliable marker of neutrophil activation, which is required for neutrophil migration in response to chemokines expressed at sites of injury and infection (34). To determine if Ad vectors activated neutrophils in a manner consistent with the observed inflammatory response in vivo, L-selectin shedding was determined. Neutrophils were incubated with unlabeled AdLuc, AdL.PB* (1 × 104 particles/cell), or vehicle, and neutrophils were analyzed by flow cytometry for surface L-selectin. At 20, 40, and 60 min, AdLuc induced L-selectin shedding that became significant at 40 min compared to that by vehicle-treated neutrophils. Despite the small reduction in uptake at 20 min, AdL.PB*-neutrophil interactions were still sufficient to induce a degree of L-selectin cleavage similar to that with AdLuc at all time points (Fig. 4C). Taken together, these results show that neutrophils interact with and are activated by Ad vectors. Although a small RGD-dependent effect cannot be ruled out, Ad vector-neutrophil interactions occur largely independent of this capsid domain.
Role of human serum in adenovirus-neutrophil interactions.
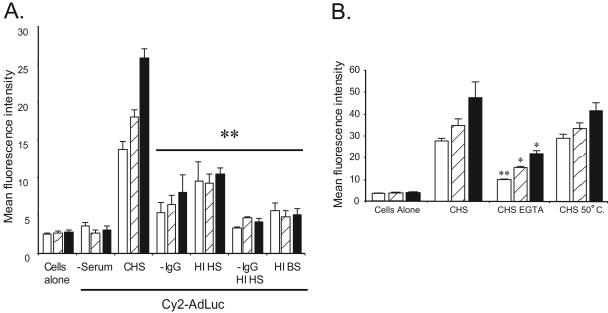
Initial experiments demonstrate that neutrophil-adenovirus interactions occur independent of CAR and capsid RGD motifs. Compared to those incubated in complete human serum, neutrophils incubated with Cy2-AdLuc (1 × 104 particles/cell) in serum-free conditions or with heat-inactivated fetal bovine serum displayed negligible Ad vector uptake over 60 min as determined by flow cytometry (Fig. 5A). These data suggest that serum proteins are required to mediate Ad vector-neutrophil interactions. Recently, a number of studies have demonstrated that the adenovirus capsid binds serum proteins in vivo, including proteins in the complement system (14, 29). Furthermore, the majority of people are immunized against the common adenovirus serotypes, raising the likelihood of antibody-dependent cellular interactions (23). To identify the possible serum factors that underlie adenovirus uptake in neutrophils, experiments were undertaken using human serum that was depleted of IgG, complement inactivated, or both. To deplete IgG, human serum was treated with protein G-Sepharose, which was successful in removing neutralizing IgG and restored cellular transduction in REC cells (data not shown). To inactivate the complement cascade, human serum was heated at 56°C for 30 min. The incubation of Cy2-AdLuc (1 × 104 particles/cell) with neutrophils in the presence of IgG-depleted serum significantly reduced neutrophil fluorescence over 60 min compared to that with complete human serum as determined by flow cytometry. Similarly, incubation of Cy2-AdLuc with heat-inactivated serum also resulted in reduced neutrophil uptake. Incubation of Cy2-AdLuc with human serum that was both heat inactivated and IgG depleted reduced neutrophil fluorescence to baseline, similar to that seen in serum-free conditions (Fig. 5A). These results suggest that adenovirus-neutrophil interactions are likely antibody and complement dependent.
FIG. 5.
Role of serum factors in neutrophil-adenovirus interaction. (A) Cy2-AdLuc was incubated with neutrophils and modified human serum. Cells were analyzed by flow cytometry at 20 (open bars), 40 (hatched bars), and 60 (closed bars) min. −Serum, no serum; CHS, complete human serum; −IgG, IgG-depleted human serum; HI HS, heat-inactivated human serum; −IgG HI HS, IgG-depleted heat-inactivated human serum; HI BS, heat-inactivated bovine serum. For CHS versus relevant time points from subsequent treatment groups, P < 0.01 (**); n = 3 or 4. (B) Cy2-AdLuc was incubated with neutrophils in the presence of EGTA (blocks classical complement pathway) or 50°C heat-inactivated human serum (blocks alternative complement pathway). Cells were analyzed by flow cytometry at 20 (open bars), 40 (hatched bars), and 60 (closed bars) min. For CHS versus corresponding EGTA treatment time points, P < 0.05 (*) or P < 0.01 (**); n = 3 or 4. Error bars indicate standard deviations.
Ad vectors activate both the classical and alternative complement pathways (5). To determine the relative contributions of the two pathways in adenovirus-neutrophil interaction, experiments were performed using human serum in the presence of EGTA (to inactivate the classical complement pathway) or serum heated at 50°C (to inactivate the alternative complement pathway) (5, 13, 28). Neutrophils incubated with Cy2-AdLuc in the presence of EGTA exhibited significantly reduced fluorescence over 60 min compared to the control. In contrast, serum heat inactivated at 50°C only slightly reduced Cy2-AdLuc uptake in neutrophils (Fig. 5B). Consistent with previous reports regarding adenovirus and complement activation, these results suggest that the classical complement pathway primarily mediates Ad vector-neutrophil interactions.
Adenovirus-neutrophil interactions occur via Fc receptors and complement receptor 1.
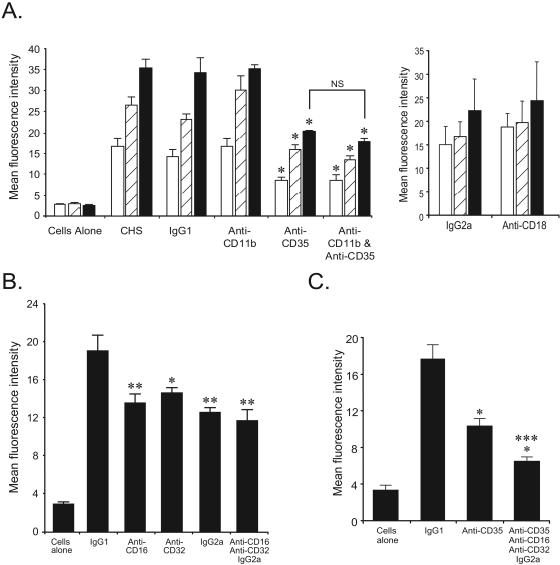
The studies described above demonstrate opsonin-dependent Ad vector-neutrophil binding that is likely mediated by complement and antibody. To further examine the mechanism by which neutrophils mediate adenovirus internalization, complement and Fc receptor blocking studies were performed. Neutrophils express complement receptors 1 (CR1, CD35), 3 (CR3, CD11b, αMβ2), and 4 (CR4, CD11c, αXβ2) (12, 17). To determine the role of these receptors, Cy2-AdLuc (1 × 104 particles/cell) was incubated with neutrophils in the presence of blocking CD35, CD11b, or CD18 (common β2-integrin subunit) antibodies or isotype controls. In the presence of human serum, blocking CD35 antibodies significantly reduced adenovirus uptake by neutrophils over 60 min compared to a mouse IgG1 isotype control as determined by flow cytometry (Fig. 6A). Blocking of CD35 was specific, since the antibody had no impact on AdLuc transduction in REC cells (data not shown). In contrast, CD11b or CD18 antibodies did not affect neutrophil fluorescence through the experimental time course, confirming that CR1, but not CR3 or CR4, mediates adenovirus uptake in neutrophils (Fig. 6A).
FIG. 6.
Role of complement and Fc receptors in neutrophil-adenovirus interaction. (A) Cy2-AdLuc was incubated with neutrophils in the presence of blocking CD35, CD11b, CD18, or isotype control antibodies. Cells were analyzed by flow cytometry at 20 (open bars), 40 (hatched bars), and 60 (closed bars) min. For anti-CD35 versus isotype control, P < 0.05 (*); n = 3 to 5. (B) Cy2-AdLuc was incubated with neutrophils in the presence of blocking CD32, CD16, mouse IgG1, or mouse IgG2a antibodies. Cells were analyzed by flow cytometry at 40 min. For treatment groups versus IgG1 control, P < 0.05 (*) and P < 0.01 (**); n = 6. For combined Fc receptor blockade versus individual blockade, P = not significant (NS). (C) Effect of combined complement and Fc receptor blockade. Cy2-AdLuc was incubated with neutrophils in the presence of blocking CD35, CD32, CD16, mouse IgG2a, or control mouse IgG1 antibodies. Cells were analyzed by flow cytometry at 40 min. For CD35 blockade or combined receptor blockade versus IgG1 control, P < 0.05 (*) and P < 0.005 (***), respectively; n = 6. For combined CD35 and Fc receptor blockade versus CD35 alone, P < 0.05 (*); n = 6. Error bars indicate standard deviations.
Neutrophils express FcγRIIA (CD32) and FcγRIIIB (CD16) and can be induced to express FcγRI (CD64) (11). To examine the role of Fc receptors, experiments were performed using blocking CD32 and CD16 antibodies. To account for any Ad vector binding to induced FcγRI, mouse IgG2a, which binds with high affinity to this receptor, was also employed (9). Cy2-AdLuc (1 × 104 particles/cell) was incubated with neutrophils and human serum in the presence of blocking antibodies or an IgG1 isotype control. At 40 min, the cells were analyzed by flow cytometry. Blocking of CD32 and CD16 significantly reduced Ad vector-neutrophil binding at 40 min compared to controls (Fig. 6B). Interestingly, mouse IgG2a also reduced neutrophil fluorescence similar to blocking CD32 and CD16, suggesting a component of Ad vector binding to FcγRI in the presence of human serum. Again, none of the Fc blocking antibodies affected AdLuc transduction in control REC cells, confirming specificity (data not shown). Combined blockade of CD16, CD32, and FcγR1 reduced neutrophil-Ad vector binding further; however, the difference was not statistically significant compared to blocking separate Fc receptors (Fig. 6B).
Antagonism of individual or combined Fc receptors was less effective than CD35 blockade, suggesting that CR1 mediates much of the Ad vector binding to neutrophils. To determine the collective role of Fc receptors and CR1 in Ad vector-neutrophil interactions, neutrophils were incubated with Cy2-AdLuc in the presence of blocking CD35, CD32, CD16, and IgG2a antibodies. At 40 min, flow cytometry demonstrated significantly reduced neutrophil fluorescence following combined receptor blockade compared to blocking of CD35 alone confirming the significance of Fc receptor-mediated Ad vector-neutrophil interactions (Fig. 6C). Taken together, these results show that neutrophils bind to and internalize Ad vectors in a complement- and IgG-dependent manner via complement receptor CR1 and Fc receptors FcγRI, FcγRII, and FcγRIII.
DISCUSSION
Adenovirus vectors activate the innate immune system, yet little is known regarding the interaction between adenovirus and innate effector cells. We demonstrate for the first time that Ad vectors interact with neutrophils, a major effector arm of innate immunity, in vitro and in vivo. Furthermore, our results show that Ad vector-neutrophil binding is opsonin dependent and mediated by complement receptor 1 and Fc receptors FcγRI, FcγRII, and FcγRIII. These data establish a novel paradigm in the host response to Ad vectors that may lead to strategies to minimize the innate immune response to these agents.
In this study we chose to evaluate neutrophil-adenovirus interactions. Neutrophils are rapidly recruited to Ad vector-transduced organs during the innate response in vivo and represent the most abundant innate effector cell in the early stages of the host response to Ad vectors (19, 24). Despite this, little is known regarding the biology of neutrophils in relation to Ad vectors. Our results show that Ad vectors interact with neutrophils in an opsonin-dependent manner via the complement receptor CR1. This is consistent with numerous recent studies that have documented the binding and the activation of complement proteins, such as C3, by the adenovirus capsid (5, 14, 29). Our studies shed further light on this biology and show that complement opsonization likely targets Ad vectors to leukocyte complement receptors. Interestingly, compared to CR1, complement receptor CR3 and β2-integrins did not mediate significant Ad vector-neutrophil binding. This is not surprising, since CR1 is the high-affinity receptor for C3b whereas β2-integrins such as CR3, in addition to having many other diverse functions, bind to the less abundant proteolytic fragment iC3b. While β2-integrins may not directly mediate Ad vector-neutrophil binding, they may be required as coreceptors to facilitate CR1- and/or Fc receptor-mediated phagocytosis (7, 10, 12).
Neutrophils express a number of Fc receptors, including FcγRIIA and FcγRIIIB. FcγRIIIB is a specific neutrophil-inhibitory Fc receptor that lacks the prototypical immunoreceptor tyrosine-based activation motif of activating Fc receptors such as FcγRIIA (11). The involvement of Fc receptors in adenovirus-leukocyte interactions has clinical significance, since the majority of humans are immunized against the common Ad vector serotypes. Studies by Wilson and colleagues with adenovirus-naive and -immunized hosts demonstrate similar levels of inflammation but reduced target tissue toxicity in preimmunized mice and nonhuman primates receiving Ad vectors (25, 33). Our findings support the hypothesis that in the presence of circulating antibodies, Ad vectors are targeted to leukocytes and the reticuloendothelial system of macrophages via Fc receptors. The observed Fc receptor usage in neutrophils mirrors studies performed with dendritic cells that demonstrate antibody-dependent interactions with Ad vectors via FcγRII and FcγRIII (23). Interestingly, our results also suggest a role for FcγRI in antibody-dependent uptake of Ad vectors in neutrophils. Monomeric mouse IgG2a binds with high affinity and effectively blocks human FcγRI, but not FcγRII or FcγRIII, which recognize only multimeric or complexed IgG (9). FcγRI receptor is not expressed on resting neutrophils but can be induced by gamma interferon. It is possible that neutrophil activation by Ad vectors upregulates FcγRI expression that subsequently facilitates binding to antibody-coated viral particles. However, where Fc receptor usage appears to be sufficient to completely mediate Ad vector binding to dendritic cells (23), a greater proportion of Ad vector interactions with neutrophils are mediated by CR1. Furthermore, the role of antiadenovirus antibodies in mediating leukocyte interactions may relate more to their ability to bind and activate the complement than to direct Fc receptor binding. In this regard, serum IgG depletion had a greater effect on neutrophil uptake of Ad vectors than blocking of Fc receptors, suggesting that Ad vector interaction with CR1 is potentiated through antibody-dependent activation of the classical complement pathway and the production of the CR1 ligand C3b.
Our studies and others illustrate the importance of considering serum factors in evaluation of Ad vector tropism and receptor interactions (23, 29, 37). These findings provide an explanation for the numerous studies that demonstrate the similar distribution and inflammatory properties of systemically administered wild-type and tropism-modified Ad vectors that are based on the traditional CAR/integrin paradigm (1, 20, 22). However, it should be noted that in the absence of leukocytes such as Kupffer cells in the liver, classical Ad vector tropism can be observed in vivo (20). Furthermore, the role of antibody or complement-mediated leukocyte interactions may not be relevant if vectors are administered to tissues locally or ex vivo. Thus, the determinants of tropism and the innate immune response to Ad vectors will be dependent on many factors, including immune status, cell type, and mode of vector administration.
Very little is known regarding the cellular and molecular impact of Ad vector interaction with innate effectors cells. In vitro, Fc receptor binding greatly enhances Ad vector internalization in dendritic cells but significantly reduces transduction (23). Phosphoinositide-3-OH kinase, but not MyD88, plays a role in Ad vector-induced dendritic cell activation in the absence of serum factors (26). In the presence of complement or antibody, the consequence of Ad vector interactions with CR1 and Fc receptors is unknown. Although Fc receptor signaling is relatively well documented, less is known regarding CR1. CR1 is not known to directly initiate cellular signaling, but it may cooperate with other cell surface molecules, including CR3 (β2-integrins) and Fc receptors that are capable of mediating downstream signals (7, 10). In vivo, although studies have examined the innate immune response to Ad vectors in various leukocyte-depleted states (21, 24, 36), the direct impact of virus-leukocyte interactions and leukocyte receptor binding by Ad vectors has yet to be determined. Future studies will be required to evaluate the role of complement and Fc receptors in Ad vector induction of innate immunity.
Our studies demonstrate a novel interaction between Ad vectors and the innate immune system. The opsonin-dependent binding of Ad vectors to phagocyte complement and Fc receptors increases the understanding of the host response to agents, which will ultimately result in strategies to modulate the innate immune response to adenovirus vectors.
Acknowledgments
This research was funded by the Canadian Institutes of Health Research (CIHR). M.J.C. is supported by an Alberta Cancer Board postdoctoral fellowship. A.K.Z. is supported by a Heart and Stroke Foundation studentship. D.A.M. is the recipient of Canadian Institutes of Health Research (CIHR) New Investigator and Alberta Heritage Foundation for Medical Research (AHFMR) Scholar Awards.
We acknowledge Laurie Robertson (Flow Cytometry Core Facility) for her expert technical assistance.
REFERENCES
- 1.Alemany, R., and D. T. Curiel. 2001. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 8:1347-1353. [DOI] [PubMed] [Google Scholar]
- 2.Becker, T. C., R. J. Noel, W. S. Coats, A. M. Gomez-Foix, T. Alam, R. D. Gerard, and C. B. Newgard. 1994. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 43A:161-189. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., A. Krithivas, L. Celi, G. Droguett, M. S. Horwitz, T. Wickham, R. L. Crowell, and R. W. Finberg. 1998. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J. Virol. 72:415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cara, D. C., J. Kaur, M. Forster, D. M. McCafferty, and P. Kubes. 2001. Role of p38 mitogen-activated protein kinase in chemokine-induced emigration and chemotaxis in vivo. J. Immunol. 167:6552-6558. [DOI] [PubMed] [Google Scholar]
- 5.Cichon, G., S. Boeckh-Herwig, H. H. Schmidt, E. Wehnes, T. Muller, P. Pring-Akerblom, and R. Burger. 2001. Complement activation by recombinant adenoviruses. Gene Ther. 8:1794-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagiolo, U., F. Kricek, C. Ruf, A. Peserico, A. Amadori, and M. Cancian. 2000. Effects of complement inactivation and IgG depletion on skin reactivity to autologous serum in chronic idiopathic urticaria. J. Allergy Clin. Immunol. 106:567-572. [DOI] [PubMed] [Google Scholar]
- 7.Fallman, M., R. Andersson, and T. Andersson. 1993. Signaling properties of CR3 (CD11b/CD18) and CR1 (CD35) in relation to phagocytosis of complement-opsonized particles. J. Immunol. 151:330-338. [PubMed] [Google Scholar]
- 8.Fine, D. P., S. R. Marney, Jr., D. G. Colley, J. S. Sergent, and R. M. Des Prez. 1972. C3 shunt activation in human serum chelated with EGTA. J. Immunol. 109:807-809. [PubMed] [Google Scholar]
- 9.Flesch, B. K., G. Achtert, and J. Neppert. 1997. Inhibition of monocyte and polymorphonuclear granulocyte immune phagocytosis by monoclonal antibodies specific for Fc gamma RI, II and III. Ann. Hematol. 74:15-22. [DOI] [PubMed] [Google Scholar]
- 10.Galon, J., J. F. Gauchat, N. Mazieres, R. Spagnoli, W. Storkus, M. Lotze, J. Y. Bonnefoy, W. H. Fridman, and C. Sautes. 1996. Soluble Fcgamma receptor type III (FcgammaRIII, CD16) triggers cell activation through interaction with complement receptors. J. Immunol. 157:1184-1192. [PubMed] [Google Scholar]
- 11.Garcia-Garcia, E., and C. Rosales. 2002. Signal transduction during Fc receptor-mediated phagocytosis. J. Leukoc. Biol. 72:1092-1108. [PubMed] [Google Scholar]
- 12.Gasque, P. 2004. Complement: a unique innate immune sensor for danger signals. Mol. Immunol. 41:1089-1098. [DOI] [PubMed] [Google Scholar]
- 13.Gotze, O., and H. J. Muller-Eberhard. 1971. The C3-activator system: an alternate pathway of complement activation. J. Exp. Med. 134:90s-108s. [PubMed] [Google Scholar]
- 14.Jiang, H., Z. Wang, D. Serra, M. M. Frank, and A. Amalfitano. 2004. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-Ad antibodies. Mol. Ther. 10:1140-1142. [DOI] [PubMed] [Google Scholar]
- 15.Jooss, K., and N. Chirmule. 2003. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther. 10:955-963. [DOI] [PubMed] [Google Scholar]
- 16.Koller, H., K. Hochegger, G. J. Zlabinger, K. Lhotta, G. Mayer, and A. R. Rosenkranz. 2004. Apoptosis of human polymorphonuclear neutrophils accelerated by dialysis membranes via the activation of the complement system. Nephrol. Dial. Transplant. 19:3104-3111. [DOI] [PubMed] [Google Scholar]
- 17.Kumar, A., E. Wetzler, and M. Berger. 1997. Isolation and characterization of complement receptor type 1 (CR1) storage vesicles from human neutrophils using antibodies to the cytoplasmic tail of CR1. Blood 89:4555-4565. [PubMed] [Google Scholar]
- 18.Leopold, P. L., B. Ferris, I. Grinberg, S. Worgall, N. R. Hackett, and R. G. Crystal. 1998. Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells. Hum. Gene Ther. 9:367-378. [DOI] [PubMed] [Google Scholar]
- 19.Li, Y., D. A. Muruve, R. G. Collins, S. S. Lee, and P. Kubes. 2002. The role of selectins and integrins in adenovirus vector-induced neutrophil recruitment to the liver. Eur. J. Immunol. 32:3443-3452. [DOI] [PubMed] [Google Scholar]
- 20.Liu, Q., A. K. Zaiss, P. Colarusso, K. Patel, G. Haljan, T. J. Wickham, and D. A. Muruve. 2003. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum. Gene Ther. 14:627-643. [DOI] [PubMed] [Google Scholar]
- 21.Liu, Z. X., S. Govindarajan, S. Okamoto, and G. Dennert. 2000. NK cells cause liver injury and facilitate the induction of T cell-mediated immunity to a viral liver infection. J. Immunol. 164:6480-6486. [DOI] [PubMed] [Google Scholar]
- 22.Martin, K., A. Brie, P. Saulnier, M. Perricaudet, P. Yeh, and E. Vigne. 2003. Simultaneous CAR- and alpha V integrin-binding ablation fails to reduce Ad5 liver tropism. Mol. Ther. 8:485-494. [DOI] [PubMed] [Google Scholar]
- 23.Mercier, S., H. Rouard, M. H. Delfau-Larue, and M. Eloit. 2004. Specific antibodies modulate the interactions of adenovirus type 5 with dendritic cells. Virology 322:308-317. [DOI] [PubMed] [Google Scholar]
- 24.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10:965-976. [DOI] [PubMed] [Google Scholar]
- 25.Nunes, F. A., E. E. Furth, J. M. Wilson, and S. E. Raper. 1999. Gene transfer into the liver of nonhuman primates with E1-deleted recombinant adenoviral vectors: safety of readministration. Hum. Gene Ther. 10:2515-2526. [DOI] [PubMed] [Google Scholar]
- 26.Philpott, N. J., M. Nociari, K. B. Elkon, and E. Falck-Pedersen. 2004. Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-alpha induction pathway. Proc. Natl. Acad. Sci. USA 101:6200-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenburg, H. F., and J. I. Gallin. 1999. Inflammation, p. 1051-1066. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 28.Salmon, D., J. L. Vilde, B. Andrieu, R. Simonovic, and J. Lebras. 1986. Role of immune serum and complement in stimulation of the metabolic burst of human neutrophils by Plasmodium falciparum. Infect. Immun. 51:801-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shayakhmetov, D. M., A. Gaggar, S. Ni, Z. Y. Li, and A. Lieber. 2005. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 79:7478-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, K. R. 2003. Gene therapy: theoretical and bioethical concepts. Arch. Med. Res. 34:247-268. [DOI] [PubMed] [Google Scholar]
- 31.Somia, N., and I. M. Verma. 2000. Gene therapy: trials and tribulations. Nat. Rev. Genet. 1:91-99. [DOI] [PubMed] [Google Scholar]
- 32.Tibbles, L. A., J. C. Spurrell, G. P. Bowen, Q. Liu, M. Lam, A. K. Zaiss, S. M. Robbins, M. D. Hollenberg, T. J. Wickham, and D. A. Muruve. 2002. Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J. Virol. 76:1559-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varnavski, A. N., R. Calcedo, M. Bove, G. Gao, and J. M. Wilson. 2005. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 12:427-436. [DOI] [PubMed] [Google Scholar]
- 34.Venturi, G. M., L. Tu, T. Kadono, A. I. Khan, Y. Fujimoto, P. Oshel, C. B. Bock, A. S. Miller, R. M. Albrecht, P. Kubes, D. A. Steeber, and T. F. Tedder. 2003. Leukocyte migration is regulated by L-selectin endoproteolytic release. Immunity 19:713-724. [DOI] [PubMed] [Google Scholar]
- 35.Villarreal, G., J. Zagorski, and S. M. Wahl. 2001. Inflammation: acute. In Encyclopedia of life sciences. [Online.] Nature Publishing Group, London, United Kingdom. http://www.els.net/doi:10.1038/npg.els.0000943.
- 36.Zhang, Y., N. Chirmule, G. P. Gao, R. Qian, M. Croyle, B. Joshi, J. Tazelaar, and J. M. Wilson. 2001. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3:697-707. [DOI] [PubMed] [Google Scholar]
- 37.Zinn, K. R., A. J. Szalai, A. Stargel, V. Krasnykh, and T. R. Chaudhuri. 2004. Bioluminescence imaging reveals a significant role for complement in liver transduction following intravenous delivery of adenovirus. Gene Ther. 11:1482-1486. [DOI] [PubMed] [Google Scholar]