Abstract
The Epstein-Barr virus (EBV) EBER transcripts are small, highly structured RNAs able to bind to and inhibit activation of the double-stranded RNA-dependent protein kinase PKR in cell-free systems, and within latently infected B-cell lines they inhibit alpha interferon-induced apoptosis that is believed to be mediated through PKR. Here, we address the consequences of EBER expression for PKR activation in vivo in response to alpha interferon. In agreement with published findings, either EBV infection or the EBERs alone protected Burkitt lymphoma cells from alpha-interferon-induced apoptosis. However, utilizing multiple phosphorylation state-specific antibodies to monitor PKR activation within cells in response to interferon, we demonstrate that the EBERs are unable to inhibit phosphorylation of either cytoplasmic or nuclear PKR. Concordantly, a direct substrate of PKR, the α subunit of eukaryotic initiation factor 2 (eIF-2α), was equally phosphorylated in EBV-positive and EBV-negative cells following interferon treatment. Therefore, EBER inhibition of alpha-interferon-induced apoptosis, and potentially other PKR-mediated events, is unlikely to be mediated through direct inhibition of PKR, as previously thought.
The Epstein-Barr virus (EBV) small RNAs EBER-1 and EBER-2 (167 and 172 nucleotides, respectively) are highly structured noncoding RNA polymerase III transcripts expressed at very high levels (∼107 copies per cell) within the nuclei of cells latently infected by EBV (1, 6, 8, 9, 20, 31). Though not essential for EBV immortalization of B lymphocytes in vitro (39), a hallmark property of this oncogenic herpesvirus, the EBER RNAs can nonetheless enhance immortalization (45) and contribute to the tumorigenic potential of Burkitt lymphoma (BL) and other B-lymphoma cell lines, as well as the growth of cells derived from gastric and nasopharyngeal carcinomas (10, 11, 14, 32, 46). The precise contribution(s) of the EBER RNAs to the tumorigenic potential of B cells is unclear (32), while enhanced growth of epithelial tumor cells has been attributed to up-regulation of insulin-like growth factor 1, though the mechanism through which this occurs is also unknown (10, 11, 32).
The EBER RNAs also enhance interleukin-10 expression (13) and inhibit apoptosis induced by alpha interferon (IFN-α) (24), functions likely to promote long-term persistence of the virus. Whereas the mechanism through which the EBERs promote interleukin-10 expression is unknown, inhibition of IFN-α-induced apoptosis has been reported to be mediated through inhibition of the double-stranded RNA (dsRNA)-dependent protein kinase PKR (24). PKR has also been implicated in various signal transduction pathways and response to stress signals (44), and dominant-negative forms of PKR have transforming potential (16, 23). Thus, an inhibition of PKR by the EBER RNAs has implications for their oncogenic role in EBV infection, as well.
PKR is a latent, IFN-inducible Ser/Thr kinase activated by dsRNA via two dsRNA-binding motifs (dsRBM I and II) present in each PKR monomer. This promotes protein dimerization and conformational changes that result in autophosphorylation-mediated activation of PKR and the subsequent phosphorylation by PKR of the α subunit of eukaryotic initiation factor 2 (eIF-2α) on Ser51, resulting in cessation of protein synthesis (reviewed in reference 44). Because viral infections can result in the production of dsRNAs capable of activating PKR, PKR is considered a significant component of the innate antiviral response, though numerous viruses have developed mechanisms to circumvent PKR's antiviral functions (5). Specifically, some viruses encode dsRNAs capable of being bound by PKR but which have an inhibitory effect on its activation, circumventing the antiviral role of PKR. Notable examples are the adenovirus VAI small RNA and (presumably) the EBV EBER RNAs (3, 17, 21, 34).
Several lines of experimental evidence support an inhibitory effect of the EBER RNAs on PKR through direct interaction with PKR. Within reticulocyte lysates, the EBERs, like the structurally comparable adenovirus VAI small RNA, bind PKR and inhibit its repression of translation in a dose-dependent manner (3, 34). Concordantly, stem-loop IV of EBER-1 is bound in a specific manner by PKR in vitro through its dsRBM I, but not dsRBM II (42), a feature apparently distinct from activating dsRNAs that interact with both sites, presumably relieving an autoinhibitory influence of dsRBM II on the PKR catalytic domain (36). The EBER RNAs, furthermore, are able to rescue replication of adenoviruses that lack the VAI RNA gene (2), suggesting that the EBERs share with the VAI RNA the ability to inhibit PKR in vivo. Finally, protein kinase assays performed with B-cell lysates have indicated that PKR activation is inhibited in the presence of endogenous EBERs in latently infected cells or by EBERs stably expressed in an EBV-negative B-cell background (24, 46).
While the EBER RNAs are able to bind to and inhibit PKR in cell-free systems and compensate for VAI RNA loss in the context of a cytolytic adenovirus infection, the different principal locations of PKR (cytoplasmic) and the EBERs (nuclear) within cells latently infected by EBV prompted us to ask whether the EBER RNAs are significant inhibitors of PKR in vivo. Here, we show, through the use of recently available phosphospecific antibodies to PKR, as well as phosphospecific antibody to its substrate eIF-2α, that the EBER RNAs are incapable of inhibiting IFN-α-induced expression and activation of PKR within BL cells. Thus, the ability of the EBERs to protect against IFN-α-induced apoptosis, as shown here and previously (24), must occur independently of a direct inhibition of PKR.
MATERIALS AND METHODS
Cell culture.
Cells were maintained in RPMI 1640 medium containing 2 mM L-glutamine (Mediatech) and 10% defined fetal bovine serum (HyClone). Akata A.15 and A.2 cell lines are EBV-positive and -negative derivatives, respectively, of the parental Akata BL cell line (33). Akata Ak− (EBV-negative) and Ak+ (EBV-positive) cells were kindly provided by K. Takada (Hokkaido University, Sapporo, Japan). Daudi, KemI, and MutuI are EBV-positive BL cell lines, the last two of which maintain a latency I program of EBV gene expression comparable to that of Akata BL cells. A.2.EBER and A.2.Vector are derivatives of A.2 Akata cells engineered to express EBER-1 and EBER-2 or that contain an empty EBER expression vector, respectively, as previously described (32). These two lines also express the EBV genome maintenance protein EBNA-1, which contributes to high-level EBER expression and maintenance of the extrachromosomal EBER expression plasmid that contains a truncated EBV origin of latent DNA replication (oriP).
Cell death assays.
Cells were seeded at 2.5 × 105 per ml in standard growth medium. At 24 h postseeding, human IFN-α (R&D Systems) was added to a final concentration of 100 or 500 U/ml. Cell viability was monitored daily by trypan blue dye exclusion, while aliquots of cells were simultaneously harvested for analysis of poly(ADP-ribose) polymerase (PARP) cleavage by immunoblotting (Roche).
RNA analysis.
Total cellular RNA was isolated from 107 cells (treated for 48 h with IFN-α [100 U/ml]) with RNA-Bee as recommended by the manufacturer (TelTest), followed by extraction with an equal volume of phenol-chloroform and then chloroform prior to ethanol precipitation. For RNA (Northern) blot analysis, duplicate samples of 10 μg of RNA were fractionated by electrophoresis in a 1.2% agarose-2.2 M formaldehyde gel and blotted onto GeneScreen Plus membrane (DuPont). 32P-labeled DNA probes specific for either EBER-1 or EBER-2 were generated by random-priming labeling. Following hybridization, the blots were washed and processed by autoradiography.
Immunoblot analysis of PKR and eIF-2α phosphorylation.
For each sample, 5 × 106 cells were washed once in phosphate-buffered saline and frozen at −86°C. The thawed cell pellets were lysed in 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, protease inhibitor (Roche Complete Mini), and phosphatase inhibitor (Calbiochem; phosphatase inhibitor cocktail set II). Following 10 min on ice, extracts were clarified by microcentrifugation at 13,200 rpm for 10 min. The protein concentration was determined by Bradford assay (Bio-Rad), and 20 μg of protein was fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to an Immobilon P membrane (Millipore), and immunoblotted using an enhanced-chemiluminescence detection system (Amersham). The blots were initially probed using phosphorylation site-specific antibodies; subsequently stripped of antibody in 62.5 mM Tris-HCl (pH 6.8), 100 mM 2-mercaptoethanol, 2% sodium dodecyl sulfate (50°C for 30 min); and reprobed with phosphorylation state-independent antibodies. The blots were stripped a second time and reprobed with antibody to β-actin as a control for protein loading. The primary antibodies utilized were phosphorylation site-specific antibodies for PKR (Thr446 and Thr451; Cell Signaling Technology) and eIF-2α (Ser51; Cell Signaling), total PKR (Cell Signaling Technology), total eIF-2α (provided by R. Kaufman, University of Michigan), and β-actin (Oncogene Research Products). Secondary antibodies conjugated to horseradish peroxidase were obtained from Chemicon. To assess the phosphorylation status of PKR in response to IFN-α, cells were seeded at 2.5 × 105 per ml, and IFN-α was added as described above. For analyses of eIF-2α phosphorylation, an initial cell density of 1.5 × 105 was employed to reduce the background level of phospho-eIF-2α (19). For analysis of cytoplasmic and nuclear extracts, cells were untreated or treated with IFN-α at 500 U/ml; 107 cells were then fractionated using the NE-PER kit (Pierce) according to the manufacturer's instructions. The integrity of the nuclear and cytoplasmic fractions was assessed by immunoblotting with an antibody to β-tubulin (provided by N. Morrissette, University of California, Irvine).
RESULTS AND DISCUSSION
EBV and EBER inhibition of IFN-α-induced apoptosis.
To determine whether the EBERs inhibit PKR in the context of a cellular response to IFN-α, we first assessed their ability to protect Akata BL cells from IFN-α-induced apoptosis. For unknown reasons, Akata cells can spontaneously lose the EBV genome in culture, resulting in the loss of tumorigenic potential and resistance to various proapoptotic stimuli that can be restored by reinfection with EBV (15, 33, 35). Further, EBV protection of BL cells (including Akata) from IFN-α-induced apoptosis has been attributed to the inhibition of PKR by the EBERs (24). Thus, the EBV-positive/negative Akata cell system offers an ideal model with which to assess EBER functions.
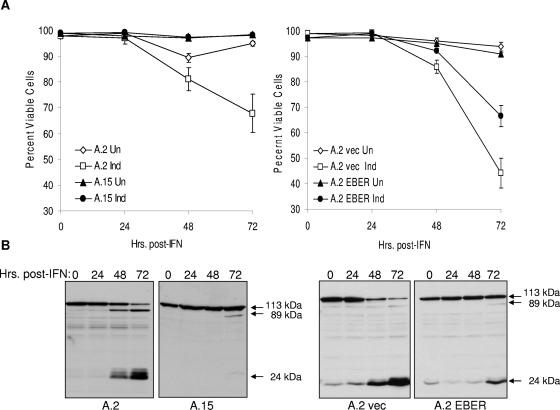
As shown in Fig. 1A (left), survival of EBV-positive (A.15) Akata cells in the presence of IFN-α (100 U/ml) was significantly higher than for EBV-negative (A.2) Akata cells. We next asked whether the EBERs alone were capable of protection. As illustrated in Fig. 1A (right), expression of EBERs 1 and 2 within EBV-negative Akata cells, at levels comparable to those of EBV-positive BL cell lines (Fig. 2), likewise promoted cell survival. Interestingly, we consistently observed an intermediate protective effect of the EBERs relative to the protection afforded by EBV infection, suggesting that another gene(s) expressed within Akata cells may also contribute to survival. Nonetheless, significant protection from IFN-α-induced cell death could be attributed to the EBERs. Virtually identical results were obtained when an IFN-α concentration of 500 U/ml was used in parallel (data not shown). To confirm that protection afforded by EBV infection and EBERs alone was due to inhibition of apoptosis, we monitored cleavage of PARP, a caspase-mediated event characteristic of apoptotic cell death (25). As shown in Fig. 1B, induction of PARP cleavage by IFN-α was nearly completely inhibited in the EBV-positive cells (A.15) and notably reduced in the presence of the EBERs alone (A.2 EBER). This was consistent with the relative levels of protection from IFN-α-mediated cell death observed in Fig. 1A and an earlier report demonstrating an antiapoptotic effect of the EBERs within Akata and other BL lines (24). Comparable results were obtained with a higher concentration (500 U/ml) of IFN-α, and little or no cleavage of PARP was observed in the absence of IFN-α over the 72-h course of the experiment (data not shown).
FIG. 1.
EBV infection and EBERs protect BL cells against IFN-α-induced apoptosis. (A) Protection of Akata BL cells from IFN-α (100 U/ml)-induced cell death by EBV infection (left) or by stable expression of the EBERs in the absence of EBV infection (right). A.2 and A.15 are EBV-negative and -positive Akata cell lines, respectively. Cell viability was measured by trypan blue dye exclusion. Un, uninduced; Ind, induced with IFN-α; vec, vector-only control. The error bars indicate standard errors of the mean. (B) Inhibition of IFN-α-induced cleavage of PARP by EBV infection (left two blots) and stable EBER expression in EBV-negative Akata cells (right two blots). Cells (from panel A) were exposed to IFN-α for the times indicated prior to detection of PARP by immunoblotting. Full-length (113-kDa) and the 89- and 24-kDa cleavage products of PARP are indicated. Virtually identical results were obtained with 500 U/ml IFN-α for both assays (data not shown).
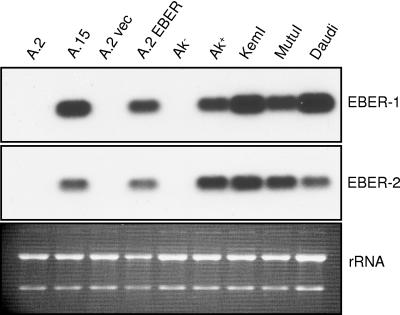
FIG. 2.
EBER-1 and EBER-2 expression in BL cells. Expression levels of the two EBERs were assessed by RNA (Northern) blot analysis of total cell RNA from the EBV-negative Akata cell lines A.2 and Ak−, EBV-positive Akata lines A.15 and Ak+, A.2 Akata cells that contain the empty EBER expression vector (A.2 vec) or that stably express the EBER RNAs (A.2 EBER), and the non-Akata BL cell lines KemI, MutuI, and Daudi. The level of rRNA in each lane that was detected by ethidium bromide staining of the gel prior to blotting is shown as an indication of RNA loading; note that the A.2 EBER lane was underloaded.
Activation of PKR by IFN-α in the presence EBV.
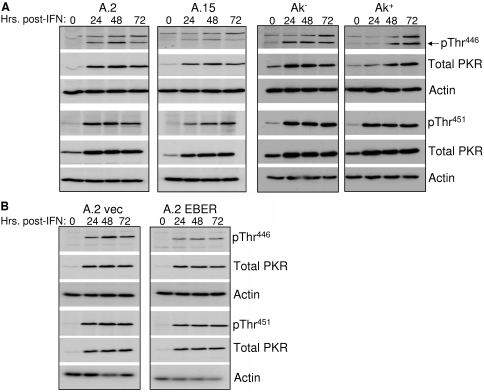
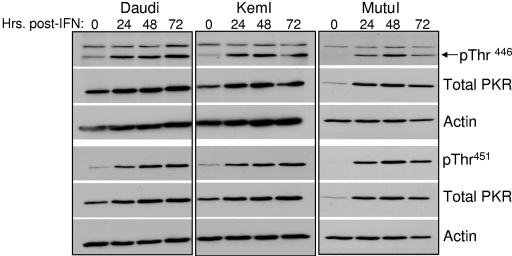
To determine whether inhibition of IFN-α-induced apoptosis was actually due to inhibition of PKR by the EBERs, the phosphorylation (and therefore activation) status of PKR within Akata BL cells upon exposure to IFN-α was monitored by immunoblotting with antibodies specific for PKR phosphorylated on either Thr446 or Thr451. Mutational analyses have indicated that Thr446 is critical for PKR function, while Thr451 is essential (30, 47). We first assessed PKR activation in two independently derived sets of EBV-negative and -positive Akata lines—one set was derived within our laboratory (A.2 and A.15), and the other (designated Ak− and Ak+) was a gift from K. Takada (Hokkaido University, Sapporo, Japan). As demonstrated in Fig. 3A, relative to total PKR, both the kinetics and levels of PKR phosphorylation at either Thr446 or Thr451 were comparable in EBV-negative and -positive cells in response to IFN-α. Likewise, expression of the EBERs alone within EBV-negative Akata cells could not prevent phosphorylation of PKR (Fig. 3B). As expected, total PKR protein levels were higher following IFN-α treatment, as the PKR gene is an IFN-stimulated gene (22). Identical results were obtained with a third phosphospecific PKR antibody (phospho-PKR Thr446/451; Cell Signaling) (data not shown). Comparable activation of PKR was observed in three other EBV-positive BL lines analyzed (Daudi, KemI, and MutuI), as shown in Fig. 4, each of which expresses EBER-1 and EBER-2 at levels comparable to those of EBV-positive Akata cells (Fig. 2). KemI and MutuI cells, like their Akata cell counterparts, maintain a strict type I latency program with respect to EBV latency gene expression. Therefore, we concluded from these results that, in vivo, the EBV EBERs are unlikely to prevent significant activation of PKR in response to IFN-α. The mechanism through which PKR is activated in response to IFN-α is unknown, though IFN-α/γ-dependent signaling to the IRF-1 promoter requires PKR, implying activation, and phosphorylation of PKR occurred when the effect of IFN-γ on PKR was assessed (18). dsRNA-independent activation of PKR by the PKR-interacting protein PACT (protein activator of PKR) occurs under various stress conditions (26, 44); however, PACT itself is not activated by IFNs (26, 27).
FIG. 3.
IFN-α-induced phosphorylation of PKR is unaffected by EBV infection or the EBERs. (A) Phosphorylation of PKR at Thr446 and Thr451 (pThr446 and pThr451) in response to IFN-α (100 U/ml) was monitored within two independently derived lines of EBV-negative (A.2 and Ak−) and EBV-positive (A.15 and Ak+) Akata cells by immunoblotting with phosphospecific PKR antibodies. The blots were subsequently stripped and reprobed with antibodies to total PKR and β-actin (loading control). (B) Phosphorylation of PKR monitored in EBV-negative Akata BL cells either containing the empty EBER expression vector (A.2 vec) or stably expressing EBER-1 and EBER-2 (A.2 EBER) at levels comparable to EBV-infected Akata cells.
FIG. 4.
IFN-α induces phosphorylation of PKR in EBV-positive BL cell lines. Phosphorylation of PKR was monitored as described in the legend to Fig. 3.
Our finding is in apparent disagreement with that of Nanbo and colleagues, who found that phosphorylation of PKR is inhibited in the presence of the EBER RNAs (24). There are two plausible reasons for this. First, instead of assessing phosphorylation of endogenous PKR in response to IFN-α (as in Fig. 3), PKR phosphorylation in the presence and absence of the EBERs was assessed by in vitro kinase assay following immunoprecipitation of PKR transiently overexpressed in BL cells. Thus, the observed phosphorylation of PKR from EBV-negative BL cells, which was inhibited in the presence of the EBERs, may not have been representative of the mechanism through which PKR is activated in response to IFN-α. Notably, a number of stress signals, including overexpression, result in activation of PKR. Second, under the conditions of immunoprecipitation and the in vitro kinase assay, corruption of EBER subcellular location and/or disruption of the interaction between the EBER RNAs and ribosomal protein L22 (formerly EBER-associated protein [EAP]) (40, 41), which has been reported to exclude interaction of EBER-1 with PKR in vitro (4), may have permitted an EBER interaction with PKR sufficient to inhibit activation. This possibility also raises concern over the physiologic relevance of an earlier report of EBER inhibition of poly(I) · poly(C)-induced phosphorylation of PKR in vitro (46).
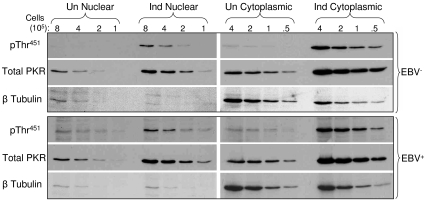
Phosphorylation of nuclear PKR is unaffected by EBV.
Approximately 20% of the total cellular pool of PKR is present within the nucleus (12). While the role of nuclear PKR is unclear, one function may be to phosphorylate protein substrates other than eIF-2α, such as DRBP76, itself a dsRNA-binding protein (28). Because the EBER RNAs reside almost exclusively within the cell nucleus (9), possibly as a consequence of stable association via their 3′ termini with the La nuclear protein (6, 20), the EBER RNAs may primarily target nuclear PKR. Because the nuclear pool of PKR is minor relative to that present in the cytoplasm, a negative effect of EBV on PKR phosphorylation within the nucleus might be masked in experiments that assess phosphorylation of total PKR (as in Fig. 3 and 4), the bulk of which would originate from the cytoplasm. We therefore fractionated EBV-positive and -negative Akata cells into nuclear and cytoplasmic fractions following IFN-α treatment (500 U/ml for 24 h) and assessed the phosphorylation of PKR on Thr451. Phosphorylation of nuclear PKR, however, mirrored that of cytoplasmic PKR, with no decrease in phosphorylation associated with EBV infection (Fig. 5). Although a small amount of the cytoplasmic β-tubulin was detected in the nuclear fractions, this could not account for a significant degree of cytoplasmic PKR contamination.
FIG. 5.
Phosphorylation of nuclear PKR is unaffected by EBV infection. Phosphorylation of PKR (pThr451) within the nuclear and cytoplasmic fractions of EBV-negative (A.2) and EBV-positive (A.15) Akata cells was monitored in the absence or presence of IFN-α treatment. The blots were reprobed with an antibody to β-tubulin, which is predominately cytoplasmic. The amount of protein loaded per lane was based on cell equivalents times 105 instead of actual protein, since protein levels within nuclear fractions were substantially lower than in corresponding cytoplasmic fractions. Un, uninduced; Ind, induced with IFN-α.
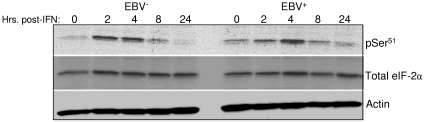
IFN-α-induced phosphorylation of eIF-2α.
We further examined the likelihood that the EBER RNAs do not inhibit PKR in vivo by assessing the influence of EBV on phosphorylation of eIF-2α, a principal substrate of PKR. If the EBER RNAs are indeed capable of inhibiting PKR function, a decrease in phosphorylation of eIF-2α in response to IFN-α should be observed within EBV-positive Akata cells relative to their EBV-negative counterparts. However, we detected no influence of EBV infection on the IFN-α-induced phosphorylation of eIF-2α on Ser51 (Fig. 6). The same result was obtained with a different phosphospecific eIF-2α antibody (data not shown) and is consistent with our observation that neither EBV infection nor the EBERs alone are capable of preventing phosphorylation of PKR on Thr446 or Thr451 (Fig. 3).
FIG. 6.
IFN-α-induced phosphorylation of eIF-2α is unaffected by EBV infection. EBV-negative (A.2) and EBV-positive (A.15) Akata cells were untreated or exposed to IFN-α (100 U/ml) for up to 24 h. Phosphorylation of eIF-2α was monitored at the indicated times by immunoblotting to detect phosphorylated (pSer51) relative to total eIF-2α. The blot was reprobed with an antibody to β-actin to monitor protein loading.
Nanbo and colleagues, however, did observe a decrease in eIF-2α Ser51 phosphorylation within EBER-expressing BL cells when phosphorylation was assessed by immunoblotting at a single time point of 12 h posttreatment with IFN-α (24). We did observe some variation in eIF-2α phosphorylation between EBV-positive and -negative cells at certain time points, e.g., an apparently smaller amount of phosphorylation in EBV-positive cells at 2 h but a greater amount of phosphorylation in the same cells at 24 h (Fig. 6). Overall, however, we found no evidence of significant inhibition in eIF-2α phosphorylation over the course of these experiments, consistent with the lack of an effect of either EBV infection or the EBER RNAs alone on PKR phosphorylation (Fig. 3). Another explanation for this apparent discrepancy may be the different cell densities at which cells were seeded in the current and previous studies (1.5 × 105 and 5 × 104 per ml, respectively). In our hands, even in the presence of 10% serum, BL cells seeded at densities lower that 105 per ml typically exhibit an extended lag period before they are able to begin proliferating. Low cell density alone, or possibly in combination with IFN-α as used in the previous study, therefore, may have contributed to stress-induced phosphorylation of eIF-2α by another eIF-2α kinase that may be negatively regulated by the EBER RNAs. Currently, it is not known if either of the EBER RNAs directly or indirectly targets another member of this family of stress-responsive kinases.
Conclusions.
Previous studies have concluded, based largely on cell-free system-based assays, that the EBER RNAs can function by direct inhibition of PKR. Here, by directly monitoring phosphorylation of endogenous PKR and its substrate, eIF-2α, within BL cells treated with IFN-α, which induces PKR-dependent apoptosis (24), we found no evidence that the antiapoptotic effect of the EBERs (or EBV infection in general) in this system is mediated by direct inhibition of PKR. Our findings, furthermore, are consistent with three previous observations that suggest PKR is not a direct target of the EBERs in vivo. First, replication of vesicular stomatitis virus, a virus sensitive to the antiviral function of PKR (37), is equivalent in B-cell lines immortalized with either a wild-type EBV or a mutant virus lacking the EBER genes and is equivalently repressed in both lines by IFN-α treatment (38). Second, the EBERs are normally in a complex with rpL22, an interaction that in vitro can exclude binding by PKR (4), and third, activation of PKR is not prevented upon infection of EBV/EBER-positive cells with mutant adenovirus that does not express the PKR-inhibiting VAI small RNA (43). We conclude, therefore, that EBER inhibition of IFN-α-induced apoptosis in latently infected cells must occur downstream of PKR activation. One possible target in this respect may be the nuclear dsRNA-binding protein DRBP76, a putative substrate of PKR (28).
Finally, while our findings and those discussed above argue against direct inhibition of PKR during latent EBV infection, as yet we have not ruled out EBER inhibition of PKR upon activation of lytic infection. Specifically, disruption of nuclear membrane integrity and/or latency-associated EBER-protein interactions upon EBV reactivation may enable the EBERs to bind to, and thus inhibit subsequent activation of, PKR, e.g., by abundant structured viral transcripts produced during the lytic cycle. Although EBER gene transcription is reduced upon reactivation, EBER levels remain relatively high through at least 72 h postinduction of lytic infection (7). Potentially, this could ensure inhibition of PKR prior to adequate expression of the EBV early protein SM, itself an inhibitor of PKR (29).
Acknowledgments
This work was supported by U.S. Public Health Service grants CA056639 and CA073544 (to J.T.S.) and Cancer Center Support Grants CA21765 (to St. Jude Children's Research Hospital) and CA62203 (to the University of California, Irvine) from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC). K.A.L. was a University of California UROP Fellow.
We thank K. Takada, R. Kaufman, and N. Morrissette for gifts of reagents and D. Henson for excellent technical assistance.
REFERENCES
- 1.Arrand, J. R., and L. Rymo. 1982. Characterization of the major Epstein-Barr virus-specific RNA in Burkitt lymphoma-derived cells. J. Virol. 41:376-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhat, R. A., and B. Thimmappaya. 1983. Two small RNAs encoded by Epstein-Barr virus can functionally substitute for the virus-associated RNAs in the lytic growth of adenovirus 5. Proc. Natl. Acad. Sci. USA 80:4789-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke, P. A., M. Schwemmle, J. Schickinger, K. Hilses, and M. J. Clemens. 1991. Binding of the Epstein-Barr virus small RNA EBER-1 to the double-stranded RNA-activated protein kinase DAI. Nucleic Acids Res. 19:243-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elia, A., J. Vyas, K. G. Laing, and M. J. Clemens. 2004. Ribosomal protein L22 inhibits regulation of cellular activities by the Epstein-Barr virus small RNA EBER-1. Eur. J. Biochem. 271:1895-1905. [DOI] [PubMed] [Google Scholar]
- 5.Gale, M., Jr., and M. G. Katze. 1998. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol. Ther. 78:29-46. [DOI] [PubMed] [Google Scholar]
- 6.Glickman, J. N., J. G. Howe, and J. A. Steitz. 1988. Structural analyses of EBER1 and EBER2 ribonucleoprotein particles present in Epstein-Barr virus-infected cells. J. Virol. 62:902-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greifenegger, N., M. Jager, L. A. Kunz-Schughart, H. Wolf, and F. Schwarzmann. 1998. Epstein-Barr virus small RNA (EBER) genes: differential regulation during lytic viral replication. J. Virol. 72:9323-9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howe, J. G., and M. D. Shu. 1989. Epstein-Barr virus small RNA (EBER) genes: unique transcription units that combine RNA polymerase II and III promoter elements. Cell 57:825-834. [DOI] [PubMed] [Google Scholar]
- 9.Howe, J. G., and J. A. Steitz. 1986. Localization of Epstein-Barr virus-encoded small RNAs by in situ hybridization. Proc. Natl. Acad. Sci. USA 83:9006-9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwakiri, D., Y. Eizuru, M. Tokunaga, and K. Takada. 2003. Autocrine growth of Epstein-Barr virus-positive gastric carcinoma cells mediated by an Epstein-Barr virus-encoded small RNA. Cancer Res. 63:7062-7067. [PubMed] [Google Scholar]
- 11.Iwakiri, D., T. S. Sheen, J. Y. Chen, D. P. Huang, and K. Takada. 2005. Epstein-Barr virus-encoded small RNA induces insulin-like growth factor 1 and supports growth of nasopharyngeal carcinoma-derived cell lines. Oncogene 24:1767-1773. [DOI] [PubMed] [Google Scholar]
- 12.Jeffrey, I. W., S. Kadereit, E. F. Meurs, T. Metzger, M. Bachmann, M. Schwemmle, A. G. Hovanessian, and M. J. Clemens. 1995. Nuclear localization of the interferon-inducible protein kinase PKR in human cells and transfected mouse cells. Exp. Cell Res. 218:17-27. [DOI] [PubMed] [Google Scholar]
- 13.Kitagawa, N., M. Goto, K. Kurozumi, S. Maruo, M. Fukayama, T. Naoe, M. Yasukawa, K. I. Hino, T. Suzuki, S. Todo, and K. Takada. 2000. Epstein-Barr virus-encoded poly(A)-RNA supports Burkitt's lymphoma growth through interleukin-10 induction. EMBO J. 19:6742-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komano, J., S. Maruo, K. Kurozumi, T. Oda, and K. Takada. 1999. Oncogenic role of Epstein-Barr virus-encoded RNA in Burkitt's lymphoma cell line Akata. J. Virol. 73:9827-9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komano, J., M. Sugiura, and K. Takada. 1998. Epstein-Barr virus contributes to the malignant phenotype and to apoptosis resistance in Burkitt's lymphoma cell line Akata. J. Virol. 72:9150-9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koromilas, A. E., S. Roy, G. N. Barber, M. G. Katze, and N. Sonenberg. 1992. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science 257:1685-1689. [DOI] [PubMed] [Google Scholar]
- 17.Kostura, M., and M. B. Mathews. 1989. Purification and activation of the double-stranded RNA-dependent eIF-2 kinase DAI. Mol. Cell. Biol. 9:1576-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar, A., Y. L. Yang, V. Flati, S. Der, S. Kadereit, A. Deb, J. Haque, L. Reis, C. Weissmann, and B. R. Williams. 1997. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 16:406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam, N., M. L. Sandberg, and B. Sugden. 2004. High physiological levels of LMP1 result in phosphorylation of eIF2α in Epstein-Barr virus-infected cells. J. Virol. 78:1657-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerner, M. R., N. C. Andrews, G. Miller, and J. A. Steitz. 1981. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA 78:805-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maran, A., and M. B. Mathews. 1988. Characterization of the double-stranded RNA implicated in the inhibition of protein synthesis in cells infected with a mutant adenovirus defective for VA RNA. Virology 164:106-113. [DOI] [PubMed] [Google Scholar]
- 22.Meurs, E., K. Chong, J. Galabru, N. S. Thomas, I. M. Kerr, B. R. Williams, and A. G. Hovanessian. 1990. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 62:379-390. [DOI] [PubMed] [Google Scholar]
- 23.Meurs, E. F., J. Galabru, G. N. Barber, M. G. Katze, and A. G. Hovanessian. 1993. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 90:232-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nanbo, A., K. Inoue, K. Adachi-Takasawa, and K. Takada. 2002. Epstein-Barr virus RNA confers resistance to interferon-α-induced apoptosis in Burkitt's lymphoma. EMBO J. 21:954-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson, D. W., A. Ali, N. A. Thornberry, J. P. Vaillancourt, C. K. Ding, M. Gallant, Y. Gareau, P. R. Griffin, M. Labelle, and Y. A. Lazebnik. 1995. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376:37-43. [DOI] [PubMed] [Google Scholar]
- 26.Patel, C. V., I. Handy, T. Goldsmith, and R. C. Patel. 2000. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J. Biol. Chem. 275:37993-37998. [DOI] [PubMed] [Google Scholar]
- 27.Patel, R. C., and G. C. Sen. 1998. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 17:4379-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel, R. C., D. J. Vestal, Z. Xu, S. Bandyopadhyay, W. Guo, S. M. Erme, B. R. Williams, and G. C. Sen. 1999. DRBP76, a double-stranded RNA-binding nuclear protein, is phosphorylated by the interferon-induced protein kinase, PKR. J. Biol. Chem. 274:20432-20437. [DOI] [PubMed] [Google Scholar]
- 29.Poppers, J., M. Mulvey, C. Perez, D. Khoo, and I. Mohr. 2003. Identification of a lytic-cycle Epstein-Barr virus gene product that can regulate PKR activation. J. Virol. 77:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romano, P. R., M. T. Garcia-Barrio, X. Zhang, Q. Wang, D. R. Taylor, F. Zhang, C. Herring, M. B. Mathews, J. Qin, and A. G. Hinnebusch. 1998. Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2α kinases PKR and GCN2. Mol. Cell. Biol. 18:2282-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosa, M. D., E. Gottlieb, M. R. Lerner, and J. A. Steitz. 1981. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol. Cell. Biol. 1:785-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruf, I. K., P. W. Rhyne, C. Yang, J. L. Cleveland, and J. T. Sample. 2000. Epstein-Barr virus small RNAs potentiate tumorigenicity of Burkitt lymphoma cells independently of an effect on apoptosis. J. Virol. 74:10223-10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruf, I. K., P. W. Rhyne, H. Yang, C. M. Borza, L. M. Hutt-Fletcher, J. L. Cleveland, and J. T. Sample. 1999. Epstein-Barr virus regulates c-MYC, apoptosis, and tumorigenicity in Burkitt lymphoma. Mol. Cell. Biol. 19:1651-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp, T. V., M. Schwemmle, I. Jeffrey, K. Laing, H. Mellor, C. G. Proud, K. Hilse, and M. J. Clemens. 1993. Comparative analysis of the regulation of the interferon-inducible protein kinase PKR by Epstein-Barr virus RNAs EBER-1 and EBER-2 and adenovirus VAI RNA. Nucleic Acids Res. 21:4483-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu, N., A. Tanabe-Tochikura, Y. Kuroiwa, and K. Takada. 1994. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt's lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J. Virol. 68:6069-6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spanggord, R. J., M. Vuyisich, and P. A. Beal. 2002. Identification of binding sites for both dsRBMs of PKR on kinase-activating and kinase-inhibiting RNA ligands. Biochemistry 41:4511-4520. [DOI] [PubMed] [Google Scholar]
- 37.Stojdl, D. F., N. Abraham, S. Knowles, R. Marius, A. Brasey, B. D. Lichty, E. G. Brown, N. Sonenberg, and J. C. Bell. 2000. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J. Virol. 74:9580-9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swaminathan, S., B. S. Huneycutt, C. S. Reiss, and E. Kieff. 1992. Epstein-Barr virus-encoded small RNAs (EBERs) do not modulate interferon effects in infected lymphocytes. J. Virol. 66:5133-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swaminathan, S., B. Tomkinson, and E. Kieff. 1991. Recombinant Epstein-Barr virus with small RNA (EBER) genes deleted transforms lymphocytes and replicates in vitro. Proc. Natl. Acad. Sci. USA 88:1546-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toczyski, D. P., and J. A. Steitz. 1991. EAP, a highly conserved cellular protein associated with Epstein-Barr virus small RNAs (EBERs). EMBO J. 10:459-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toczyski, D. P., and J. A. Steitz. 1993. The cellular RNA-binding protein EAP recognizes a conserved stem-loop in the Epstein-Barr virus small RNA EBER 1. Mol. Cell. Biol. 13:703-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vuyisich, M., R. J. Spanggord, and P. A. Beal. 2002. The binding site of the RNA-dependent protein kinase (PKR) on EBER1 RNA from Epstein-Barr virus. EMBO Rep. 3:622-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, Y., S. A. Xue, G. Hallden, J. Francis, M. Yuan, B. E. Griffin, and N. R. Lemoine. 2005. Virus-associated RNA I-deleted adenovirus, a potential oncolytic agent targeting EBV-associated tumors. Cancer Res. 65:1523-1531. [DOI] [PubMed] [Google Scholar]
- 44.Williams, B. R. G. 1999. PKR; a sentinel kinase for cellular stress. Oncogene 18:6112-6120. [DOI] [PubMed] [Google Scholar]
- 45.Yajima, M., T. Kanda, and K. Takada. 2005. Critical role of Epstein-Barr virus (EBV)-encoded RNA in efficient EBV-induced B-lymphocyte growth transformation. J. Virol. 79:4298-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto, N., T. Takizawa, Y. Iwanaga, N. Shimizu, and N. Yamamoto. 2000. Malignant transformation of B lymphoma cell line BJAB by Epstein-Barr virus-encoded small RNAs. FEBS Lett. 484:153-158. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, F., P. R. Romano, T. Nagamura-Inoue, B. Tian, T. E. Dever, M. B. Mathews, K. Ozato, and A. G. Hinnebusch. 2001. Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J. Biol. Chem. 276:24946-24958. [DOI] [PubMed] [Google Scholar]