Abstract
The program of gene expression exhibited by herpes simplex virus during productive infection of cultured cells is well established; however, less is known about the regulatory controls governing reactivation from latency in neurons. One difficulty in examining gene regulation during reactivation lies in distinguishing between events occurring in initial reactivating cells versus events occurring in secondarily infected cells. Thus, two inhibitors were employed to block production of infectious virus: acyclovir, which inhibits viral DNA synthesis, and WAY-150138, which permits viral DNA synthesis but inhibits viral DNA encapsidation. Latently infected murine ganglia were explanted in the presence of either inhibitor, and then amounts of RNA, DNA, or infectious virus were quantified. In ganglia explanted for 48 h, the levels of five immediate-early and early RNAs did not exhibit meaningful differences between acyclovir and WAY-150138 treatments when analyzed by in situ hybridization or quantitative reverse transcription-PCR. However, comparative increases in viral DNA and RNA content in untreated ganglia suggested that virus was produced before 48 h postexplant. This was confirmed by the detection of infectious virus as early as 14 h postexplant. Together, these results suggest that high levels of viral gene expression at 48 h postexplant are due largely to the production of infectious virus and subsequent spread through the tissue. These results lead to a reinterpretation of previous results indicating a role for DNA replication in immediate-early and early viral gene expression; however, it remains possible that viral gene expression is regulated differently in neurons than in cultured cells.
A remarkable feature of herpes simplex virus (HSV) is its ability to establish a lifelong latent infection in hosts following acute disease. This latent stage and subsequent reactivations of infections, causing recurrent disease at peripheral sites, account for the high transmission rate and prevalence of HSV in the general population and are an attractive target for drug therapy. However, the molecular events that promote and regulate the shift from latency to reactivation are not well understood.
In the mouse eye model of infection, virus applied to the cornea gains access to axon termini and establishes latency in neurons of the trigeminal ganglia (TG) (44). In most mouse models, infectious virus is not detected during latency (38). The viral genome is detectable at the level of one to several thousand copies per neuron (15, 29, 31), but evidence of viral antigens during latency is rare (10, 16, 32). Only the LAT (latency-associated transcript) region of the viral genome produces abundant transcripts (39), although transcripts from other genes can be detected by sensitive reverse transcription-PCR (RT-PCR) assays (2, 3, 19, 43).
Compared with latency, the productive replication cycle of HSV in fully permissive cells is robust and dynamic, ending in destruction of host cells (reviewed in reference 30). The virus directs a regulated cascade of gene expression that can be grouped into three main kinetic classes (14). Upon entry into a cell, immediate-early (IE) genes are induced by a combination of virion components and cellular transcription factors. These genes, including ICP27, ICP4, and ICP0, act in part to up-regulate the early (E) genes. ICP8 and thymidine kinase (TK), along with the products of other early genes, carry out functions involved in viral DNA synthesis. The third gene class, the late (L) genes, is dependent upon DNA replication for maximal expression. Although this regulated program of gene expression has been studied extensively during in vitro productive infection, its relevance to events during reactivation from latency remains unclear.
Several investigations focusing on this issue have suggested that the progression of gene expression in neurons during initial infection and/or reactivation differs from that in permissive cells. Previous studies of ours, which measured gene expression during a 48-h explant reactivation from murine TG by in situ hybridization (ISH), observed that inhibition of viral DNA synthesis by pharmacologic inhibitors or genetic mutations resulted in an unexpected decrease in IE and E gene expression, events which precede viral DNA synthesis in productive infection (17). Thus, these results suggested that DNA synthesis might play a key role during reactivation. In uninhibited explants, the progressive detection of IE, E, and L genes over a 48-h period conformed to other reports suggesting that the reactivation process took approximately 48 h to complete (36, 37, 47). This, coupled with the detection of LATs in the same cells as IE, E, and L gene RNA hybridization, made it seem likely that the chronicled events were occurring during a single, initial replicative cycle. However, the possibility that secondary infections could occur within this time period and account for the high gene expression levels was not ruled out. Therefore, whether the decreased gene expression was a direct result of blocking DNA synthesis or was due to the attendant blocking of production of infectious virus and spread to neighboring cells was not resolved.
Nevertheless, more data in support of a role for DNA replication in gene expression in neurons were reported. In vitro studies comparing levels of HSV gene expression in Vero cells and rat peripheral neurons found that inhibition of viral DNA synthesis greatly reduced IE and E gene expression in neurons but not in Vero cells (26). Inhibition of DNA synthesis was also reported to reduce IE and E promoter activity in primary cells but not in Vero cells (41). In addition, it has been reported that during explant reactivation from murine TG, E and leaky late transcripts are detected before IE transcripts (43). On the other hand, a recent investigation of this question with rabbit TG found no deviation from the canonical regulatory order (28).
To enable examination of gene expression during reactivation in more detail, we have employed acyclovir (ACV), a well-established inhibitor of viral DNA replication, and WAY-150138, a newer inhibitor of viral DNA cleavage and packaging (45). The latter compound appears to preclude the incorporation of the UL6 portal protein and UL15 into capsids (25). Because WAY-150138 inhibits progeny virus production at a stage subsequent to viral DNA replication, it allows examination of a possible role of viral DNA synthesis in reactivation without the confounding effects of infectious virus production and spread. Here, we describe the use of this inhibitor in individual, explanted TG to distinguish between gene expression events confined to initial reactivating neurons and those events associated with spread through explanted tissue.
MATERIALS AND METHODS
Cells and viruses.
Strain Patton wild-type and 138R/5 drug-resistant viruses were gifts from M. van Zeijl and T. R. Jones, Wyeth Research. Procedures for propagation and assay of HSV on Vero cell monolayers have been described previously (7).
Compounds.
Acyclovir (acycloguanosine; Sigma) and WAY-150138 (a gift from M. van Zeijl and T. R. Jones, Wyeth Research) were dissolved in dimethyl sulfoxide (DMSO). Because WAY-150138 requires DMSO for solubility, all media were supplemented with 0.5% DMSO.
Plaque reduction assay.
Antiviral activity was assessed essentially as described previously (7), with the following modifications. After adsorption of virus, Vero cell monolayers were overlaid with media containing 2% newborn calf serum, 0.8% methylcellulose, 0.5% DMSO, and WAY-150138. After 3 days of incubation, the cells were fixed and then stained with crystal violet.
Dot blot assay for viral DNA replication.
Following a published method (20) with slight modifications, Vero cells in 24-well plates were infected at a multiplicity of infection of 5 or mock infected. After 1 h, cells were washed three times with cold phosphate-buffered saline (PBS) and then incubated a further 2 or 14 h in media containing 5 to 40 μg/ml WAY-150138, 0.5% DMSO; 200 μM ACV, 0.5% DMSO; only 0.5% DMSO; or no additives. After lysis in 0.4 N NaOH-10 mM EDTA, lysates were applied to a nylon membrane (GeneScreen Plus; PerkinElmer Life Sciences) with a dot blot apparatus (Schleicher & Schuell) and hybridized with a 32P-labeled ICP0 oligonucleotide probe, ICP0-3 (oligonucleotide sequence can be found in the supplemental material at http://coen.med.harvard.edu). The amount of hybridization was quantified with a Personal Molecular Imager FX and Quantity One software (Bio-Rad) and compared with that obtained with a dilution series of lysates from untreated, infected cells.
Mouse infection.
Male ICR mice (Harlan) were infected as described previously (21), with minor changes. Eight-week-old mice were anesthetized with 85 mg sodium pentobarbital/kg of body weight (Nembutal; Abbott Laboratories) or a mixture delivering 120 mg/kg ketamine and 18 mg/kg xylazine (Sigma). Either 2 × 105 or 2 × 106 PFU of virus was dropped onto both eyes in a volume of 4 μl. Animal housing and all procedures involving mice were approved by the Harvard Medical School Institutional Animal Care and Use Committee in accordance with federal guidelines.
Explant reactivation.
At a minimum of 30 days postinfection, mice were sacrificed, and TG were removed, bisected, and placed in 1.5 ml reactivation media (Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 200 U/ml penicillin, 200 μg/ml streptomycin, 0.25 μg/ml amphotericin B [Fungizone], and 0.5% DMSO) with or without inhibitors (17, 37). Within 70 min, TG were transferred to a 37°C incubator. Hours postexplant were counted from the time of sacrifice.
In situ hybridization.
Explanted TG were serially cryosectioned lengthwise to a thickness of 8 μm and hybridized with DNA probes (17) labeled with α-35S-dATP and α-35S-dCTP, as described previously (12).
RNA/DNA isolation.
Explanted TG were quickly rinsed in cold PBS, frozen in liquid nitrogen, and stored at −80°C. Frozen TG were homogenized in 650 μl of guanidine thiocyanate solution (4). For DNA analysis, 1/10 volume of the homogenate was removed and mixed with 150 μl water, 20 μl 3 M sodium acetate, and 600 μl ethanol. Precipitates were treated with proteinase K as described previously (19), except that samples were vigorously vortexed every 30 min and the addition of Tween and the 80°C incubation were omitted. Aliquots were taken directly for real-time PCR. RNA was purified from the remaining 9/10 of the TG homogenate essentially as described previously (4).
DNA and RNA standards.
(i) Mouse DNA standards. Mouse brain DNA was isolated and treated with RNase as described previously (40) and quantified by spectrophotometry at 260 nm. Diluted aliquots were used directly in real-time PCRs. (ii) HSV DNA standards. Transfection-quality HSV DNA was purified from strain Patton-infected Vero cells, as described previously (22). Concentrations were measured by comparison to mass standards electrophoresed on an agarose gel. Tenfold serial dilutions were mixed with 1/10 of a mock-infected TG lysate and then processed along with the experimental sample lysates, as described above. (iii) Mouse RNA standards. Mouse brain RNA was purified in a scaled-up version of the protocol for ganglionic RNA, and concentrations were determined by spectrophotometry at 260 nm. Serial dilutions of this RNA were subjected to DNase, cDNA synthesis, and PCR protocols, as described below. (iv) HSV RNA standards. Plasmid pKS + 5′ICP8 (kindly provided by M. F. Kramer) was constructed by cloning a 2.3-kb BamHI fragment from plasmid p8B-S (11), containing sequences in the 5′ region of the ICP8 gene, into the BamHI site of pBluescript II KS (Stratagene), downstream from the T3 RNA polymerase promoter. Similar transcription plasmids containing ICP0, ICP4, or tk sequences have been described previously (19). Synthetic viral transcripts of approximately 1.5 to 2.6 kb were generated from these plasmids via in vitro transcription using T7 or T3 RNA polymerases (Promega) as described previously (19), with the following modifications. Linearized plasmids were transcribed for 4 h at 37°C in the presence of 600 Ci/mmol α-35S-CTP (MP Biomedicals) and an RNase inhibitor, SUPERase•In (Ambion). Afterwards, reactions were treated with DNase and RNA was purified with an RNeasy kit (QIAGEN). The percentage of α-35S-CTP incorporated into trichloroacetic acid-precipitable material was used to calculate RNA concentration (19). Synthetic HSV RNAs representing intended target genes were combined and serially diluted. Several aliquots of each dilution were mixed with 5 μg of mouse brain RNA to generate replicate standard sets. These standards were then subjected to DNase, cDNA synthesis, and PCR protocols along with TG samples, as described below. In some cases, preparation of standards was scaled up while ensuring that efficiency and recovery were not altered.
DNase digestion.
To completely digest the large amount of viral DNA resulting from reactivated virus, the following DNase protocol was devised. Purified TG RNA or RNA standards (20 μl) were mixed with 10 μl 10× buffer, 5 μl SUPERase•In, and 5 μl TURBO DNase (all Ambion reagents) in a total volume of 95 μl. The reactions were incubated at 37°C for 90 min, with the addition of 5 μl more DNase after 45 min. The reactions were stopped by adding 10.7 μl of 58 mM EDTA-850 μg/ml baker's yeast tRNA (as carrier; Roche)-11 μl 2 M sodium acetate, pH 4; and 120 μl acid phenol:chloroform (5:1). These mixtures were extracted once and then back-extracted following the addition of 80 μl 0.2 M sodium acetate, pH 4. The pooled aqueous layers were extracted with 200 μl chloroform:isoamyl alcohol (49:1) and precipitated with ethanol at −80°C.
RT-PCR.
DNase-treated, precipitated RNA was collected by centrifugation, washed with 70% ethanol, air dried for 8 min, and resuspended in water:SUPERase•In (100:1). Annealing of reverse primers and reverse transcription were carried out essentially as described previously (19). Each annealed sample was split equally between reaction mixtures with and without avian myeloblastosis virus reverse transcriptase (Promega). Mouse cDNA was assayed by real-time PCR, as described below. PCR assays for HSV cDNA were performed according to the general parameters described previously (19), except that the reaction volumes were 50 μl and included 2 or 3 μl of cDNA, depending on the gene assayed. Conditions for the ICP8 assay were as follows: 3 mM MgCl2, 10% glycerol, and a 5 min, 94°C incubation followed by 30 cycles comprising 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C. Primer sequences are reported in the supplemental material (http://coen.med.harvard.edu). Conditions and primer sequences for other assays have been described previously (3, 19). Replicate sets of cDNA standards were subjected to PCR alongside batches of experimental TG samples.
Real-time PCR.
Real-time PCR assays, based on those developed by M. F. Kramer (unpublished data), were performed as follows to quantify viral DNA, mouse DNA, and mouse cDNA; primer and probe sequences can be found in the supplemental material (http://coen.med.harvard.edu). Viral DNA was assayed in 20 μl using 2.5 μl of TG DNA or standards, 0.1 μM primers for the tk gene, and SYBR green PCR master mix (PerkinElmer Life Sciences). Mouse DNA was assayed in 20 μl using 2 μl of purified TG DNA or standards, 0.1 μM primers for the mouse glyceraldehyde-3-phosphate dehydrogenase (mGAPDH) gene, and SYBR green PCR master mix. Mouse RNA was assayed in 20 μl using 1 μl of cDNA made from TG or mouse RNA standards, 0.1 μM mGAPDH primers, 0.1 μM 6-carboxyfluorescein/6-carboxytetramethylrhodamine dual-labeled probe (Integrated DNA Technologies), and a Brilliant QPCR core reagent kit (Stratagene). All reactions were performed by an ABI Prism 7700 sequence detection system. Semilog plots of threshold cycle (fractional cycle at which an arbitrary amount of product is detected) versus input DNA were analyzed by linear regression, and the nucleic acid contents of unknown samples were interpolated (see the supplemental material at http://coen.med.harvard.edu).
Gel transfers and dot blots.
Southern blotting of polyacrylamide gels was performed as described previously (19). Dot blotting was performed essentially as recommended by the GeneScreen manufacturer. In brief, membranes were soaked in 0.4 M Tris-HCl, pH 7.5, for 5 min and briefly subjected to a vacuum before 20 μl of each PCR premixed with 80 μl cold 0.5 N NaOH-12.5 mM EDTA was loaded onto the dot blot apparatus (see above). After 25 min, vacuum was applied to empty the wells, followed by a 5-min incubation with 200 μl 0.4 N NaOH and final vacuum. Blots were neutralized in 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) and cross-linked in a UV Stratalinker (Stratagene). Gel blots and dot blots were incubated with 32P-end-labeled oligonucleotide probes as previously described (19). ICP8 probe sequence can be found in the supplemental material (http://coen.med.harvard.edu); ICP4, tk, and ICP0 probes have been published (3, 19). Membranes were exposed to phosphor screens for quantification of hybridized probe. Log-log plots of hybridization signal (in phosphorimager units) versus input RNA copies were analyzed by linear regression, and the RNA contents of unknown samples were interpolated. Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, Calif.).
Detection of infectious virus after explant.
After explant incubations, TG were washed twice on ice with PBS, 0.5% DMSO for 10 min to remove replication inhibitors. (Washes at 25°C for 15 to 60 min were detrimental because they allowed a fraction of TG to support reactivation after the explant period). TG were then frozen in culture media at −80°C, thawed at 37°C, homogenized, and sonicated as previously described (21). The homogenates were centrifuged for 15 min at 1,300 × g, and the supernatants were plated on Vero cell monolayers. After 1 h of incubation, methyl cellulose-containing medium was overlaid, and plaques were allowed to form for 3 days.
RESULTS
To investigate if viral DNA synthesis could account for the elevated gene expression observed with previous ISH experiments (17), a method to allow viral DNA synthesis to proceed while blocking production of infectious virus was sought. Attempts to address this question by using a temperature-sensitive HSV mutant blocked at a late stage of replication were hindered by deterioration of the ganglion tissue (unsuitable for ISH) and inhibition of reactivation of wild-type virus at elevated temperatures (D. A. Garber and D. M. Knipe, unpublished data). As the explant model is well suited for pharmacological studies, the development of an inhibitor of a late event in viral replication provided a solution. This compound, WAY-150138, along with the wild-type strain Patton virus and a WAY-150138-resistant derivative, 138R/5 (with a resistance-conferring mutation mapped to the UL6 gene), were kindly provided by T. R. Jones and M. van Zeijl, Wyeth Research (45).
Specificity and efficacy of WAY-150138 in vitro.
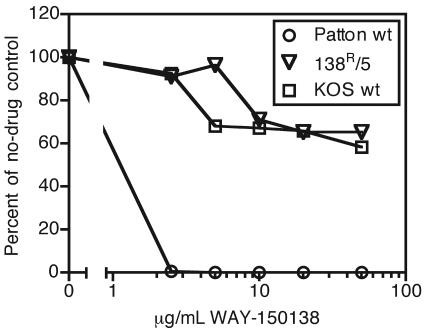
WAY-150138 inhibits the replication of some strains of HSV-1, including Patton, with a 50% inhibitory concentration of 0.2 to 0.7 μg/ml (45). Plaque reduction assays demonstrated that wild-type strain Patton was prevented from forming plaques in 10 μg/ml WAY-150138, while the drug-resistant derivative and wild-type strain KOS were subject to no more than a 40% reduction in plaque number in up to 50 μg/ml (Fig. 1).
FIG. 1.
Plaque reduction assay with WAY-150138. Patton wild-type (wt); 138R/5 mutant, which is resistant to WAY-150138; and KOS wt viruses were assayed for plaque formation in the presence of WAY-150138. Average plaque numbers from triplicate wells were calculated as percentages of plaques formed relative to DMSO vehicle treatment.
The value of WAY-150138 in our proposed experiments lay in its reported ability to block production of progeny virus while allowing viral DNA replication to proceed (45). To confirm that this compound had little or no effect on viral DNA replication, the viral DNA content of cells infected in the presence of WAY-150138 was measured by dot blotting and Southern hybridization (Fig. 2). Both wild-type strain Patton and the 138R/5 resistant mutant virus exhibited no more than a 35% reduction in viral DNA levels when treated with WAY-150138 (Fig. 2). This effect, which did not correlate with the difference in susceptibility of these two viruses, was minor compared to the inhibition achieved by acyclovir (greater than 95%) (Fig. 2) and to the effect of WAY-150138 on plaque formation by wild-type strain Patton. In addition, two groups have reported finding no effect of WAY-150138 on synthesis of certain viral proteins (25, 45).
FIG. 2.
Effects of WAY-150138 and ACV on viral DNA replication. (A) Cells were mock infected (first row) or infected (all other rows) with wild-type virus (left) or 138R/5 virus (right) in triplicate and incubated for 3 h (second row) or 15 h (all other rows) in media containing DMSO alone, ACV (plus DMSO), or WAY-150138 (plus DMSO), as indicated at the far left. Lysates were applied to a dot blot and hybridized with a probe specific for viral DNA sequences. The mean percentage of hybridization, representing the amount of viral DNA synthesized under each treatment relative to the DMSO-treated control, is labeled to the right of each blot. (B) Amounts of hybridization were quantified using 1:3 serial dilutions of triplicate, untreated, infected cell lysates. For wild-type and 138R/5 infections, the lowest detectable serial dilutions represented 5% and 4% of the DMSO-treated controls, respectively. h.p.i., hours postinfection.
Specificity and efficacy of WAY-150138 and behavior of Patton viruses in vivo.
In moving from in vitro tests to in vivo studies, we needed to assess (i) the ability of the wild-type Patton strain to establish latency and subsequently reactivate in our murine eye model and (ii) the efficacy of WAY-150138 when used to treat whole ganglion tissue. Conditions for animal studies were designed to achieve reactivatable latent infections that were comparable to those achieved via our standard protocol using KOS strain virus while limiting the mortality of the more virulent Patton viruses (unpublished data). To assess the ability of these viruses to reactivate and the ability of the drugs to prevent reactivation, latently infected TG were harvested and incubated for 48 h with or without drugs and then scored for reactivation by detection of infectious virus in TG homogenates (Table 1). TG latently infected with wild-type virus or 138R/5 virus and not subjected to drug treatment reactivated 100% of the time, indicating that all TG were latently infected and reactivation could be readily and reliably induced over 48 h. Six latently infected TG (frozen in liquid nitrogen directly after harvest) yielded no virus, demonstrating that detected virus was a product of reactivation (data not shown). In initial experiments (Table 1, third column), reactivation by wild-type virus showed sensitivity to both ACV and WAY-150138. However, reactivation by 138R/5 showed sensitivity only to ACV, with 100% of TG yielding virus when treated with WAY-150138. Although reactivated virus was observed for 4 of 15 wild-type-infected TG treated with ACV and 3 of 15 treated with WAY-150138, only a few PFU were detected for each TG (while we estimated that >1,500 PFU were produced by each untreated TG). Further testing indicated these few PFU were likely produced after the explant incubation, during procedures to wash out the drugs before freezing (data not shown). In subsequent experiments in which mice were infected with 10-fold-lower inocula (to reduce mortality), inhibitor concentrations were doubled and shorter washes were performed on ice. Under these conditions, complete inhibition of wild-type virus production was reproducibly achieved by both compounds (Table 1, last column), confirming that the drugs penetrated the tissue and were effective for the duration of explant.
TABLE 1.
Performance of drugs and viruses in 48-h explants
| Virus | Drug | Fraction of TG reactivated
|
|
|---|---|---|---|
| Earlier conditionsa | Later conditionsb | ||
| Wild type | None | 15/15 | 5/5 |
| Wild type | ACV | 4/15 | 0/21 |
| Wild type | WAY-150138 | 3/15 | 0/28 |
| 138R/5 | None | 5/5 | NDc |
| 138R/5 | ACV | 0/3 | ND |
| 138R/5 | WAY-150138 | 7/7 | ND |
| Mock | None | 0/8 | 0/4 |
PFU inoculated, 2 × 106; 100 μM ACV or 10 μg/ml WAY-150138; 15- to 60-min washes at 25°C; data collated from three experiments.
PFU inoculated, 2 × 105; 200 μM ACV or 20 μg/ml WAY-150138; 5- to 15-min washes at 4°C; data collated from four experiments.
ND, not done.
In situ hybridization of explanted TG in the presence of WAY-150138.
To evaluate the possible contribution of secondary infections to viral gene expression in reactivation experiments, we used wild-type Patton or 138R/5 virus to infect mice and WAY-150138 or ACV to inhibit viral multiplication. Latently infected TG were explanted for 48 h in the presence or absence of these inhibitors and then frozen and cryosectioned. ISH was performed using probes for RNA transcripts from a marker of latent infection (LATs), an immediate-early gene (ICP27) or an early gene (ICP8). Hybridization with the LAT probe yielded an average of 4.0 to 7.0 signals (positive cells) per section under all three treatment conditions (data not shown), consistent with past experiments using strain KOS (17). Untreated wild-type-infected and 138R/5-infected TG displayed high levels of hybridization with both productive-cycle gene probes: 9.1 and 9.8 signals/section, respectively, for ICP27; and 16 and 11 signals/section, respectively, for ICP8 (Table 2). High signal levels were also observed for WAY-150138-treated, 138R/5 samples (Table 2), confirming that the mutant virus was exhibiting resistance to WAY-150138 and that the drug did not cause any nonspecific suppression of gene expression in the tissue.
TABLE 2.
In situ hybridization of TG undergoing reactivation in the presence of inhibitors
| Virus | Drug | No. of TG |
ICP27 probe
|
ICP8 probe
|
||||
|---|---|---|---|---|---|---|---|---|
| Sections | Signalsa | Signals/section | Sections | Signalsa | Signals/section | |||
| Wild type | None | 3 | 48 | 439 | 9.1 | 43 | 708 | 16 |
| Wild type | ACV | 4 | 48 | 4 | 0.08 | 60 | 5 | 0.08 |
| Wild type | WAY-150138 | 5 | 48 | 10 | 0.21 | 56 | 16 | 0.29 |
| 138R/5 | None | 1 | 18 | 176 | 9.8 | 32 | 353 | 11 |
| 138R/5 | WAY-150138 | 2 | 26 | 559 | 22 | 24 | 619 | 26 |
Each signal corresponds to hybridization observed in a single cell.
Consistent with the results of Kosz-Vnenchak et al. (17), ACV treatment resulted in a large reduction in hybridization for ICP27 and ICP8 probes: only 0.08 signals per section were counted for both, more than a 2-log reduction compared with untreated sections (Table 2). However, a large reduction was also observed for samples treated with WAY-150138, the encapsidation inhibitor (Table 2). These data were bolstered by observation of the intensity of hybridization: in the drug-treated, wild-type virus sections, only weak hybridization in individual neurons was seen, suggestive of restricted viral activity, whereas in the untreated sections, neurons contained strong hybridization signals, similar to the results of Kosz-Vnenchak et al. (17).
Viral DNA quantification in explanted TG.
This ISH study suggested that similarly low levels of IE and E RNA were expressed over 48 h of reactivation when infectious virus production and viral spread were blocked. Therefore, for more sensitive and quantitative assessments of viral gene activity during reactivation, explanted TG were analyzed for DNA and RNA content by using real-time PCR and RT-PCR. Following infection and explant protocols similar to those used for the ISH study, individual TG homogenates were divided for use in DNA and RNA quantification procedures.
For DNA analyses, viral and mouse DNA standards were amplified by real-time PCR alongside DNA from experimental ganglia, using primers for sequences within the viral tk or mouse GAPDH genes. Standard curves were used to interpolate the amounts of DNA in unknown samples and furnish a measure of amplification efficiency (detailed in the supplemental material at http://coen.med.harvard.edu). To account for differential recovery, for each TG, viral DNA was normalized to mouse DNA detected and measured in a similar manner.
The levels of viral DNA achieved with ACV-treated and untreated TG were similar for wild-type and mutant viruses, providing evidence that they established comparable latent infections. For wild-type virus, the mean viral DNA levels in the presence of ACV over 48 h did not rise above latent levels (0 explant hours), which confirmed the activity of ACV in blocking viral DNA synthesis during explant (Fig. 3). A statistically significant sevenfold increase in DNA content seen for TG treated with WAY-150138 for 48 h likely represented the amount of viral DNA replicated in cells stimulated to reactivate. By comparison, there was a nearly-50-fold difference in DNA content between WAY-150138-treated and untreated ganglia, which could be evidence of secondary infections within 48 h. In 138R/5-infected TG, the amount of DNA detected in the presence of WAY-150138 was similar to that for the no-drug control. This was expected since 138R/5 displays considerable resistance to the concentrations of WAY-150138 used in this assay (Fig. 1). Similar results were obtained when mice were infected with a 10-fold-lower inoculum and TG were treated with doubled concentrations of inhibitors (unpublished data).
FIG. 3.
Viral genome copies per TG during explant reactivation. TG from mice infected with 2 × 106 PFU wild-type Patton or 138R/5 virus were harvested and either frozen directly (latent; 0 h) or incubated for 48 h with 100 μM ACV, 10 μg/ml WAY-150138, or no drug. Viral DNA content was measured by quantitative, real-time PCR and normalized to mouse DNA content. Means ± standard deviations are plotted. For wild type, n = 6 for 0 h, and n = 9 for 48 h; for 138R/5, n = 3. Mock-infected TG were negative for viral DNA. *, P = 0.0104 by two-sided Student's t test. The nine WAY-150138-treated, wild-type-infected ganglia comprised seven ganglia with viral DNA levels above those present in any of the ACV-treated, wild-type-infected ganglia, and two ganglia whose lower viral DNA contents resulted in relatively large error bars for this group.
Viral RNA quantification in explanted TG.
To confirm and extend the RNA expression data gathered in the ISH study, we measured the levels of two IE RNAs (ICP4 and ICP0) and two E RNAs (ICP8 and tk) for the same TG analyzed in Fig. 3. Total RNA was isolated from individual TG and reverse transcribed with a battery of gene-specific primers. Every RNA sample was divided into two aliquots, one to which reverse transcriptase was added and one where it was omitted. Each cDNA sample was then divided among several PCRs for amplification of viral transcripts or mouse GAPDH transcripts. The assays for ICP0, ICP4, and tk have been described previously (3, 19), but the ICP8 assay was newly developed. All assays were optimized to yield a single major amplified species, confirmed by gel electrophoresis and Southern blot hybridization (Fig. 4A). This permitted quantification of large numbers of samples by using dot blots of the PCR products (Fig. 4B). For all four PCR assays, the primer regions and intervening cDNA sequences contained no mismatches when comparing Patton viral DNA to the plasmids used to make the RNA standards. Standard curves generated from ICP8 (Fig. 4C) and mGAPDH (see the supplemental material at http://coen.med.harvard.edu) RT-PCR displayed a linearity and reproducibility typical of all of the RT-PCR assays.
FIG. 4.
Quantitative ICP8 RT-PCR. (A) Southern blot of polyacrylamide gel containing ICP8 RT-PCR products. (Left) TG samples (either infected or mock infected, as indicated). (Right) RNA standards representing the indicated number of copies per 90% of a TG. RT indicates whether reverse transcriptase was added (+) to one-half of the cDNA synthesis reaction or omitted (−). The DNA size marker is shown in the center, and the sizes of certain DNA markers are labeled in bp. ntc, no template control for PCR. (B) Portions of dot blots with RT-PCR products from TG samples (either infected or mock infected, as indicated in panel a) and standards. Panels b to e contain replicate sets of RNA standards representing the indicated number of RNA copies per 90% of a TG. RT indicates whether reverse transcriptase was added (+) to one-half of the cDNA synthesis reaction or omitted (−). (C) Standard curve of ICP8 RT-PCR products. Data are derived from panel B in the following manner: filled and open triangles/solid lines represent data from the same cDNA series subjected to separate PCRs and applied to separate blots (panels c and e, respectively); circles/dashed line (panel b) and squares/dotted line (panel d) represent two further, independent cDNA series, each loaded on one of the two blots described above. This standard range encompassed all experimental samples. PIU, phosphorimager units.
The mean numbers of copies of viral RNAs were measured for latently infected TG and for TG that had been explanted for 48 h in the presence of ACV, WAY-150138, or no drug (Fig. 5). Wild-type-infected TG left untreated and 138R/ 5-infected TG left untreated or treated with WAY-150138 exhibited the highest RNA contents. For some of these TG, RNA levels were beyond the linear range of the RT-PCR assays, which were designed to optimize detection of possible differences between the lower RNA levels expected with drug-treated samples. These TG were assigned an RNA content equivalent to the upper quantification limit of the assay, yielding values that represent minimum mean RNA copies per TG. Thus, this experiment likely underestimates the increase in RNA levels in untreated versus treated or latently infected ganglia.
FIG. 5.
Viral RNA copies per TG in latent and reactivating TG. TG from mice infected with 2 × 106 PFU wild-type Patton (A) or 138R/5 (B) were harvested and either frozen directly (latent) or incubated for 48 h with 100 μM ACV, 10 μg/ml WAY-150138, or no drug. Indicated viral RNA species were measured by quantitative RT-PCR. Undetectable samples were calculated as zero. Means ± standard errors of the means are plotted. (A) n = 6 for latent, and n = 11 for others; (B) n = 3. Mock-infected TG were negative for viral RNA. ‡, representation of minimum values, because one or more TG contained RNA levels greater than the linear range of the assay.
For wild-type-infected TG (Fig. 5A), all IE and E RNA species showed a clear increase over latent levels during explant. However, compared to TG in which viral DNA replication was blocked (ACV treated), TG in which viral DNA replication was free to proceed (WAY-150138 treated) did not exhibit measurable increases in IE or E gene expression. This result is similar to those observed with cell culture, in which immediate-early and early gene expression were not reduced by inhibition of viral DNA synthesis (14). Compared to results for these drug-treated ganglia, increases were readily apparent with ganglia that were untreated and allowed to freely reactivate over a 48-h period, substantiating the ISH results and showing again that both ACV and WAY-150138 were restricting some source of viral transcripts.
The same assays were also performed on TG latently infected with the drug-resistant 138R/5 virus. Figure 5B shows that levels of RNA expressed in the presence of ACV were similar to those achieved by the wild-type virus with the same drug and that all four RNAs displayed a marked increase in expression in the presence of WAY-150138. These results indicated that WAY-150138 did not exhibit nonspecific, suppressive effects on the expression of IE and E RNAs.
Timing of replication cycle during reactivation.
The ISH, PCR, and RT-PCR studies all suggested that secondary infection could be under way by 48 h postexplant (h p.e.). To investigate how early infectious virus could be detected during reactivation in our system, we explanted TG latently infected with either wild-type Patton or KOS virus for between 2 and 26 h and then measured virus yield. Both strains of virus achieved 100% reactivation by 24 h p.e. (Table 3), much earlier than the 4 days previously reported for 100% reactivation in a similar explant model (37), on which interpretations of earlier ISH data had been based (17). Additionally, virus could be reproducibly detected in a fraction of TG explanted for only 14 h: 27% of Patton-infected TG and 22% of KOS-infected TG. Although none of the KOS-infected TG were positive for virus at 16 h p.e., two independent experiments yielded TG positive for virus at 14 h p.e. The difference in the fractions of positive TG at 14 and 16 h p.e. is not statistically significant. Overall, the data support the authenticity of these early reactivation events. In addition, this early detection of reactivated virus is concordant with similar experiments using heat shock for induction of reactivation in vivo, in which virus was also detected at 14 h postinduction (34).
TABLE 3.
Infectious virus production during explant reactivation
| Virus production in TG | Result for virus strain or mock infection at indicated h p.e.
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KOS
|
Mka
|
Patton
|
Mk
|
|||||||||||
| 2 | 14 | 16 | 18 | 24 | 24 | 2 | 14 | 16 | 18 | 20 | 24 | 26 | 26 | |
| No. positive | 1 | 4 | 0 | 4 | 6 | 0 | 0 | 6 | 10 | 4 | 7 | 4 | 6 | 0 |
| No. negative | 19 | 14 | 18 | 4 | 0 | 3 | 8 | 16 | 12 | 4 | 1 | 0 | 0 | 2 |
| Total no. tested | 20 | 18 | 18 | 8 | 6 | 3 | 8 | 22 | 22 | 8 | 8 | 4 | 6 | 2 |
| % Positive | 5 | 22 | 0 | 50 | 100 | 0 | 0 | 27 | 46 | 50 | 88 | 100 | 100 | 0 |
Mk, mock infected.
The ranges of PFU found in individual explanted TG are depicted in Fig. 6. To test the recovery of infectious virus in this protocol, we spiked uninfected TG with 10 to 50 PFU of virus just before homogenization and continued to process as usual. The number of PFU recovered from spiked samples was no more than 50% lower than the titer of the spiked virus (data not shown). Virus could have been lost during the freeze/thaw, homogenization, or sonication procedures or by association with membranes in the spun-out cellular debris. This protocol aimed to ensure that all TG cells were destroyed, preventing any from assembling virus after drugs were removed. Numbers of PFU from TG that produced virus ranged from 1 to 552, with no obvious differences in distribution between KOS and Patton. Although very low amounts of PFU were detected in some TG even at 24 h p.e., there is clearly a time-dependent increase in mean PFU produced. The yield of infectious virus at 24 h p.e. was lower than that produced by TG explanted for 48 h, which typically achieved complete obliteration of the monolayer in the same protocol (>1,500 PFU). This suggested that by 48 h p.e., virus had time to significantly multiply and spread within TG. We intended the 2-h explant treatment to be an additional negative control but were surprised to find 1 PFU of virus detected in a single TG for strain KOS (Table 3 and Fig. 6). Possible sources of this virus are considered in Discussion.
FIG. 6.
Infectious virus production during explant reactivation. TG latently infected with wild-type KOS (A) or Patton (B) virus (inoculated with 2 × 106 and 2 × 105 PFU per eye, respectively) were harvested and incubated for the indicated times before being washed, frozen, and processed for infectious virus assay. Circles represent only TG which were positive for infectious virus, but bars represent mean values which include zeros for negative TG. Data are from two experiments for each viral strain.
The demonstration that virus was reproducibly present in some explanted TG as early as 14 h p.e. and present in all TG by 24 h p.e. suggested that reactivating virus could complete two or even three rounds of replication within 48 h. This was in agreement with the data from ISH and RT-PCR studies, which indicated that removing blocks to DNA replication and packaging led to much higher levels of expression over 48 h, whereas comparing ACV treatment to WAY-150138 treatment showed little or no difference. Viral DNA content of explanted TG also displayed large increases in untreated TG samples compared to drug-treated samples. Taken together, these data strongly suggest that production of infectious virus, followed by viral spread, accounts for the bulk of increase in RNA expression detected after 24 h p.e. in untreated, explanted TG.
DISCUSSION
In early, classic studies, investigators used metabolic inhibitors of DNA replication, transcription, or protein synthesis to probe the gene expression patterns of HSV in cell culture (14, 27, 42, 46). Such pharmacological interference can identify events that are critical to measured variables and avoid the complexity of overlapping activities. In this report, we employed ACV and WAY-150138 to test the hypothesis that DNA replication augments IE and E gene expression during reactivation from latency over 48 h and to distinguish events occurring in initial reactivating cells from those presumably resulting from secondary infections.
While viral DNA levels displayed a significant increase between ACV and WAY-150138 treatments at 48 h p.e., suggestive of viral DNA that is replicated in primary reactivating cells, IE and E gene RNA levels measured by ISH or RT-PCR exhibited little or no differences. However, there were large increases in viral DNA and RNA in untreated ganglia compared to latent or drug-treated ganglia, which strongly suggest that progeny virus initiated secondary infections well before 48 h p.e. This was confirmed by the reproducible detection of infectious virus as early as 14 h p.e.
We have repeated and extended the results of Kosz-Vnenchak et al. (17). In those ISH studies, when untreated TG were compared to TG in which viral DNA replication was inhibited, a large decrease in IE and E gene expression was observed and interpreted as evidence that DNA replication potentiated gene expression during reactivation. Our use of WAY-150138 in ISH and RT-PCR studies provides strong evidence that the great majority of gene expression detected by ISH in untreated tissues at 48 h p.e. is due not to viral DNA replication in primary reactivating cells but to productive infection of secondary cells by reactivated virus. The ISH signals were much less intense in cells from drug-treated TG than in cells from untreated TG (data not shown), which suggests restricted expression in primary reactivating cells and more typically robust, productive infections in secondary cells. This is consistent with the notion that expression from the latent, nucleosomal genome is limited, perhaps by the absence of VP16 or other facilitating factors. Kosz-Vnenchak et al. (17) had disfavored the possibility of spread for several reasons. It had been reported that viral DNA replication (36), production of infectious virus (37), and staining of multiple, adjacent cells with anti-HSV-1 sera (47) were not observed before 48 h p.e., suggesting that 48 h marked the end of the first cycle of viral replication. The inability to detect DNA replication or HSV antigens at times before 48 h p.e. was likely due to a lack of sensitivity, but the inability to detect virus at 24 h p.e. in a nearly identical system is harder to explain, because we easily detected virus between 14 and 24 h p.e. The hybridization of both LATs and productive-cycle genes in the same ganglion cells in studies by Kosz-Vnenchak et al. (17) was also offered as evidence that events were occurring in initial reactivating cells. It was assumed that LATs would decrease after initial reactivation and adopt a pattern wherein they would be regulated as a late gene in secondarily infected cells, as occurs during productive infection of cell culture (35; S. A. Rice and D. M. Knipe, unpublished data). Instead, the finding of relatively strong hybridization of LATs in the same cells as IE and E genes interestingly suggests that the division between latent and reactivating gene programs is not a hard line and leaves the door open for LATs to play a role in the reactivation process.
In addition to supporting our ISH results, the RT-PCR experiments provided quantitative information on RNA expression levels in individual TG. Whereas some studies have relied on pooling ganglia (24, 28, 43) and have rarely detected productive-cycle gene expression during latency (9, 24, 28, 43), our sensitive assays have detected genes from all kinetic classes in individual TG in this study and others (2, 3, 18, 19). Although the variability observed between individual TG can impede achievement of statistical significance for certain comparisons, it provides a clear picture of the natural variation among latent and reactivating ganglia.
We had previously detected ICP4, ICP0, and tk RNA in ganglia latently infected with strain KOS (2, 3, 19) and herein have extended these analyses to strain Patton. To our knowledge, this is the first reported detection and quantification of ICP8 RNA during HSV latency and reactivation, although transcripts from the varicella-zoster virus ICP8 homolog have been detected in human ganglia latently infected with varicella-zoster virus (23). It is interesting to note that ICP8 was expressed at the highest levels of any productive-infection gene during latent infection. ICP8 is essential for productive infection, playing vital roles in DNA replication as well as transcription (1, 8, 13).
Increases in viral DNA by 24 h p.e. (9) or 48 h p.e. (36) have been reported previously for similar systems; however, these data were not strictly quantitative and could not distinguish between primary reactivation and spread. The use of WAY-150138 permitted such a distinction. The increases measured in viral DNA and RNA over 48 h in the presence of WAY-150138 could be due to accumulation of molecules in a few initial reactivating cells or to accruing numbers of reactivating cells. Similarly, the upward trend in PFU over time in untreated TG could result from either a few cells with large burst sizes or many cells with small burst sizes. Immunohistochemical studies of explanted, ACV-treated ganglia have indicated that the number of single, HSV-antigen-staining neurons increases over the course of 4 days of explant (33). Thus, at least a portion of the increased expression is likely due to new neurons being induced to reactivate over time.
Production of infectious virus was shown to occur reproducibly as early as 14 h p.e. Although this contradicts one report of a similar explant system (37), it concurs with another (33) and with HSV reactivation induced by hyperthermia (34). We were surprised to detect a single PFU produced by a KOS-infected TG explanted for only 2 h. Although contamination is a possible source of the virus in this sample, homogenates in other wells on the same 6-well plate were negative for virus and additional negative controls revealed no PFU. Survival of a latently infected cell which produced virus after the explant period is unlikely because such a cell would have had to withstand freezing, homogenization, and sonication. Three other potential explanations are spontaneous reactivation, a new infection (perhaps transmitted by a cage-mate with reactivated virus), or evolution of a mutated virus unable to maintain latency. These all would be controversial, in that 20 years of studies have failed to find evidence for infectious virus in murine TG latently infected with most viral strains (enumerated in reference 19). However, two TG latently infected with strain 17syn+ were recently reported to each yield a single PFU (32) (although these TG were neither frozen nor sonicated, making the survival of a latently infected cell more plausible, as described above). Various avenues of study have suggested that low levels of HSV antigens exist in latently infected TG (2, 10, 16, 19). Based on these results, we are intrigued by the possibility that, in rare instances, 2 h is sufficient time for infectious virus to be assembled in reactivating neurons where viral genes have been expressed. Studies with a temperature-sensitive packaging mutant in cell culture have shown that approximately 1 h permits recovery of virus after downshift from a nonpermissive temperature to a permissive one (6). Follow-up studies indicate that viral DNA can be packaged even in the presence of ACV, suggesting that active DNA replication is not a requirement for genome packaging (5). Thus, we suggest that a latently infected cell could be primed for virus production with low but sufficient levels of progeny genomes and virion components synthesized and thus could theoretically complete assembly within 2 h of the stimulus. As interesting as the possibilities are, in all cases this event would be considered extremely rare, and we have not observed it more than once.
Although our results have precipitated a reinterpretation of the results of Kosz-Vnenchak et al. (17), the model they put forth for novel regulatory mechanisms and a possible role for viral DNA replication in gene expression in neurons still may be viable at early times following reactivation stimuli. There is already supporting evidence for the ideas they presented, with several other reports suggesting a novel program of gene expression during reactivation (9, 43) and supporting a role for viral DNA replication in potentiating gene expression in certain cells (26, 41). Tal-Singer et al. (43) detected E and leaky L genes (tk, ICP6, and VP5) at 4 h p.e., before IE genes (ICP4, ICP0, ICP27, ICP22, and ICP47), during HSV reactivation from latently infected TG. Devi-Rao et al. (9) also detected VP5 early, by 1 to 4 h p.e., but detected ICP4 and ICP27 at 2 and 4 h p.e., respectively. Working in vitro, Nichol et al. (26) found that ACV inhibited IE and E gene expression in primary neuronal cultures but not in Vero cells, while Summers and Leib (41) observed decreased IE and E promoter activity in primary cells (mouse embryo fibroblasts and dissociated ganglia) but not Vero cells when both were treated with DNA synthesis inhibitors. The explanations for these observations and how to reconcile such results remain to be elucidated.
Acknowledgments
We thank M. van Zeijl and T. R. Jones (Wyeth Research) for the generous gifts of WAY-150138, strain Patton wild-type virus, and 138R/5 virus. We thank A. Griffiths for assistance with animal experiments, J. W. Carbone for DNA sequencing, and M. F. Kramer for plasmid pKS + 5′ICP8 and the design of several PCR assays. We also thank S. H. Chen and M. F. Kramer for helpful advice.
This work was supported by NIH grant PO1 NS35138.
REFERENCES
- 1.Challberg, M. D. 1986. A method for identifying the viral genes required for herpesvirus DNA replication. Proc. Natl. Acad. Sci. USA 83:9094-9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, S. H., M. F. Kramer, P. A. Schaffer, and D. M. Coen. 1997. A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 71:5878-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, S.-H., L. Y. Lee, D. A. Garber, P. A. Schaffer, D. M. Knipe, and D. M. Coen. 2002. Neither LAT nor open reading frame P mutations increase expression of spliced or intron-containing ICP0 transcripts in mouse ganglia latently infected with herpes simplex virus. J. Virol. 76:4764-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chomczynski, P., and N. Sacchi. 1996. Single-step RNA isolation from cultured cells or tissues, p. 421-422. In V. B. Chanda (ed.), Current protocols in molecular biology, vol. 1. John Wiley & Sons, Inc., New York, N.Y. [Google Scholar]
- 5.Church, G. A., A. Dasgupta, and D. W. Wilson. 1998. Herpes simplex virus DNA packaging without measurable DNA synthesis. J. Virol. 72:2745-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Church, G. A., and D. W. Wilson. 1997. Study of herpes simplex virus maturation during a synchronous wave of assembly. J. Virol. 71:3603-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coen, D. M., H. E. Fleming, L. K. Leslie, and M. J. Retondo. 1985. Sensitivity of arabinosyladenine-resistant mutants of herpes simplex virus to other antiviral drugs and mapping of drug hypersensitivity mutations to the DNA polymerase locus. J. Virol. 53:477-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conley, A. J., D. M. Knipe, P. C. Jones, and B. Roizman. 1981. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of γ polypeptides. J. Virol. 37:191-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devi-Rao, G. B., D. C. Bloom, J. G. Stevens, and E. K. Wagner. 1994. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J. Virol. 68:1271-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman, L. T., A. R. Ellison, C. C. Voytek, L. Yang, P. Krause, and T. P. Margolis. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. USA 99:978-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao, M., and D. M. Knipe. 1989. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J. Virol. 63:5258-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garber, D. A., P. A. Schaffer, and D. M. Knipe. 1997. A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. J. Virol. 71:5885-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godowski, P. J., and D. M. Knipe. 1986. Transcriptional control of herpesvirus gene expression: gene functions required for positive and negative regulation. Proc. Natl. Acad. Sci. USA 83:256-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz, J. P., E. T. Bodin, and D. M. Coen. 1990. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J. Virol. 64:4288-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna, K. M., R. H. Bonneau, P. R. Kinchington, and R. L. Hendricks. 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18:593-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosz-Vnenchak, M., J. Jacobson, D. M. Coen, and D. M. Knipe. 1993. Evidence for a novel regulatory pathway for herpes simplex virus gene expression in trigeminal ganglion neurons. J. Virol. 67:5383-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer, M. F., S. H. Chen, D. M. Knipe, and D. M. Coen. 1998. Accumulation of viral transcripts and DNA during establishment of latency by herpes simplex virus. J. Virol. 72:1177-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer, M. F., and D. M. Coen. 1995. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 69:1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krosky, P. M., M. C. Baek, and D. M. Coen. 2003. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leib, D. A., D. M. Coen, C. L. Bogard, K. A. Hicks, D. R. Yager, D. M. Knipe, K. L. Tyler, and P. A. Schaffer. 1989. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 63:759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLean, A. R. 1998. Preparation of HSV-DNA and production of infectious virus, p. 19-25. In S. M. Brown and A. R. MacLean (ed.), Methods in molecular medicine: herpes simplex virus protocols, vol. 10. Humana Press, Totowa, N.J. [DOI] [PubMed] [Google Scholar]
- 23.Meier, J. L., R. P. Holman, K. D. Croen, J. E. Smialek, and S. E. Straus. 1993. Varicella-zoster virus transcription in human trigeminal ganglia. Virology 193:193-200. [DOI] [PubMed] [Google Scholar]
- 24.Minagawa, H., S. Tanaka, Y. Toh, and R. Mori. 1994. Detection of herpes simplex virus type 1-encoded RNA by polymerase chain reaction: different pattern of viral RNA detection in latently infected murine trigeminal ganglia following in vitro or in vivo reactivation. J. Gen. Virol. 75:647-650. [DOI] [PubMed] [Google Scholar]
- 25.Newcomb, W. W., and J. C. Brown. 2002. Inhibition of herpes simplex virus replication by WAY-150138: assembly of capsids depleted of the portal and terminase proteins involved in DNA encapsidation. J. Virol. 76:10084-10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichol, P. F., J. Y. Chang, E. M. Johnson, Jr., and P. D. Olivo. 1996. Herpes simplex virus gene expression in neurons: viral DNA synthesis is a critical regulatory event in the branch point between the lytic and latent pathways. J. Virol. 70:5476-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell, K. L., D. J. Purifoy, and R. J. Courtney. 1975. The synthesis of herpes simplex virus proteins in the absence of virus DNA synthesis. Biochem. Biophys. Res. Commun. 66:262-271. [DOI] [PubMed] [Google Scholar]
- 28.Rezuchova, I., M. Kudelova, V. Durmanova, A. Vojvodova, J. Kosovsky, and J. Rajcani. 2003. Transcription at early stages of herpes simplex virus 1 infection and during reactivation. Intervirology 46:25-34. [DOI] [PubMed] [Google Scholar]
- 29.Rock, D. L., and N. W. Fraser. 1983. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature 302:523-525. [DOI] [PubMed] [Google Scholar]
- 30.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 31.Sawtell, N. M. 1997. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J. Virol. 71:5423-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawtell, N. M. 2003. Quantitative analysis of herpes simplex virus reactivation in vivo demonstrates that reactivation in the nervous system is not inhibited at early times postinoculation. J. Virol. 77:4127-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawtell, N. M., and R. L. Thompson. 2004. Comparison of herpes simplex virus reactivation in ganglia in vivo and in explants demonstrates quantitative and qualitative differences. J. Virol. 78:7784-7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawtell, N. M., and R. L. Thompson. 1992. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J. Virol. 66:2150-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spivack, J. G., and N. W. Fraser. 1988. Expression of herpes simplex virus type 1 (HSV-1) latency-associated transcripts and transcripts affected by the deletion in avirulent mutant HFEM: evidence for a new class of HSV-1 genes. J. Virol. 62:3281-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spivack, J. G., and N. W. Fraser. 1988. Expression of herpes simplex virus type 1 latency-associated transcripts in the trigeminal ganglia of mice during acute infection and reactivation of latent infection. J. Virol. 62:1479-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spivack, J. G., D. R. O'Boyle II, and N. W. Fraser. 1987. Novobiocin and coumermycin A1 inhibit viral replication and the reactivation of herpes simplex virus type 1 from the trigeminal ganglia of latently infected mice. J. Virol. 61:3288-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens, J. G., and M. L. Cook. 1971. Latent herpes simplex virus in spinal ganglia of mice. Science 173:843-845. [DOI] [PubMed] [Google Scholar]
- 39.Stevens, J. G., E. K. Wagner, G. B. Devi-Rao, M. L. Cook, and L. T. Feldman. 1987. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235:1056-1059. [DOI] [PubMed] [Google Scholar]
- 40.Strauss, W. M. 1998. Preparation of genomic DNA from mammalian tissue, p. 221-223. In V. B. Chanda (ed.), Current protocols in molecular biology, vol. 1. John Wiley & Sons, Inc., New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 41.Summers, B. C., and D. A. Leib. 2002. Herpes simplex virus type 1 origins of DNA replication play no role in the regulation of flanking promoters. J. Virol. 76:7020-7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swanstrom, R. I., K. Pivo, and E. K. Wagner. 1975. Restricted transcription of the herpes simplex virus genome occurring early after infection and in the presence of metabolic inhibitors. Virology 66:140-150. [DOI] [PubMed] [Google Scholar]
- 43.Tal-Singer, R., T. M. Lasner, W. Podrzucki, A. Skokotas, J. J. Leary, S. L. Berger, and F. W. Fraser. 1997. Gene expression during reactivation of herpes simplex virus type 1 from latency in the peripheral nervous system is different from that during lytic infection of tissue cultures. J. Virol. 71:5268-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tenser, R. B., and M. E. Dunstan. 1979. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology 99:417-422. [DOI] [PubMed] [Google Scholar]
- 45.van Zeijl, M., J. Fairhurst, T. R. Jones, S. K. Vernon, J. Morin, J. LaRocque, B. Feld, B. O'Hara, J. D. Bloom, and S. V. Johann. 2000. Novel class of thiourea compounds that inhibit herpes simplex virus type 1 DNA cleavage and encapsidation: resistance maps to the UL6 gene. J. Virol. 74:9054-9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward, R. L., and J. G. Stevens. 1975. Effect of cytosine arabinoside on viral-specific protein synthesis in cells infected with herpes simplex virus. J. Virol. 15:71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wroblewska, Z., K. Savage, J. G. Spivack, and N. W. Fraser. 1989. Detection of HSV-1 proteins prior to the appearance of infectious virus in mouse trigeminal ganglia during reactivation of latent infection. Virus Res. 14:95-106. [DOI] [PubMed] [Google Scholar]