Abstract
The Spanish influenza pandemic of 1918 to 1919 swept the globe and resulted in the deaths of at least 20 million people. The basis of the pulmonary damage and high lethality caused by the 1918 H1N1 influenza virus remains largely unknown. Recombinant influenza viruses bearing the 1918 influenza virus hemagglutinin (HA) and neuraminidase (NA) glycoproteins were rescued in the genetic background of the human A/Texas/36/91 (H1N1) (1918 HA/NA:Tx/91) virus. Pathogenesis experiments revealed that the 1918 HA/NA:Tx/91 virus was lethal for BALB/c mice without the prior adaptation that is usually required for human influenza A H1N1 viruses. The increased mortality of 1918 HA/NA:Tx/91-infected mice was accompanied by (i) increased (>200-fold) viral replication, (ii) greater influx of neutrophils into the lung, (iii) increased numbers of alveolar macrophages (AMs), and (iv) increased protein expression of cytokines and chemokines in lung tissues compared with the levels seen for control Tx/91 virus-infected mice. Because pathological changes in AMs and neutrophil migration correlated with lung inflammation, we assessed the role of these cells in the pathogenesis associated with 1918 HA/NA:Tx/91 virus infection. Neutrophil and/or AM depletion initiated 3 or 5 days after infection did not have a significant effect on the disease outcome following a lethal 1918 HA/NA:Tx/91 virus infection. By contrast, depletion of these cells before a sublethal infection with 1918 HA/NA:Tx/91 virus resulted in uncontrolled virus growth and mortality in mice. In addition, neutrophil and/or AM depletion was associated with decreased expression of cytokines and chemokines. These results indicate that a human influenza H1N1 virus possessing the 1918 HA and NA glycoproteins can induce severe lung inflammation consisting of AMs and neutrophils, which play a role in controlling the replication and spread of 1918 HA/NA:Tx/91 virus after intranasal infection of mice.
The influenza pandemic of 1918 was exceptional in that it resulted in an estimated 20 to 50 million deaths worldwide (9, 32, 50, 59). Approximately 30% of the world's population was clinically infected during the pandemic (15), and there was an unusually high lethality rate among healthy adults aged 15 to 34 years, a finding that has not been observed in subsequent pandemics or epidemics caused by influenza A viruses (40). Histopathological analysis of lung tissues from individuals who died from primary influenza pneumonia in 1918 showed massive pulmonary edema with unique destruction of the alveolar architecture (35, 58, 72). The retrieval of viral RNA from archived autopsy materials and from the body of an Alaskan influenza victim buried in permafrost has revealed the genetic code of this H1N1 subtype influenza virus. Analyses of the complete coding sequences of the 1918 hemagglutinin (HA), neuraminidase (NA), matrix, nonstructural, and nucleoprotein genes have not revealed the molecular basis for its extreme virulence (4, 48-52, 58).
Plasmid-based reverse genetics has allowed for the generation of recombinant viruses containing one or more of the 1918 influenza virus genes entirely from cloned cDNAs (4, 19, 30, 34, 64, 65). Using this approach, we previously generated recombinant viruses possessing the 1918 HA and/or NA segments rescued on the genetic background of the mouse-adapted A/WSN/33 (1918 HA/NA:WSN) virus (30, 64, 65). Although the full virulence of the 1918 pandemic virus is most likely due to the constellation of virus genes, initial emphasis was placed on the HA and NA glycoproteins (30, 34, 64, 65), since they are the major viral surface antigens and are important virulence factors in birds and mammals (22, 25, 44, 74). Infection with the 1918 HA/NA:WSN virus resulted in severe morbidity and mortality in BALB/c mice, whereas a recombinant control virus possessing the HA and NA of the contemporary A/New Caledonia/20/99 (H1N1) virus (N. Cal. HA/NA:WSN) induced minimal lung pathology and was not lethal. Histological characterization of 1918 HA/NA:WSN-infected mouse lungs demonstrated severe pulmonary lesions consisting of severe bronchitis and alveolitis as early as 72 h following an intranasal infection (30). More-recent studies by Kobasa et al. (34) confirmed these results and also indicated that the 1918 HA alone confers increased replication properties and lethality in mice to human influenza viruses. Additional studies are needed to further characterize the pulmonary inflammation and the functional roles of inflammatory cells that contribute to the unique pathogenic properties observed with the 1918 HA and NA recombinant influenza viruses.
In its uncomplicated form, a human influenza A virus infection of the H1 or H3 subtype results in viral replication in the respiratory epithelium and leads to the induction of an inflammatory infiltrate comprised mainly of mononuclear leukocytes, with lower levels of polymorphonuclear leukocytes (neutrophils) (1). Recovery from influenza infection is dependent on virus-specific T and B cells (10, 20, 74). However, neutrophils and alveolar macrophages (AMs) can respond more quickly to virus infection and play important roles in the pulmonary disease process (23, 54, 56). AMs are the primary mononuclear phagocytes of the uninflamed lung alveoli and are considered the first cells of the immune system to encounter inhaled organisms and antigens. Therefore, they function as the major scavenger cells in the lower respiratory tract, and upon appropriate stimulation, these cells can release proinflammatory cytokines and chemokines (5, 39, 54). Neutrophils may also contribute to the release of cytokines, and although they traditionally have been viewed as being important in fighting bacterial infections, these cells can be involved in virally induced pathology (46, 62, 67).
The basis for the exceptional virulence of the 1918 pandemic influenza virus remains elusive but most certainly involves host responses to influenza infection. Because little is known about the mechanism(s) by which the 1918 influenza virus caused severe lung pathology and death, this study was undertaken to investigate the pathogenesis in mice of a recombinant influenza virus possessing the 1918 HA and NA glycoproteins. The genetic background of the remaining influenza genes used for the recombinant 1918 virus was derived from a human influenza A H1N1 1991 strain, A/Texas/36/91 (1918 HA/NA:Tx/91) virus, which has been characterized in humans under experimental settings (26) and is nonpathogenic in mice. Characterization of the 1918 HA/NA:Tx/91 virus revealed a high pathogenicity phenotype in mice in comparison to that of the parental Tx/91 virus. An additional feature of the lethal 1918 HA/NA:Tx/91 virus was its ability to induce elevated levels of cytokines, AMs, and neutrophils in the infected mouse lung. While increased numbers of AMs and neutrophils in lungs contribute to the inhibition of virus replication as part of the host innate immune response, they might also contribute to the enhanced immunopathology and, therefore, be partially responsible for the lethal phenotype of the 1918 HA/NA:Tx/91 virus. Using depletion techniques, we found that these cells play a role in the initial stages of the innate immune response in limiting virus replication, virus spread, and lethality during 1918 HA/NA:Tx/91 virus infection.
MATERIALS AND METHODS
Generation of 1918 HA and NA cDNAs and recombinant viruses.
The 1918 HA and NA cDNAs were constructed by PCR with overlapping deoxyoligonucleotides corresponding to the published sequence of the influenza A/South Carolina/1/18 (H1N1) virus HA (48) open reading frame or the influenza A/Brevig Mission/1/18 (H1N1) virus NA open reading frame (49). The noncoding regions of each segment are identical to those of the corresponding segment of influenza A/WSN/33 (WSN) H1N1 virus. Recombinant viruses were generated using the reverse genetics system of Fodor et al. (14) following the methods of Basler et al. (4). Viruses possessing 1918 HA and NA genes in a WSN (1918 HA/NA:WSN) or 1918 HA/NA:Tx/91 virus background were generated under biosafety level 3 (BSL-3 with enhancements) containment (3) to ensure the safety of laboratory workers, the environment, and the public. All subsequent laboratory and animal work with live virus also was performed under these high-stringency containment conditions. The control viruses included a whole parental Tx/91 virus rescued (rTx/91) in a similar manner and a wild-type Tx/91 stock provided by A. Klimov (Centers for Disease Control and Prevention, Atlanta, Georgia). The identity of the 1918 influenza virus genes in the recombinant viruses was confirmed by reverse transcription-PCR and sequencing. Primer sequences and PCR conditions are available upon request.
Infection of mice.
Female BALB/c mice, 6 to 7 weeks old (Charles River Laboratories, Wilmington, Mass.), were anesthetized with an intraperitoneal (i.p.) injection of 0.2 ml of 2,2,2-tribromoethanal in tert-amyl alcohol (Avertin; Aldrich Chemical Co., Milwaukee, Wis.), and 50 μl of infectious virus diluted in phosphate-buffered saline (PBS) was inoculated intranasally (i.n.). Fifty-percent lethal dose (LD50) titers were determined by inoculating groups of three mice i.n. with serial 10-fold dilutions of virus. LD50 titers were calculated by the method of Reed and Muench (47). For determination of lung virus titers, morbidity (measured by weight loss), and mortality at multiple time points during the first week of infection, 24 additional mice were infected i.n. with the highest inoculating dose (106 PFU) of virus. Individual body weights from eight mice were recorded for each group on days 0, 2, 4, 6, 8, and 10 postinfection (p.i.) and monitored daily for disease signs and death for 14 days p.i. On days 2, 4, 6, and 8 p.i., four mice from each group were euthanatized, and whole lungs, spleen, and brain tissues were removed aseptically and homogenized in 1 ml of sterile PBS. After this process, homogenates were titrated for virus infectivity in 10-day-old embryonated eggs (36). The statistical significance of virus titer data was determined by using analysis of variance (ANOVA). Fifty-percent egg infectious dose (EID50/ml) titers were calculated by the method of Reed and Muench (47).
Lung homogenates along with bronchoalveolar lavage (BAL) samples (described below) were also individually tested for bacterial infection by routine bacteriological methods for the isolation and identification of bacteria. Briefly, serial 10-fold dilutions of lung homogenates (day 4 p.i.) or BAL samples (days 0, 3, 5, and 7 p.i.) were transferred to a tryptic soy agar plate, blood agar, chocolate agar, or a MacConkey agar plate (Fisher Scientific, Pittsburgh, PA). Five lung homogenates collected on day 4 p.i. and 20 BAL samples collected on p.i. days 0, 3, 5, and 7 per group were tested from both the mock control and infected groups. Three individual bacteriological loops were plated from each sample, and the number of CFU was determined after 72 h of incubation at 37°C.
Histopathology.
Three mice from each group were euthanatized on day 5 p.i. A 5-mm lung sample from the ventral end of each left lobe was collected for histopathology, as were brain, lung, heart, spleen, liver, kidney, thymus, and bronchial lymph node samples. Duplicate sections were immunohistochemically stained to demonstrate influenza A viral protein. The primary antibody was produced in chickens that had been immunized twice with A/chicken/Wisconsin/68 (H5N9) virus and was used at a 1:100 dilution. This antibody recognizes conserved internal virus proteins of multiple subtypes. The secondary antibody was a goat anti-chicken immunoglobulin G (IgG) tagged with horseradish peroxidase (Southern Biotech, Birmingham, AL), and the chromogen diaminobenzidine (DAB Plus kit; Zymed, San Francisco, CA) was used for visualization. For the lung sections, the numbers of all viral antigen-positive respiratory epithelial cells and macrophages in a minimum of five high-powered fields (HPF) were determined by counting, and averages were determined for individual mice and for treatment groups.
BAL sample and blood cell counts.
BAL sample cells were obtained from euthanatized mice as previously described (31). Briefly, the BAL sample cells were washed from the lungs of anesthetized mice in 1 ml of PBS containing 1% bovine serum albumin (Sigma, St. Louis, MO). Absolute cell counts were performed by diluting cell suspensions 1:5 in Turks solution (2% acetic acid, 0.01% methylene blue). The cell numbers from five individual mice per group were determined in triplicate by counting in a hemocytometer. Portions were cytospun onto glass microscope slides and stained with Hema 3 stain (Fisher Scientific), and the percentages of monocytes, polymorphonuclear neutrophils, and lymphocytes were determined. At least 100 cells were counted for each slide; counts were performed in triplicate at a magnification of ×1,000. For differential cell counts, peripheral blood samples (20 to 40 μl) were obtained from the orbital plexus of two or three mice on days 1, 3, 5, and 7 p.i., and smears were stained with Hema 3 stain. For each sample, at least 100 cells were counted at a magnification of ×1,000. The percentage of leukocytes was determined following treatment with monoclonal antibody (MAb) RB6-8C5 or control rat IgG antibody (described below).
Depletion of neutrophils and AMs.
The hybridoma RB6-8C5, which produces a rat IgG2b anti-mouse granulocyte MAb, was a gift from R. Coffman, DNAX Research Institute (Palo Alto, CA). The antibody was purified from hybridoma culture medium by affinity chromatography with protein G-Sepharose Fast Flow (Pharmacia, LKB Biotechnology, Piscataway, NJ) and quantified by use of a radial immunodiffusion kit (ICN Immunobiologicals, Costa Mesa, Calif.). Each mouse received one i.p. injection of 0.5 mg of purified antibody 5 h before infection and another on day 3 p.i. To maintain sufficient depletion of neutrophils, mice received 1 mg of antibody on days 5 and 7 p.i. Control mice received rat IgG antibody (Sigma) in place of MAb RB6-8C5. The same treatment regimen also was followed for mice that received MAb RB6-8C5 treatment after infection, except that the first i.p. injection of MAb RB6-8C5 was initiated on day 3 or 5 p.i. Thus, the first and second i.p. injections consisted of 0.5 mg of MAb followed by 1 mg of antibody every other day. For depletion of AMs, liposome-encapsulated dichloromethylene diphosphonate (clodronate) (L-CL2MDP) and liposomes containing PBS (L-PBS) were prepared as previously described (66). Dichloromethylene diphosphonate was a kind gift of Roche Diagnostics, Mannheim, Germany. BALB/c mice were anesthetized with Avertin before i.n. inoculation with L-CL2MDP or L-PBS in a 50-μl volume on days −4 and −2 before virus infection. In experiments where AMs were depleted after infection, mice received L-CL2MDP or L-PBS on days 3 and 5 p.i or on days 5 and 7 p.i. This procedure results in the selective depletion of AM so that other cells remain unaffected after the elimination procedure (60, 69, 70). The toxicity of L-CL2MDP was tested in five mice that were not later infected with virus. In comparison to naïve or L-PBS-treated mice, no overt signs of clinical illness, including weight loss, were observed among animals that received L-CL2MDP.
Cytokine and chemokine quantitation.
To determine the in vivo levels of cytokine or chemokine proteins, five mice per group were exsanguinated from the axilla and euthanatized, and lung tissues were removed from naïve and infected mice (day 5 p.i.). Individual whole-lung samples were immediately frozen at −70°C. On the day of analysis, tissues were thawed, homogenized in 1 ml of cold PBS, and centrifuged at 150 × g for 5 min. The clarified cell lysates were assayed for alpha interferon (IFN-α; assay sensitivity, 12.5 pg/ml), IFN-γ (assay sensitivity, 2 pg/ml), tumor necrosis factor alpha (TNF-α; assay sensitivity, 5.1 pg/ml), macrophage inflammatory protein-1 alpha (MIP-1α; assay sensitivity, 1.5 pg/ml), and MIP-2 (assay sensitivity, 1.5 pg/ml) by use of enzyme-linked immunosorbent assay (ELISA) kits purchased from R & D Systems (Minneapolis, MN). Statistical significance of lung cytokine data was determined by ANOVA.
RESULTS
Construction and characterization of recombinant viruses with 1918 HA and NA influenza virus genes.
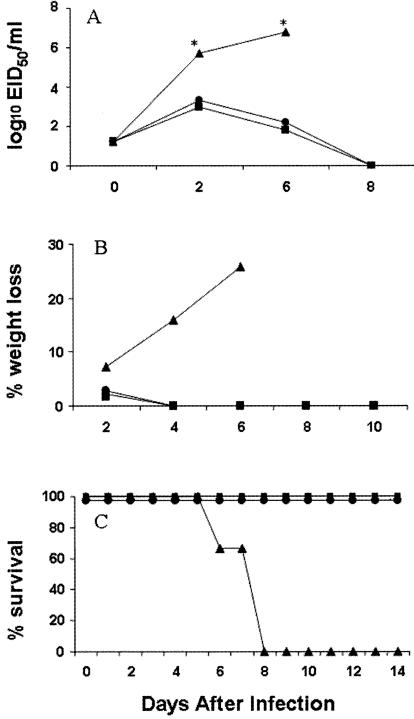
Genes encoding the 1918 pandemic influenza virus were reconstructed from deoxyoligonucleotides and corresponded to the reported 1918 virus coding sequences (48, 49, 58). The HA and NA genes of the 1918 virus rescued in the genetic background of Tx/91 (1918 HA/NA:Tx/91) or WSN (1918 HA/NA:WSN) virus had high infectivity titers in MDCK cells and 10-day-old embryonated chicken eggs, similar to those of the wild-type Tx/91, rTx/91, and parental rescued WSN (rWSN) viruses (Table 1). BALB/c mice were inoculated i.n., and weight loss, virus replication, and LD50 titers were determined. For comparison, groups of mice were infected with the highly pathogenic 1918 HA/NA:WSN or rWSN virus, both of which were previously shown to have high lethality in mice (65). Infection of mice with the 1918 HA/NA:Tx/91 recombinant virus resulted in lung virus titers that were approximately 250-fold higher than those for rTx/91-infected mice on day 4 p.i. (Table 1). The 1918 HA/NA:WSN and rWSN viruses were more lethal (LD50 ≤102.75) than the 1918 HA/NA:Tx/91 virus (LD50 of 104.75). In contrast to the lethal outcomes of infection with the 1918 recombinant viruses, the rescued Tx/91 virus and its wild-type counterpart did not kill mice and resulted in only minimal weight reduction. To better understand the differences in pathogenicity among the H1N1 recombinant viruses, we examined the kinetics of virus replication in lung, brain, and spleen, the percent weight loss, and overall survival throughout the course of 1918 HA/NA:Tx/91 virus infection. Mice infected i.n. with 106 PFU of virus were sacrificed on days 2, 6, and 8 p.i. to determine virus titers (Fig. 1A). On days 2 and 6 p.i., virus titers recovered from the lungs of mice infected with the Tx/91 control viruses were significantly lower than those from the 1918 HA/NA:Tx/91-infected mice. 1918 HA/NA:Tx/91-infected mice were unable to clear the infection, showed signs of illness (e.g., ruffled fur and hunched posture), and began to lose significant amounts of weight 4 days after infection (Fig. 1B). Weight loss continued in 1918 HA/NA:Tx/91-infected mice, and mortality reached 100% by day 8, with a mean death time (MDT) of 7.3 days (Fig. 1C). In contrast, all Tx/91-infected mice survived the infection and displayed minimal weight reduction on day 2 p.i. only. We examined whether the 1918 HA/NA:Tx/91 virus replicated systemically in the mouse by removing brain, heart, and spleen tissues on day 6 p.i. (four samples per tissue), when virus titers in these tissues are maximal following infection with highly pathogenic influenza viruses (36). All eight mice infected with 1918 HA/NA:Tx/91 or Tx/91 control viruses had undetectable levels of virus (≤100.8 EID50/ml) in the brain, heart, and spleen tissues (not shown).
TABLE 1.
Properties of recombinant influenza viruses used in this study
| Virusa | Titerb
|
% Weight lossc | Lung titer (log10 EID50/ ml ± SE)d | LD50e | |
|---|---|---|---|---|---|
| log10 EID50/ml | PFU/ml | ||||
| Wild-type Tx/36/91 | 8.2 | 2.0 × 107 | 0.7 | 3.3 ± 0.2 | >6 |
| Parental rTx/36/91 | 8.7 | 4.4 × 107 | 0.1 | 3.6 ± 0.3 | >6 |
| Parental rWSN | 8.2 | 2.2 × 107 | 21.8 | 7.2 ± 0.2 | 2.75 |
| 1918 HA/NA:Tx/36/91 | 8.7 | 2.5 × 107 | 15.9 | 6.0 ± 0.2 | 4.75 |
| 1918 HA/NA:WSN | 8.5 | 2.1 × 107 | 21.7 | 7.0 ± 0.3 | 2.25 |
All viral genomic segments derived from the WSN or Tx/91 virus unless otherwise indicated.
Calculated by the method of Reed and Muench (47) from titrations in eggs and MDCK cells.
Average percent weight loss on day 4 postinfection (five mice per group).
Average lung titers of four mice on day 4 postinfection.
Expressed as the log10 PFU required to give 1 LD50.
FIG. 1.
Comparison of lung virus titers (A), weight loss rates (B), and mortality rates (C) of BALB/c mice infected with 106 PFU of 1918 HA/NA:Tx/91 (▴), rescued parental Tx/91 (•), and wild-type Tx/91 (▪) virus. Four mice from each virus-infected group were euthanatized on the indicated days p.i., and titers of individual lungs were determined in eggs. The limit of virus detection was 101.2 EID50/ml. The remaining eight mice from each group were observed for weight loss and mortality through a 14-day observation period. Asterisks indicate that the 1918 HA/NA:Tx/91 lung titers are significantly (P < 0.05) different than those of both Tx/91 controls, as determined by ANOVA.
Mouse lung pathology.
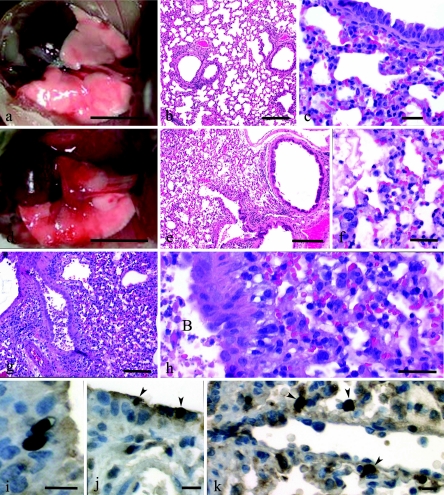
The presence of gross and histopathologic changes and virus antigen in the mouse lung was determined at 5 days p.i., a time that immediately preceded the death of mice infected with the lethal 1918 HA/NA:Tx/91 virus. The three mice infected with the control Tx/91 viruses had normal lungs in terms of color, texture, and size, with only occasional small dark red foci of pneumonia (Fig. 2a). Histologically, the lungs were normal or had minimal-to-mild bronchitis with lymphocytic-to-histiocytic peribronchitis and mild histiocytic alveolitis (Fig. 2b and c). By contrast, mice infected with the 1918 HA/NA:Tx/91 recombinant virus generally had within the lungs large pneumonic areas primarily affecting the ventral portions of each of the lung lobes (Fig. 2d). Histologically, a mild-to-severe necrotizing bronchitis with associated moderate-to-severe alveolitis and moderate-to-severe pulmonary edema was observed in these lungs (Fig. 2e). A striking feature of the 1918 HA/NA:Tx/91-infected lungs was the prominent number of neutrophils in the peribronchium and alveoli (Fig. 2f). In comparing the two viruses, lungs from mice infected with rTx/91 and from those infected with wild-type Tx/91 virus groups had 14.2 and 9.9 neutrophils/HPF of alveoli, respectively, while lungs from mice infected with 1918 HA/NA:Tx/91 had 68.3 neutrophils/HPF of alveoli. To demonstrate that a similar pathology could be induced with a second 1918 HA/NA recombinant virus, three mice were infected with the 1918 HA/NA:WSN virus, and histopathologic changes were determined at day 5 p.i. The lungs from the 1918 HA/NA:WSN-infected mice also showed a severe necrotizing bronchitis with associated alveolitis (Fig. 2g). The moderate-to-severe alveolitis was characterized by abundant AMs and neutrophils (Fig. 2h). Viral antigen commonly could be seen in necrotic cellular debris within bronchial lumina and alveoli, and frequently, within AMs. The virus-positive respiratory epithelial cells (Fig. 2i and j) were infrequent in all groups, as evidenced by the presence of only 0.05, 0.28, and 0.3 infected epithelial cells/HPF in the rTx/91, wild-type Tx/91, and 1918 HA/NA:Tx/91 virus-infected mouse lungs, respectively. The frequencies of virus-positive macrophages in the alveoli were also low in the Tx/91 control virus groups (0 and 1.35/HPF) (Fig. 2k), but the frequency was at least threefold higher in the lungs of 1918 HA/NA:Tx/91 virus-infected mice (4.5/HPF). These histological results indicate that neutrophils and AMs are prominent cell types associated with and potentially mediating the severe lung pathology, especially in alveoli, following lethal 1918 HA/NA recombinant virus infection.
FIG. 2.
Pathologies of mice intranasally inoculated with 106 PFU of rescued parental Tx/91, 1918 HA/NA:Tx/91, or 1918 HA/NA:WSN recombinant influenza viruses. Gross photographs (a and d) and photomicrographs (b, c, and e through h) of hematoxylin and eosin-stained tissue sections are shown. (a) Lungs of a mouse inoculated with Tx/91 virus; lungs at day 5 p.i. appear normal except for a small focus of pneumonia in left lung lobe. Bar = 5 mm. (b) Mild lymphocytic peribronchitis with mild alveolitis in a mouse inoculated with Tx/91; day 5 p.i. Bar = 200 μm. (c) Predominant peribronchial histiocytic alveolitis with iatrogenic atelectasis in a mouse inoculated with Tx/91; day 5 p.i. Bar = 25 μm. (d) Large areas of pneumonia affecting most of the left lung and cardiac lobe of the right lung. Smaller areas of pneumonia in apical and azygous lobes of the right lung of a mouse inoculated with 1918 HA/NA:Tx/91; day 5 p.i. Bar = 5 mm. (e) Diffuse severe alveolitis in a mouse inoculated with 1918 HA/NA:Tx/91; day 5 p.i. Bar = 200 μm. (f) Severe neutrophilic-to-histiocytic alveolitis adjacent to bronchiole with necrotizing bronchitis. Furthermore, some alveoli have pulmonary edema evident as protein crescents in a mouse inoculated with 1918 HA/NA:Tx/91; day 5 p.i. Bar = 20 μm. (g) Severe necrotizing bronchitis with severe alveolitis in a mouse inoculated with 1918 HA/NA:WSN; day 5 p.i. Bar = 100 μm. (h) Higher magnification of panel g showing prominent necrotic debris in bronchial lumen (labeled “B” within panel) and abundant neutrophils and histiocytes in alveoli. Bar = 20 μm. (j) Two virus-positive bronchial epithelia (arrowheads) in a mouse inoculated with 1918 HA/NA:Tx/91; day 5 p.i. Bar = 10 μm. (k) Virus-positive AMs (arrowheads) in a mouse inoculated with 1918 HA/NA:Tx/91; day 5 p.i. Bar = 10 μm.
Because of the observed increases of lung neutrophils and their documented association with secondary bacterial infections, it was of interest to determine whether the 1918 HA/NA:Tx/91 virus-infected lungs harbored levels of bacteria greater than those in the Tx/91-infected or uninfected lungs. Twenty individual samples from the BAL specimens collected on days 0, 3, 5, and 7 p.i. (five per time period) and lung homogenates collected on day 4 p.i (five mice per group) were evaluated for the presence of bacteria. In general, there was no tendency for bacteria to be isolated more frequently or at higher levels from the 1918 HA/NA:Tx/91-infected mouse lungs than from rTx/91-infected or uninfected lungs (data not shown). For BAL specimens, 15% (3 of 20) of Tx/91-infected and 20% (4 of 20) of uninfected mice were colonized with bacteria, whereas 5% (1 of 20) of BAL samples collected from the 1918 HA/NA:Tx/91-infected lungs demonstrated bacterial growth. Among the positive BAL samples, bacterial growth ranged from 102 to 103 CFU/ml. The proportions of lung homogenates that yielded growth of bacteria were also similar for all three groups tested. In these samples, no viable bacteria were present in either normal or 1918 HA/NA:Tx/91-infected lungs, and only one of five rTx/91-infected lung homogenates presented low bacterial growth, which ranged from <101 to 102 CFU/ml among the triplicate samples tested. Taken together, these results indicated that the HA and NA genes of the 1918 virus enhanced the virulence of the Tx/91 virus in mice and that this enhancement does not appear to be due to a higher incidence of coinfection with bacteria.
Neutrophils and alveolar macrophages contribute to host defense after 1918 HA/NA:Tx/91 recombinant virus infection.
To better characterize the cellular components in the lung following a primary 1918 HA/NA:Tx/91 virus infection, total and differential cell counts of BAL sample infiltrates were collected and analyzed from infected mice at 0.75 (18 h), 3, 5, and 7 days p.i. (Table 2). The total BAL sample cells recovered from both the 1918 HA/NA:Tx/91 virus-infected and control Tx/91-infected mice showed increases of infiltrates as early as 18 h p.i., with cell numbers increasing 1.5- to 6.7-fold by day 7 p.i. However, much larger increases in total BAL sample cells were observed in 1918 HA/NA:Tx/91 virus-infected lungs. During 1918 HA/NA:Tx/91 virus infection, peak cellular infiltration in the BAL samples on days 5 and 7 p.i. was associated with a substantial increase in the number of infiltrating neutrophils compared with that for control Tx/91-infected mice. The lymphocyte and macrophage cell counts of the 1918 HA/NA:Tx/91 virus-infected lungs generally followed patterns similar to those of counts for control mice, except elevated levels of BAL sample macrophages were observed on days 3 and 5 p.i. These results suggest that 1918 HA/NA:Tx/91 virus induces a predominant neutrophil infiltrate and moderate macrophage increases into the mouse lung that may be contributing to the pathogenesis of the enhanced disease associated with 1918 HA/NA:Tx/91 virus infection.
TABLE 2.
Total and differential counts in BAL specimens following infection with 1918 recombinant viruses
| Infectiona | Day p.i. | Total cell yield (× 105)b | Mean cell no. (± SE)/mousec
|
||
|---|---|---|---|---|---|
| Neutrophils (× 103) | Lymphocytes (× 104) | Macrophages (× 104) | |||
| rTx/91 | 0 | 1.7 | 10.2 ± 4.9 | 5.6 ± 0.9 | 10.5 ± 0.9 |
| 1918 HA/NA:Tx/91 | 0 | 1.8 | 10.8 ± 4.3 | 5.9 ± 1.2 | 10.9 ± 1.2 |
| rTx/91 | 0.75 | 2.3 | 12.8 ± 3.1 | 6.7 ± 0.6 | 14.9 ± 1.5 |
| 1918 HA/NA:Tx/91 | 0.75 | 2.9 | 31.9 ± 6.6 | 10.4 ± 0.9 | 15.4 ± 1.4 |
| rTx/91 | 3 | 2.7 | 27 ± 5.1 | 7.5 ± 2.1 | 16.7 ± 1.7 |
| 1918 HA/NA:Tx/91 | 3 | 5.0 | 60 ± 19 | 15.0 ± 2.3 | 28.5 ± 3.6 |
| rTx/91 | 5 | 3.7 | 51.8 ± 10.5 | 15.4 ± 1.5 | 15.9 ± 1.9 |
| 1918 HA/NA:Tx/91 | 5 | 7.8 | 249.6 ± 24.2 | 15.6 ± 1.8 | 37.4 ± 3.7 |
| rTx/91 | 7 | 5.8 | 69.6 ± 26.6 | 22.0 ± 1.5 | 29.0 ± 2.2 |
| 1918 HA/NA:Tx/91 | 7 | 12.1 | 798.6 ± 73.2 | 13.3 ± 4.2 | 27.8 ± 5.5 |
| 1918 HA/NA:Tx/91, RB6-8C5 treatedd | 7 | 6.7 | 1.2 ± 2.6 | 27.8 ± 1.6 | 47.3 ± 1.7 |
| 1918 HA/NA:Tx/91, L-Cl2MBPe | 7 | 9.3 | 856 ± 65.6 | 29.2 ± 1.4 | 1.8 ± 1.6 |
Mice were i.n. infected with 106 PFU of the indicated viruses.
BAL specimens were collected from five mice per group on the days indicated. Data are the mean absolute cell numbers in the BAL specimens.
In each differential from five mice per group. Differential counts were performed on LeukoStat (Fisher Scientific)-stained cytospins.
For neutrophil depletion, mice received repeated i.p. injections of MAb RB6-8C5 beginning 5 h before i.n. virus infection and on days 3 and 5 p.i.
For AM depletion, mice received L-Cl2MBP i.n. on days −4 and −2 relative to the time of i.n. virus infection.
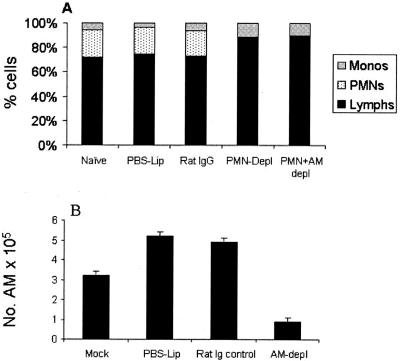
The role of neutrophils and AMs in 1918 HA/NA recombinant virus-induced lung pathology was investigated by eliminating such cells before and/or after infection with 1918 HA/NA:Tx/91 virus. Profound neutrophil depletion could be achieved in BALB/c mice by MAb RB6-8C5 treatment. Blood samples from two or three animals were collected 1 h before each i.p. injection in order to monitor the neutrophil count. The depletion protocol produced and maintained a depletion of blood neutrophils in a range from 96 to 99% with no apparent adverse affect on the percentages of lymphocytes and macrophages (Fig. 3A). The MAb RB6-8C5 protocol of depleting neutrophils was also efficient in the BAL samples without noticeably affecting the percentages of lymphocytes and macrophages (Table 2). Moreover, RB6-8C5 MAb was unable to bind CD4+ or CD8+ T cells, thymocytes, monocytes/macrophages, or B cells in flow cytometric experiments (38, 62; also data not shown), suggesting that RB6-8C5 MAb has no detectable cross-reactivity with these other cell types. Depletion of AM was performed by i.n. instillation of L-Cl2MBP twice (days −4 and −1) before infection. This procedure resulted in an 82.7% reduction in AM numbers for BAL samples compared with those for L-PBS-treated control mice on day 2 p.i. (Fig. 3B) without adversely affecting the blood monocyte percentages (Fig. 3A) or neutrophil numbers (Table 2). We initially investigated whether depleting neutrophils and/or AMs after infection would reduce the pathogenesis of the 1918 recombinant virus. Groups of 12 mice were i.n. infected with a lethal dose (20 LD50) of 1918 HA/NA:Tx/91 virus, and treatment with MAb RB6-8C5 or L-Cl2MBP was initiated on day 3 p.i. Control mice received rat IgG in place of MAb RB6-8C5 or L-PBS in place of L-Cl2MBP. Virus replication, weight loss, and mortality rates among the neutrophil- and AM-depleted mice were compared with those among their IgG- and L-PBS-treated counterparts. Although neutrophils and AMs were barely detectable in the lungs of the treated mice, depletion of these two cell types initiated after 1918 HA/NA:Tx/91 virus infection failed to reduce morbidity and mortality in comparison with those for control animals (data not shown). Furthermore, the lethalities for the neutrophil-depleted, AM-depleted, and control groups were 100%, with MDTs of 7.4, 7.1, and 7.9, respectively. The amounts of infectious virus recovered from the lungs of neutrophil-depleted and AM-depleted mice were significantly (P < 0.05) higher (7.1 ± 0.2 log10 EID50/ml and 7.4 ± 0.2 log10 EID50/ml, respectively) in comparison to the amount from untreated control mice (6.0 ± 0.3 log10 EID50/ml), suggesting that these cells suppress influenza growth in the lung. In a second independent experiment, delaying neutrophil and/or AM depletion until day 5 p.i also resulted in levels of morbidity and mortality for treated animals that were comparable to those for control animals (data not shown).
FIG. 3.
Efficiency of neutrophil and AM depletion (depl) in vivo. (A) Effect of neutrophil and AM depletion on blood leukocyte counts. For neutrophil depletion, BALB/c mice received repeated i.p. injections of MAb RB6-8C5 or control rat IgG beginning 5 h before i.n. virus infection and on days 3, 5, and 7 after infection to maintain depletion. Neutrophil depletion was monitored 1 h before each MAb injection by performing differential cell counts on blood smears. The results at day 7 p.i. are shown and are expressed as the mean percentages of monocytes (monos), neutrophils (PMNs), and lymphocytes (lymphs). (B) For AM depletion, mice received 50-μl doses of L-Cl2MBP i.n. on days −4 and −2 relative to the time of i.n. virus infection. Control mice received 50-μl doses of L-PBS (PBS-Lip). AM depletion was monitored on days 1, 3, 5, and 7 p.i. by collecting cells from lung BAL samples from infected mice, as described in Materials and Methods. The results at day 7 p.i. are shown and are expressed as the number of AMs in the BAL sample.
We next examined what effect neutrophil and/or AM depletion would have if depletion was initiated before a sublethal (0.2 LD50) 1918 HA/NA:Tx/91 virus infection. Accordingly, groups of mice (20 per group) were depleted of neutrophils (by MAb RB6-8C5 treatment) and/or depleted of AMs. At various times following sublethal virus infections with 1918 HA/NA:Tx/91 virus, the lung and brain tissues of MAb RB6-8C5- and L-Cl2MBP-treated mice were collected for determination of infectious virus titers. Figure 4A shows that the mean virus titers in the lung were significantly higher at each time period examined in neutrophil- and/or AM-depleted animals compared with those of the controls. Infectious virus was also present in the brains of MAb RB6-8C5-treated and/or L-Cl2MBP-treated mice (Fig. 4B) but was not detected in the control animals. At the sublethal infectious dose of 1918 HA/NA:Tx/91 virus, symptoms of hunched posture, lethargy, and death were not observed in untreated control animals. However, we found that most neutrophil- and/or AM-depleted mice lost significant amounts of weight and were listless during the first week of infection; these symptoms were taken as signs of morbidity (data not shown). All AM-depleted mice succumbed to infection by day 8, whereas 4 of 10 mice depleted of neutrophils died (Fig. 4C). All mice depleted of both AMs and neutrophils rapidly succumbed to virus infection, and death occurred slightly sooner than it did in groups depleted of AM or neutrophils only. In contrast, all L-PBS and rat IgG control mice survived the sublethal virus infection. In a second independent experiment, both neutrophils and AMs were depleted before a sublethal virus infection with 0.2 LD50 of 1918 HA/NA:Tx/91 virus or 106 PFU of rTx/91 virus. All depleted mice challenged with 104 PFU 1918 HA/NA:Tx/91 virus died. In contrast, we did not observe any change in morbidity or mortality in mice challenged with 106 PFU of rTx/91 virus. These results demonstrate that AMs and neutrophils play a significant role in controlling the replication and spread of 1918 HA/NA:Tx/91 virus after infection.
FIG. 4.
Depletion (depl.) of neutrophils (PMN) and AM results in increased lung virus titers (A), brain virus titers (B), and lethality (C). Neutrophils were depleted in BALB/c mice (20 mice/group) by repeated i.p. injections of MAb RB6-8C5 (▴) or control rat IgG (•) 5 h before a 1918 HA/NA:Tx/91 virus infection and on days 3, 5, and 7 p.i to maintain depletion. For AM depletion, i.n. administration of 50 μl of L-Cl2MBP (□) on days −4 and −2 relative to the time of 1918 HA/NA:Tx/91 virus infection was performed. Control mice received 50-μl doses of L-PBS (○). An additional group of mice was depleted of both AMs and neutrophils (⧫). Mice were i.n. infected with sublethal doses of 0.2 LD50 of 1918 HA/NA:Tx/91 virus. On the days indicated, mice (four per group) were sacrificed, and individual lung and brain tissues were homogenized in 1 ml of PBS. The titers of the lysates for virus infectivity in eggs were determined. The remaining eight mice were followed daily for survival. The key corresponds to panels A and B. Asterisks indicate that the control lung titers are significantly (P < 0.05) different from lung titers from depleted mice, as determined by ANOVA.
Cytokine and chemokine expression in lungs of 1918 HA/NA-infected mice.
Following primary infection of mice with influenza A viruses, many cytokines and chemokines are produced in the lung (7, 27, 63) and are believed to contribute to the recruitment/activation of protective immune cells, thus facilitating the antiviral defense against influenza infection. Individual lung tissues were collected on day 5 p.i., a time point with maximal lung cytokine/chemokine levels following infection with highly pathogenic influenza strains (63). Tissues were homogenized, and lysates were assayed for cytokines and chemokines by ELISA. Determination of IFN-α, IFN-γ, TNF-α, MIP-1α, and MIP-2 levels demonstrated that all cytokines and chemokines were produced above their constitutive levels 5 days after infection with both 1918 HA/NA:Tx/91 and rTx/91 virus (Fig. 5A). Although the lung IFN-α levels were similar for both virus-infected groups, IFN-γ, TNF-α, and both MIP-1α and MIP-2 chemokines were detected at significantly higher levels in 1918 HA/NA:Tx/91-infected mice than in Tx/91-infected mice. Concentrations of TNF-α were low compared with concentrations of the other cytokines measured, but protein levels of this cytokine in 1918 HA/NA:Tx/91-infected mice were fourfold higher than those in Tx/91-infected mice.
FIG. 5.
Lung cytokine/chemokine activity in 1918 HA/NA:Tx/91 virus-infected lungs (A) and AM- and/or neutrophil (PMN)-depleted (depl.) mice (B). At day 5 p.i., five individual lung tissues were removed from each treated group and frozen at −70°C. Samples were thawed and homogenized in 1 ml of PBS, after which the clarified cell lysates were assayed by ELISA. The mean cytokine level ± standard deviation for each cytokine was determined. In addition, the constitutive cytokine level present in each tissue was determined by harvesting individual lung tissues from four uninfected BALB/c mice. The constitutive lung cytokine levels were 22, 27, and 35 pg/ml for IFN-α, IFN-γ, and TNF-α, respectively. The constitutive lung chemokine levels were 14 and 17 pg/ml for MIP-1α and MIP-2, respectively. (A) Asterisks indicate that the levels for the 1918 HA/NA:Tx/91-infected group were significantly (P < 0.05) different from those for the Tx/91-infected control group, as determined by ANOVA. (B) For AM depletion, mice received 50-μl doses of L-Cl2MBP i.n. on days −4 and −2 relative to the time of 1918 HA/NA:Tx/91 virus infection. Control mice received 50-μl doses of L-PBS. For neutrophil depletion, mice received i.p. injections of MAb RB6-8C5 or control rat IgG beginning 5 h before 1918 HA/NA:Tx/91 virus infection and on day 3 after infection to maintain depletion until the day of tissue collection (day 5 p.i.). Asterisks indicate that the control lung cytokine/chemokine levels are significantly (P < 0.05) different than those detected in depleted mice, as determined by ANOVA.
Since the previous experiments established that AM and neutrophils play a significant role in helping control the replication and spread of the 1918 HA/NA:Tx/91 virus after i.n. infection, we next determined whether these cells influenced the expression of cytokines/chemokines associated with virus infection. We found that neutrophil depletion was accompanied by a significant reduction of cytokine and chemokine expression in the infected lungs (Fig. 5B). Compared with expression in rat IgG control mice, IFN-α and IFN-γ levels were decreased by 81% and 87%, respectively, and TNF-α and MIP-2 levels fell by 24% and 37%, respectively. The level of the beta chemokine MIP-1α was also significantly lower (55%) in mice depleted of neutrophils than that in nondepleted control mice. Treatment of naïve mice with L-PBS or L-Cl2MBP alone did not increase the production of detectable lung cytokines or chemokines above the constitutive levels. AM-depleted mice also had decreased cytokine levels in the lungs following 1918 HA/NA:Tx/91 virus infection. Marked decreases in IFN-α, IFN-γ, TNF-α, and MIP-1α levels were observed in AM-depleted mice, whereas the level of MIP-2 was moderately reduced compared with cytokine levels in the L-PBS control mice. These results suggest that the 1918 HA/NA glycoproteins possess molecular determinants that are capable of inducing high levels of cytokines/chemokines in the mouse lung and that AMs and neutrophils are crucial for their optimal production.
DISCUSSION
During the 1918 influenza pandemic, pathologists observed at autopsy severe destruction of lung tissue unlike that typically seen in cases of pneumonia. Histological observations of archived fixed tissues from 1918 showed heavy infiltrations of white blood cells into influenza-infected lungs, including the alveoli, suggesting that such cells were involved in the pathogenesis of disease (35, 71, 72). The studies described herein were based on a previous observation that the lethal 1918 HA/NA:WSN recombinant influenza virus and the nonlethal New Caledonia HA/NA:WSN control virus induced markedly different patterns of mouse lung inflammation (30). Although both viruses induced a necrotizing bronchitis, which is characteristic of uncomplicated influenza virus infections, histological samples of the lungs of 1918 HA/NA:WSN-infected mice also displayed moderate-to-severe alveolitis characterized by increases of AMs and neutrophils. Kobasa et al. (34) also observed histologically a massive recruitment of neutrophils into the mouse lung following an intranasal infection of a 1918 HA recombinant virus. In the current study, a recombinant virus possessing the 1918 HA and NA genes on the genetic background of a Tx/91 virus replicated efficiently in the mouse lung and was lethal for this species, with an MDT of 7.3 to 7.9 days. Severe disease following 1918 HA/NA:Tx/91 virus infection was dose dependent, with only high doses of virus (≥105 PFU) causing significant morbidity and mortality. In contrast, the parental Tx/91 H1N1 virus did not cause mortality in mice at any virus dose and replicated to only low titers in mouse lungs. Thus, the 1918 HA/NA genes conferred upon the Tx/91 internal gene complex the property to grow to high titers in the respiratory tract without the need for prior adaptation, which is generally required for the replication of human H1N1 viruses in mice (28). In comparison to the Tx/91-infected control lungs, the 1918 HA/NA:Tx/91-infected lungs displayed a heavy inflammatory infiltration marked by a predominance of neutrophils associated with an increase of AMs and cytokines shortly before the death of these mice. To address the functional role of neutrophils and AMs during 1918 HA/NA:Tx/91 virus infection, we depleted mice of these cells and determined the severities of disease and degrees of virus clearance. In comparison to immunocompetent controls, AM- and neutrophil-depleted mice displayed higher virus titers in the lung tissue, which spread systemically to the brain after infection from an otherwise sublethal dose in nondepleted animals. In addition, depletion of neutrophils and/or AMs resulted in significantly lower levels of cytokines and chemokines in infected lung tissues. As a consequence, AM- and/or neutrophil-depleted mice displayed greater morbidity and mortality following sublethal 1918 HA/NA:Tx/91 virus infection.
It is unclear why the lethal 1918 HA/NA:Tx/91 and parental Tx/91 strains of influenza vary in their capacities to induce neutrophil migration into the mouse lung, particularly the alveoli. In the recipients of the Tx/91 control virus infections, lung tissues displayed a relatively modest inflammatory response accompanied by clearance of infectious virus and viral antigen by day 8 p.i. The inflammatory cells in these lungs appear restricted to the level of the bronchial epithelium and parabronchial area. In contrast, 1918 HA/NA:Tx/91 virus infection induced an increase in neutrophils that could be detected as early as 18 h after infection, and by day 5 the neutrophil influx was evident both in the peribronchium and alveoli. Furthermore, the pulmonary neutrophilia did not appear to be due to a higher incidence of coinfection with bacteria but was associated with higher titers of infectious virus and greater detected levels of viral antigen, including in the alveoli. However, the extent of viral replication alone does not appear to account for the enhanced mouse lung pathology observed among 1918 recombinant viruses (30, 34). Further studies to elucidate the actual stimuli for neutrophil accumulation into the mouse lung are under way. A variety of inflammatory mediators, including complement factors (29) and chemokines (7, 55), could cause neutrophil migration. Chemokine expression closely follows the level of influenza virus replication and affects the type of inflammatory response to infection (68). We focused on MIP-1α and MIP-2, since these chemokines have been shown to activate and exert chemotactic effects on neutrophils, although MIP-1α is also a potent chemoattractant for monocytes and activated T cells (73, 75). It has been demonstrated that influenza-infected mice carrying a disrupted MIP-1α (−/−) gene have reduced inflammation and delayed clearance of the virus when compared with infected wild-type (+/+) mice (7); thus, MIP-1α may play an important role during influenza virus infection. The greater expression of MIP-1α and MIP-2 chemokines in the 1918 HA/NA:Tx/91-infected lungs relative to those in Tx/91-control lungs correlates well with the increased neutrophil influx observed on days 5 and 7 p.i.
Conceivably, excessive accumulation of neutrophils and AMs in a vital organ, such as the lung, could contribute to tissue damage by causing vascular injury and destruction of the parenchymal cells (2, 37). The resulting loss of functional alveolar surface area would result in inadequate gas exchange, lower respiration, and, ultimately, death. In fact, deterioration of pulmonary function has been observed in patients with prolonged neutropenia (2). Furthermore, the overproduction of cytokines, such as IFN-γ and TNF-α, may be detrimental to the host and may be responsible for the enhanced morbidity and mortality by influenza virus infection (8, 11, 57, 60). Increased expression of TNF-α has been associated with highly pathogenic influenza H5N1 viruses in vitro and has been noted in postmortem lung tissue of H5N1 fatal cases (6, 42). TNF-α can also function to enhance neutrophilic infiltration into the lung tissue (33). We found that the expression of TNF-α was two- to threefold higher in the 1918 HA/NA:Tx/91-infected lungs preceding the death of these mice (day 5 p.i.) than that in to Tx/91-infected control lungs. Analysis of IFN-γ cytokine levels in 1918 HA/NA:Tx/91-infected mouse lungs also demonstrated a significant increase over that in Tx/91-infected control lungs. Neutrophils elicit numerous responses in the presence of IFN-γ; these responses may include increased oxidative burst, induction of antigen presentation, and chemokine production (12, 13). The neutrophils in the 1918 HA/NA:Tx/91-infected lungs may have been one source of the cytokines and chemokines that were observed, since neutrophil depletion resulted in significantly lower levels of TNF-α, IFN-γ, and both MIP chemokines. Migration of neutrophils into the lung is thought to occur in a cascading manner. In a cycle that repeats itself, infiltrating neutrophils and AMs produce more cytokines/chemokines, which in turn chemoattract additional cells into the lung and thus increase lung inflammation (54, 77).
The notion of neutrophils and AMs driving the increased lung pathology and contributing to mortality following 1918 HA/NA:Tx/91 virus infection was addressed in our initial depletion studies. However, we found that the depletion of neutrophils and/or AMs after the initiation of lung inflammation had no effect on the overall disease outcome following lethal challenge with the 1918 HA/NA recombinant virus. Thus, despite effective and substantial depletion of neutrophils and AMs in the lungs of treated animals, these mice displayed severe morbidity and had MDTs in the range of those of untreated control animals (7.1 to 7.4 days). However, it was observed in these initial experiments that delayed depletion caused slightly greater viral burden in the infected lungs, suggesting that these cells may function either directly or indirectly to control virus replication in the lung. We subsequently found that if mice were depleted of neutrophils and/or AMs before a sublethal infection with 1918 HA/NA:Tx/91 virus, the animals displayed significant weight loss and increased mortality. Furthermore, the virus spread systemically to the brain, a property not seen with the immunocompetent control mice infected with the 1918 HA/NA:Tx/91 virus. These results suggest that although neutrophils are the predominant cell in the 1918 HA/NA:Tx/91-infected lung and may contribute to the pathology, they are essential for host defense by controlling the replication and spread of virus after a primary infection. This antiviral role of neutrophils extends the findings of previous studies (16, 17, 41, 61), including those that identified antiviral substances with IFN-like activity against human and bovine herpes viruses (53). It remains unresolved how neutrophils exert their antiviral effect against influenza, as their defense capabilities are quite diverse. Neutrophils have been shown to adhere to influenza virus-infected cells and to phagocytose influenza virions in vitro (16, 46). It has become clear that mature neutrophils, although limited in their capacity to synthesize and secrete proteins, nevertheless produce a variety of mediators, some of which may have antiviral activity. The production of immune mediators, such as myeloperoxidase (76), defensins (18), and reactive oxygen and/or nitrogen species (16, 56), by activated neutrophils may directly inhibit influenza growth in the lung. The production of IFN-α or antiviral cytokines, such as TNF-α, has antiviral activity against influenza (45, 75).
Our results also show that the elimination of AM has a dramatic effect on the host defense to 1918 HA/NA:Tx/91 virus infection. While only some of the neutrophil-depleted mice died following a sublethal 1918 HA/NA:Tx/91 virus infection, 100% mortality was observed for AM-depleted mice. Similar to the response with neutrophil depletion, we found that in the absence of AMs, mice were unable to control 1918 HA/NA:Tx/91 virus infection or its spread to the brain tissues. This does not appear to be due to impaired neutrophil recruitment in AM-depleted lungs, because neutrophils are recruited in large numbers in 1918 HA/NA:Tx/91-infected lungs but cannot functionally compensate for the antiviral functions of the depleted AM. The exact mode of action of AMs in controlling 1918 HA/NA:Tx/91 virus replication remains to be revealed. Most likely, AMs can phagocytose virus particles in the lung (54) and synthesize critical immune mediators, including nitric oxide (43) and cytokines (77). AMs can also regulate cell-mediated immune responses to influenza infection (60, 69). The significant reduction of cytokines and chemokines in AM-depleted mice would suggest that AMs play an important role as a cytokine source during the early stages of infection.
One explanation for the apparent alveolar tropism of the 1918 recombinant viruses may be that it is partly due to the ability of the 1918 HA to target certain cell types in the mouse lung alveoli. The HA surface glycoprotein possesses receptor-binding properties that determine the host range of influenza by binding to cell surface glycoproteins containing terminal sialic acid residues. The nature of the receptor sialic acid linkage to a neighboring galactose can occur in the alpha 2,3 linkage or the alpha 2,6 linkage. Although both Tx/91 and 1918 HAs have a preference for binding 2,6-linked sialic acids (21), differences in binding affinities or in binding specificities to different glycosugars might be responsible for an expanded cell tropism of the 1918 recombinant viruses that results in increased replication in the lungs. Alternatively, the 1918 HA and/or NA proteins might confer upon the virus an increased ability to evade the antiviral host response. In fact, influenza H1 and H3 subtypes have been shown to deactivate neutrophil functions that appear to be mediated by HA binding to neutrophil surface sialic acid-bearing sites (24). Further studies are needed to determine the composition of the sialic acid in the deeper airways of the alveoli and to determine whether the receptor affinity of the 1918 HA increases the cellular tropism for resident cells of the alveoli.
Arguably, the 1918 pandemic influenza virus was successful because of its ability to replicate efficiently in hosts with little or no immunity to the virus of the H1N1 subtype. However, its ability to induce severe lung pathology, particularly in young adults, suggests that host factors contributed to the high virulence of this virus. In this study, a recombinant virus containing the 1918 HA and NA glycoproteins prepared on the genetic background of a nonlethal influenza H1N1 1991 strain gave the virus a lethal phenotype in mice. More striking still were the high titers of virus in the lungs of these mice, which were associated with an influx of substantial numbers of neutrophils and macrophages into the infected lung, particularly the alveoli. While the AMs and neutrophils may be contributing to the overall pathogenesis, due to their presence in the airways, their role is crucial in controlling the growth and promoting clearance of this highly virulent virus. These results may have important implications in defining the pathogenesis of severe influenza virus infections caused by highly virulent influenza strains.
Acknowledgments
This work was partially supported by grants from the NIH to P.P., A.G.-S., C.F.B., and J.K.T. This work was partially supported by USDA/ARS CRIS project number 6612-32000-039-00D and NIH grant PO1 AI058113-01. P.P. is a Senior Fellow of the Ellison Medical Foundation. C.F.B. is a New Scholar of the Ellison Medical Foundation Program in Global Infectious Diseases.
We thank Randy A. Albrecht and Claudia Chesley for critical reviews of the manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agencies.
REFERENCES
- 1.Ada, G. L., and P. D. Jones. 1986. The immune response to influenza infection. Curr. Top. Microbiol. Immunol. 128:1-54. [DOI] [PubMed] [Google Scholar]
- 2.Azoulay, E., M. Darmon, C. Delclaux, F. Fieux, C. Bornstain, D. Moreau, H. Attalah, J.-R. Le Gall, and B. Schlemmer. 2002. Deterioration of previous acute lung injury during neutropenia recovery. Crit. Care Med. 30:781-786. [DOI] [PubMed] [Google Scholar]
- 3.Barbeito, M. S., G. Abraham, M. Best, P. Cairns. P. Langevin, W. G. Sterritt, D. Barr, W. Meulepas, J. M. Sanchez-Vizcaino, and M. Saraza. 1995. Recommended biocontainment features for research and diagnostic facilities where animal pathogens are used. First International Veterinary Biosafety Workshop. Rev. Sci. Tech. 14:873-887. [DOI] [PubMed] [Google Scholar]
- 4.Basler, C. F., A. H. Reid, J. K. Dybing, T. A. Janczewski, T. G. Fanning, H. Zheng, M. Salvatore, M. L. Perdue, D. E. Swayne, A. Garcia-Sastre, P. Palese, and J. K. Taubenberger. 2001. Sequence of the 1918 pandemic influenza virus nonstructural gene (NS) segment and characterization of recombinant viruses bearing the 1918 NS genes. Proc. Natl. Acad. Sci. USA 98:2746-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brough-Holub, E., G. B. Toews, J. F. van Iwaarden, R. M. Strieter, S. L. Kunkel, R. Paine, and T. J. Standiford. 1997. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 65:1139-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung, C. Y., L. L. Poon, A. S. Lau, W. Luk, Y. L. Lau, K. F. Shortridge, S. Gordon, Y. Guan, and J. S. Peiris. 2002. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 360:1831-1837. [DOI] [PubMed] [Google Scholar]
- 7.Conn, C. A., J. L. McClellan, H. F. Maassab, C. W. Smitka, J. A. Majde, and M. J. Kluger. 1995. Cytokines and the acute phase response to influenza virus in mice. Am. J. Physiol. 268:R78-R84. [DOI] [PubMed] [Google Scholar]
- 8.Cook, D. N., M. A. Beck, T. M. Coffman, S. L. Kirby, J. F. Sheridan, I. B. Pragnell, and O. Smithies. 1995. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science 269:1583-1585. [DOI] [PubMed] [Google Scholar]
- 9.Crosby, A. 1989. America's forgotten pandemic. Cambridge University Press, Cambridge, United Kingdom.
- 10.Doherty, P. C., W. Allan, M. Eichelberger, and S. R. Carding. 1992. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu. Rev. Immunol. 10:123-151. [DOI] [PubMed] [Google Scholar]
- 11.Dunn, A. J., J. Wang, and T. Ando. 1999. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv. Exp. Med. Biol. 461:117-127. [DOI] [PubMed] [Google Scholar]
- 12.Ellis, T. N., and B. L. Beaman. 2004. Interferon-gamma activation of polymorphonuclear neutrophil function. Immunology 112:2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrar, M. A., and R. D. Schreiber. 1993. The molecular cell biology of interferon-gamma and its receptor. Annu. Rev. Immunol. 11:571. [DOI] [PubMed] [Google Scholar]
- 14.Fodor, E., L. Devenish, O. G. Engelhardt, P. Palese, G. G. Brownlee, and A. Garcia-Sastre. 1999. Rescue of influenza A virus from recombinant DNA. J. Virol. 73:9679-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost, W. 1920. Statistics of influenza morbidity. Public Health Rep. 35:584-597. [Google Scholar]
- 16.Fujisawa, H., S. Tsuru, M. Taniguchi, Y. Zinnaka, and K. Nomoto. 1987. Protective mechanisms against pulmonary infection with influenza virus. I. Relative contribution of polymorphonuclear leukocytes and of alveolar macrophages to protection during the early phase of intranasal infection. J. Gen. Virol. 68:425-432. [DOI] [PubMed] [Google Scholar]
- 17.Fujisawa, H. 2001. Inhibitory role of neutrophils on influenza virus multiplication in the lungs of mice. Microbiol. Immunol. 45:679-688. [DOI] [PubMed] [Google Scholar]
- 18.Ganz, T., M. E. Selsted, D. Szklarek, S. S. Harwig, K. Daher, D. F. Bainton, and R. I. Lehrer. 1985. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Investig. 76:1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geiss, G. K., M. Salvatore, T. M. Tumpey, V. S. Carter, X. Wang, C. F. Basler, J. K. Taubenberger, R. E. Bumgarner, P. Palese, M. G. Katze, and A. Garcia-Sastre. 2002. Cellular transcriptional profiling in influenza A virus-infected lung epithelial cells: the role of the nonstructural NS1 protein in the evasion of the host innate defense and its potential contribution to pandemic influenza. Proc. Natl. Acad. Sci. USA 99:10736-10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerhard, W., K. Mozdanowska, M. Furchner, G. Washko, and K. Maiese. 1997. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol. Rev. 159:95-103. [DOI] [PubMed] [Google Scholar]
- 21.Glaser, L., J. Stevens, D. Zamarin, I. A. Wilson, A. García-Sastre, T. M. Tumpey, C. F. Basler, J. K. Taubenberger, and P. Palese. 2005. A single amino acid substitution in the 1918 influenza virus hemagglutinin changes the receptor binding specificity. J. Virol. 79:11533-11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goto, H., and Y. Kawaoka. 1998. A novel mechanism for the acquisition of virulence by a human influenza A virus. Proc. Natl. Acad. Sci. USA 95:10224-10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harmsen, A. G., B. A. Muggenburg, M. B. Snipes, and D. E. Bice. The role of macrophages in particle translocation from lungs to lymph nodes. Science 230:1277-1280. [DOI] [PubMed]
- 24.Hartshorn, K. L., L. S. Liou, M. R. White, M. M. Kazhdan, J. L. Tauber, and A. I. Tauber. 1995. Neutrophil deactivation by influenza A virus. Role of hemagglutinin binding to specific sialic acid-bearing cellular proteins. J. Immunol. 154:3952-3960. [PubMed] [Google Scholar]
- 25.Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293:1840-1842. [DOI] [PubMed] [Google Scholar]
- 26.Hayden, F. G, R. S. Fritz, M. Lobo, W. G. Alvord, W. Strober, and S. E. Straus. 1998. Local and systemic cytokine responses during experimental human influenza A virus infection. J. Clin. Investig. 101:643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hennet, T., H. J. Ziltener, K. Frei, and E. Peterhans. 1992. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J. Immunol. 149:932-939. [PubMed] [Google Scholar]
- 28.Hoyle, L. 1968. Adaptation of virus to mice, p. 170-171. In S. Gard, C. Hallauer, and K. F. Meyer (ed.), The influenza viruses. Springer-Verlag, New York, N.Y.
- 29.Jaeschke, H., A. Farhood, A. P. Bautista, Z. Spolarics, and J. J. Spitzer. 1993. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am. J. Physiol. 264:G801-G809. [DOI] [PubMed] [Google Scholar]
- 30.Kash, J. C., C. F. Basler, A. Garcia-Sastre, V. Carter, R. Billharz, D. E. Swayne, R. M. Przygodski, J. K. Taubenberger, M. Katze, and T. M. Tumpey. 2004. Global host immune response: pathogenesis and transcriptional profiling of type A influenza viruses expressing the hemagglutinin and neuraminidase genes from the 1918 pandemic virus. J. Virol. 78:9499-9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz, J. M., X. Lu, S. A. Young, and J. C. Galphin. 1997. Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine. J. Infect. Dis. 175:352-363. [DOI] [PubMed] [Google Scholar]
- 32.Kilbourne, E. D. 1977. Influenza pandemics in perspective. JAMA 237:1225-1228. [PubMed] [Google Scholar]
- 33.Kips, J. C., J. H. Tavernier, and R. A. Pauwels. 1992. Tumor necrosis factor causes bronchial hyperresponsiveness in rats. Am. Rev. Respir. Dis. 145:332-336. [DOI] [PubMed] [Google Scholar]
- 34.Kobasa, D., A. Takada, K. Shinya, M. Hatta, P. Halfmann, S. Theriault, H. Suzuki, H. Nishimura, K. Mitamura, N. Sugaya, T. Usui, T. Murata, Y. Maeda, S. Watanabe, M. Suresh, T. Suzuki, Y. Suzuki, H. Feldmann, and Y. Kawaoka. 2004. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature 431:703-707. [DOI] [PubMed] [Google Scholar]
- 35.LeCount, E. R. 1919. The pathologic anatomy of influenzal bronchopneumonia. JAMA 72:650-652. [Google Scholar]
- 36.Lu, X., T. M. Tumpey, T. Morken, S. R. Zaki, N. J. Cox, and J. M. Katz. 1999. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 73:5903-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulligan, M. S., S. R. Watson, C. Fennie, and P. A. Ward. 1993. Protective effects of selectin chimeras in neutrophil-mediated lung injury. J. Immunol. 151:6410-6417. [PubMed] [Google Scholar]
- 38.Nagendra, S., and A. J. Schlueter. 2004. Absence of cross-reactivity between murine Ly-6C and Ly-6G. Cytometry 58:195-200. [DOI] [PubMed] [Google Scholar]
- 39.Nathan, C. F. 1987. Secretory products of macrophages. J. Clin. Investig. 79:319-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noble, G. R. 1982. Epidemiological and clinical aspects of influenza, p. 11-50. In A. S. Beere (ed.), Basic and applied influenza research. CRC Press, Baton Rouge, Fla.
- 41.Ohmann, H. B., M. Campos, D. R. Fitzpatrick, N. Rapin, and L. A. Babiuk. 1989. A. neutrophil-derived antiviral protein: induction requirements and biological properties. J. Virol. 63:1916-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peiris, J. S., W. C. Yu, C. W. Leung, C. Y. Cheung, W. F. Ng, J. M. Nicholls, T. K. Ng, K. H. Chan, S. T. Lai, W. L. Lim, K. Y. Yuen, and Y. Guan. 2004. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363:617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pendino, K. J., J. D. Laskin, R. L. Shuler, C. J. Punjabi, and D. L. Laskin. 1993. Enhanced production of nitric oxide by rat alveolar macrophages after inhalation of a pulmonary irritant is associated with increased expression of nitric oxide synthase. J. Immunol. 151:7196-7205. [PubMed] [Google Scholar]
- 44.Perdue, M. L., and D. L. Suarez. 2000. Structural features of the avian influenza virus hemagglutinin that influence virulence. Vet. Microbiol. 74:77-86. [DOI] [PubMed] [Google Scholar]
- 45.Pestka, S., J. A. Langer, K. C. Zoon, and C. E. Samuel. 1987. Interferons and their actions. Annu. Rev. Biochem. 56:727. [DOI] [PubMed] [Google Scholar]
- 46.Ratcliffe, D. R., S. L. Nolin, and E. B. Cramer. 1988. Neutrophil interaction with influenza-infected epithelial cells. Blood 72:142-149. [PubMed] [Google Scholar]
- 47.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 48.Reid, A. H., T. G. Fanning, J. V. Hultin, and J. K. Taubenberger. 1999. Origin and evolution of the 1918 “Spanish” influenza virus hemagglutinin gene. Proc. Natl. Acad. Sci. USA 96:1651-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reid, A. H., T. G. Fanning, T. A. Janczewski, and J. K. Taubenberger. 2000. Characterization of the 1918 “Spanish” influenza virus neuraminidase gene. Proc. Natl. Acad. Sci. USA 97:6785-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reid, A. H., J. K. Taubenberger, and T. G. Fanning. 2001. The 1918 Spanish influenza: integrating history and biology. Microbes Infect. 3:81-87. [DOI] [PubMed] [Google Scholar]
- 51.Reid, A. H., T. G. Fanning, T. A. Janczewski, S. McCall, and J. K. Taubenberger. 2002. Characterization of the 1918 “Spanish” influenza virus matrix gene segment. J. Virol. 76:10717-10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reid, A. H., T. G. Fanning, T. A. Janczewski, R. M. Lourens, and J. K. Taubenberger. 2004. Novel origin of the 1918 pandemic influenza virus nucleoprotein gene. J. Virol. 78:12462-12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rouse, B. T., L. A. Babiuk, and P. M. Henson. 1980. Neutrophils in antiviral immunity: inhibition of virus replication by a mediator produced by bovine neutrophils. J. Infect. Dis. 141:223-232. [DOI] [PubMed] [Google Scholar]
- 54.Sibille, Y., and H. Y. Reynolds. 1990. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am. Rev. Respir. Dis. 141:471-501. [DOI] [PubMed] [Google Scholar]
- 55.Simonet, W. S., T. M. Hughes, H. Q. Nguyen, L. D. Trebasky, D. M. Danilenko, E. S. Medlock, and S. Chapman. 1995. Long-term impaired neutrophil migration in mice overexpressing human interleukin-8. J. Clin. Investig. 94:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith, J. A. 1994. Neutrophils, host defense, and inflammation: a double-edged sword. J. Leukoc. Biol. 56:672-686. [DOI] [PubMed] [Google Scholar]
- 57.Swiergiel, A. H., and A. J. Dunn. 1999. The roles of IL-1, IL-6, and TNF-alpha in the feeding responses to endotoxin and influenza virus infection in mice. Brain Behav. Immun. 13:252-265. [DOI] [PubMed] [Google Scholar]
- 58.Taubenberger, J. K., A. H. Reid, A. E. Krafft, K. E. Bijwaard, and T. G. Fanning. 1997. Initial genetic characterization of the 1918 “Spanish” influenza virus. Science 275:1793-1796. [DOI] [PubMed] [Google Scholar]
- 59.Taubenberger, J. K., A. H. Reid, T. G. Fanning, and T. A. Janczewski. 2001. Integrating historical, clinical and molecular genetic data in order to explain the origin and virulence of the 1918 Spanish influenza virus. Philos. Trans. R. Soc. Lond. B 356:1829-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thepen, T., N. van Rooijen, and G. Kraal. 1989. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J. Exp. Med. 170:499-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsuru, S., H. Fujisawa, M. Taniguchi, Y. Zinnaka, and K. Nomoto. 1987. Mechanism of protection during the early phase of a generalized viral infection. II. Contribution of polymorphonuclear leukocytes to protection against intravenous infection with influenza virus. J. Gen. Virol. 68:419-424. [DOI] [PubMed] [Google Scholar]
- 62.Tumpey, T. M., S.-H. Chen, J. E. Oakes, and R. N. Lausch. 1996. Neutrophil-mediated suppression of virus replication following herpes simplex virus type 1 infection of the murine cornea. J. Virol. 70:898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tumpey, T. M., X. Lu, T. Morken, S. R. Zaki, and J. M. Katz. 2000. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J. Virol. 74:6105-6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tumpey, T. M., A. Garcia-Sastre, A. Mikulasova, J. K. Taubenberger, D. E. Swayne, P. Palese, and C. F. Basler. 2002. Existing antivirals are effective against influenza viruses with genes from the 1918 pandemic virus. Proc. Natl. Acad. Sci. USA 99:13849-13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tumpey, T. M., A. Garcia-Sastre, J. K. Taubenberger, P. Palese, D. E. Swayne, and C. F. Basler. 2004. Pathogenicity and immunogenicity of influenza viruses with genes from the 1918 pandemic virus. Proc. Natl. Acad. Sci. USA 101:3166-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174:83-93. [DOI] [PubMed] [Google Scholar]
- 67.van Strijp, J. A., K. P. van Kessel, L. A. Miltenburg, A. C. Fluit, and J. Verhoef. 1988. Attachment of human polymorphonuclear leukocytes to herpes simplex virus-infected fibroblasts mediated by antibody-independent complement activation. J. Virol. 62:847-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wareing, M. D., A. B. Lyon, B. Lu, C. Gerard, and S. R. Sarawar. 2004. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J. Leukoc. Biol. 76:886-895. [DOI] [PubMed] [Google Scholar]
- 69.Wijburg, O. L., S. DiNatale, J. Vadolas, N. van Rooijen, and R. A. Strugnell. 1997. Alveolar macrophages regulate the induction of primary cytotoxic T-lymphocyte responses during influenza virus infection. J. Virol. 71:9450-9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wijburg, O. L., J. Vadolas, A. Sanders, R. A. Strugnell, and N. van Rooijen. 1998. The role of macrophages in the induction and regulation of immunity elicited by exogenous antigens. Eur. J. Immunol. 28:479-487. [DOI] [PubMed] [Google Scholar]
- 71.Winternitz, M. C., I. M. Wason, and F. P. McNamara. 1920. The pathology of influenza. Yale University Press, New Haven, Conn.
- 72.Wolbach, S. B. 1919. Comments on the pathology and bacteriology of fatal influenza cases, as observed at Camp Devens, Mass. Johns Hopkins Hosp. Bull. 30:104. [Google Scholar]
- 73.Wolpe, S. D., B. Sherry, D. Juers, G. Davatelis, R. W. Yurt, and A. Cerami. 1989. Identification and characterization of macrophage inflammatory protein 2. Proc. Natl. Acad. Sci. USA 86:612-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wright, P. F., and R. G. Webster. 2001. Orthomyxoviruses, p. 1533-1579. In D. M. Knipe, P. M. Howley, et al. Fields virology. Lippincott, Williams, and Wilkins, Philadelphia, Pa.
- 75.Xing, Z., M. Jordana, H. Kirpalani, K. E. Driscoll, T. J. Schall, and J. Gauldie. 1994. Cytokine expression by neutrophils and macrophages in vivo: endotoxin induces tumor necrosis factor-alpha, macrophage inflammatory protein-2, interleukin-1 beta, and interleukin-6 but not RANTES or transforming growth factor-beta 1 mRNA expression in acute lung inflammation. Am. J. Respir. Cell Mol. Biol. 10:148-153. [DOI] [PubMed] [Google Scholar]
- 76.Yamamoto, K., T. Miyoshi-Koshio, Y. Utsuki, S. Mizuno, and K. Suzuki. 1994. Virucidal activity and viral protein modification by myeloperoxidase: a candidate for defense factor of human polymorphonuclear leukocytes against influenza virus infection. J. Infect. Dis. 164:8-14. [DOI] [PubMed] [Google Scholar]
- 77.Zhang, P., W. R. Summer, G. J. Bagby, and S. Nelson. 2000. Innate immunity and pulmonary host defense. Immunol. Rev. 173:39-51. [DOI] [PubMed] [Google Scholar]