Abstract
The activity of five simulated antifungal regimens for eradication of catheter-related bloodstream Candida infection was evaluated with an in vitro pharmacodynamic model. Single-lumen central venous catheters were colonized with Candida species by sequentially incubating central venous catheters in plasma and then in growth medium (RPMI plus morpholinepropanesulfonic acid) containing a standardized suspension (105 CFU/ml) of Candida albicans, Candida glabrata, or slime-producing Candida parapsilosis. Colonized central venous catheters were then placed in a one-compartment pharmacodynamic model where five antifungal regimens (plus control) were simulated: amphotericin B, 1.0 mg/kg every 24 h; amphotericin B, 0.5 mg/kg every 24 h; fluconazole, 400 mg every 24 h; fluconazole, 800 mg every 24 h; and voriconazole, 4 mg/kg every 12 h. During exposure to the simulated clinical regimens, samples were serially removed from the model over 48 h for quantitation of viable organisms. All antifungal regimens suppressed fungal counts by both peripheral and catheter sampling versus control (P = 0.001). Overall, antifungal activity ranked amphotericin B (1 mg/kg) > amphotericin B (0.5 mg/kg) ≥ voriconazole > fluconazole (800 mg) ≥ fluconazole (400 mg). No regimen, however, completely eradicated (by culture and electron microscopy) central venous catheter colonization. Regrowth was noted in the model during therapy against C. glabrata and C. parapsilosis but was not associated with an increase in the MICs for the isolates. Lack of in vitro antifungal activity against biofilm-encased organisms appeared to be the primary reason for mycological failure of antifungal regimens in the model.
Intravascular catheters have become an essential component in the supportive care of patients undergoing intensive medical and surgical treatments. Use of these devices, however, is compromised by increased patient risk for the development of life-threatening bloodstream infections, which affect 4 to 8% of all central venous catheters used in the United States (14, 21). The most common pathogens responsible for catheter-related bloodstream infection are coagulase-negative staphylococci, Staphylococcus aureus, and Candida species (14). Of these organisms, Candida bloodstream infections have been associated with the highest rates of morbidity and attributable mortality (38%) (7, 26).
Current guidelines for the treatment of catheter-related Candida bloodstream infection advocate removal of the central venous catheter when a bloodstream infection is documented (14, 25). However, prompt removal of an infected central venous catheter is not always possible, especially in a patient who is hemodynamically unstable or in a cytopenic patients who is at high risk for bleeding. In these situations, clinicians are often forced to treat catheter-related Candida bloodstream infection in situ with systemic antifungal therapy until the removal or replacement of the catheter is medically feasible (14, 16). This treatment strategy attempts to reduce the burden of organisms on the catheter lumen and prevent metastatic seeding until the catheter can be removed. The optimal antifungal therapy for treating catheter-related Candida bloodstream infection in this setting is unknown.
Because clinical trials examining therapies for the treatment of catheter-related Candida bloodstream infection in situ would be difficult to complete, alternative methods are needed to identify optimal treatment strategies for catheter-related Candida bloodstream infection. In vitro models represent one such alternative, as they have been used successfully to identify promising treatment regimens for bacterial catheter-related bloodstream infection (3, 4, 9). More recently, an in vitro pharmacodynamic model of Candida bloodstream infection was used to examine the activity of combination antifungal therapy for Candida infections (13). To that end, we developed an in vitro model of catheter-related Candida bloodstream infection and compared the ability of simulated amphotericin B, fluconazole, and voriconazole dosing regimens to suppress and/or eradicate Candida species from experimentally infected central venous catheters.
(This work was presented at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 2001, abstr. J-108.)
MATERIALS AND METHODS
Fungal isolates.
Candida albicans (ATCC 90028), Candida glabrata (ATCC 582), and a clinical isolate of Candida parapsilosis (OY8-68), previously characterized as a heavy slime-producing organism (19), were tested in the model. All isolates were subcultured twice on potato dextrose agar (Remel, Lenexa, Kans.) prior to testing.
Antifungal agents.
Amphotericin B solution (5 mg/ml) was prepared fresh prior to each experiment by reconstituting a 10-ml vial of amphotericin B-deoxycholate lyophilized powder (Pharma-Tek, Inc., Huntington, N.Y.) with sterile water. Fluconazole solution for injection (2 mg/ml) and voriconazole (UK-109,496) powder were provided by Pfizer-Roerig Pharmaceuticals (New York, N.Y.). Voriconazole powder was dissolved in 20% (vol/vol) dimethyl sulfoxide-sterile water (10 mg/ml) and then diluted in sterile water to a concentration of 0.5 mg/ml.
Antifungal susceptibility testing.
MICs were determined by the National Committee for Clinical Laboratory Standards (NCCLS) broth microdilution technique (M27-A) (14a). Two strains, Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258, served as quality control isolates. The growth medium for MIC determination was RPMI 1640 (Sigma Chemicals, St. Louis, Mo.) buffered to a pH of 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS). All susceptibility tests were incubated for 48 h at 35°C in a dark, humid chamber. MICs were determined with a visual endpoint as recommended by the NCCLS M27-A methods. For isolates recovered from the model, susceptibility testing was performed directly by adjusting the inoculum in RPMI medium by either centrifugation at 6,000 × g (concentration) or dilution with fresh RPMI medium to a 0.5 MacFarlane standard (0.80 to 0.82 transmittance) on a spectrophotometer with uninoculated RPMI medium as a blank. Testing was then performed as described in the M27-A document.
Establishment of catheter colonization.
Sterile, single-lumen, 7-French polyurethane catheters (Cook Critical Care, Bloomington, Ind.) were infected by a modification of the method proposed by Guggenbichler et al. (9). In preliminary studies, we determined that this procedure resulted in the colonization of polyurethane catheters with biofilm-embedded organisms. Catheters were placed in sterile culture tubes, and 10 ml of human plasma (alanine aminotransferase elevated; Gulf Coast Blood Blank, Houston, Tex.) was injected via the catheter lumen into the culture tube. The tube and catheter were then incubated at 35°C for 24 h.
Catheters were removed and placed in a separate sterile tube, and a standardized inoculum (1 × 105 to 5 × 105 CFU/ml) of each Candida isolate prepared in RPMI growth medium was injected (10 ml) via the catheter lumen. After an additional 24 h of incubation, catheters were removed from the growth medium and rinsed with sterile saline. To remove nonadherent organisms from the inside of the catheter lumen, 60 ml of sterile saline was rapidly injected via the catheter lumen. Preliminary studies demonstrated that this method resulted in a colonization burden (as measured by sonicated catheter cultures) of approximately 3 × 103 ± 1 × 103 CFU/ml for each catheter-isolate combination tested.
In vitro infection model.
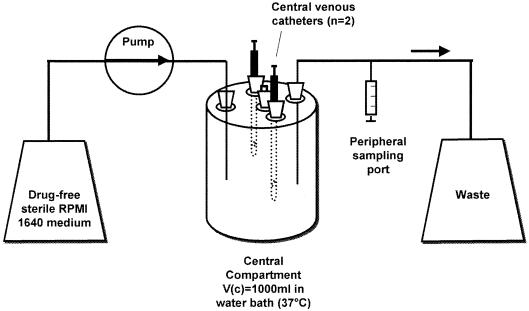
A one-compartment in vitro infection model (Fig. 1) was used to simulate the serum pharmacokinetics of clinical antifungal dosing regimens (13). The central glass compartment (Vc) of the model (1,000 ml) contained a magnetic stirbar for continuous mixing and sample ports to allow the insertion of infected catheters directly into the central compartment. The entire central compartment was placed in a water bath maintained at 37°C. Sterile drug-free RPMI growth medium was pumped into the central compartment with a computerized peristaltic pump (Masterflex LS, Vernon Hills, Ill.) at a fixed rate to simulate the 24-h (fluconazole, amphotericin B) or 6-h (voriconazole) half-lives of the antifungal regimens tested. After the desired flow rate was established in the model, two infected catheters were inserted into the central compartment, and the model was allowed to equilibrate for 30 min.
FIG. 1.
Schematic of the in vitro catheter-related bloodstream infection model.
Antifungals were then administered as bolus injections through the catheter lumens to achieve target pharmacokinetic parameters in the model (see Table 1). Six antifungal regimens were tested in the model for each isolate: control (sterile saline); fluconazole, 400 mg administered every 24 h (every 24 h); fluconazole. 800 mg every 24 h; amphotericin B, 0.5 mg/kg (simulated body weight of 70 kg) every 24 h; amphotericin B, 1.0 mg/kg every 24 h; and voriconazole, 4 mg/kg every 12 h. All experiments were carried out for 48 h and performed in duplicate.
TABLE 1.
Target and actual antifungal pharmacokinetic values
| Dose | Target (actual)
|
|||
|---|---|---|---|---|
| Cmax (mg/liter) | Cmin (mg/liter) | t1/2 (h) | AUC0-24 (mg · h/liter) | |
| Amphotericin B | 1.2 (1.2) | 0.5 (0.12) | 24 (12) | 20 (16) |
| 0.5 mg/kg every 24 h | ||||
| 1.0 mg/kg every 24 h | 2.4 (2.6) | 1.2 (0.4) | 24 (11) | 43 (37) |
| Fluconazole | 20 (17) | 10 (8) | 24 (18) | 360 (314) |
| 400 mg every 24 h | ||||
| 800 mg every 24 h | 40 (40) | 20 (16) | 24 (18) | 720 (672) |
| Voriconazole, 4 mg/kg every 12 h | 3.5 (3.9) | 0.8 (0.7) | 6 (8) | 51 (57) |
Antifungal assays.
Antifungal concentrations in the model were verified by stability-indicating high-performance liquid chromatography (HPLC) assays. Concentrations of amphotericin B, voriconazole, and fluconazole were determined with the modified reversed-phase high-pressure liquid chromatographic methods of Ng (15) for amphotericin B and of Perea (18) for voriconazole and fluconazole. Samples of RPMI were diluted 1:1 with acetonitrile before injection onto the analytical column. A Shimadzu (Shimadzu Corp., Kyoto, Japan) isocratic system comprising a peristaltic pump, UV-VIS detector, autoinjector, and integrator was used for all compounds. Briefly, the amphotericin B assay employed a mobile phase of acetonitrile-ammonium acetate with 0.2% diethylamine (25 mM, 38:62 [vol/vol]) delivered at 1 ml/min. Detection of amphotericin B and the internal standard, 1-amino-4-nitro-naphthylene, was performed at 406 nm with an elution time of 4.5 and 2.7 min, respectively. A Nova-Pak C18 (Waters, Milford, Mass.) analytical column, 5 μm (150 by 4.6 mm), was used.
For voriconazole and fluconazole, an Alltima C18 (Alltech, Deerfield, Ill.), 5-μm column was used. Voriconazole and fluconazole, with its internal standard, tinidazole, were detected at 260 nm. The mobile phase consisted of acetonitrile-ammonium dihydrogen phosphate (40 mM, pH 6.0, 68:32 [vol/vol]) for voriconazole and acetonitrile-ammonium dihydrogen phosphate (40 mM, pH 6.0, 32:68 [vol/vol]) for fluconazole and tinidazole. Flow rates were 0.8 ml/min. Voriconazole, fluconazole, and tinidazole eluted at 10.8, 6.5, and 5.8 min, respectively.
Quantification was based on the ratio of peak heights for amphotericin B, fluconazole, and their respective internal standards. The peak height was used alone for voriconazole. The standard curve concentration range was 0.25 to 4.0 mg/liter, 0.5 to 5.0 mg/liter, and 2.5 to 40 mg/liter for amphotericin B, voriconazole, and fluconazole, respectively. Five-point standard curves including a blank sample in RPMI were linear, with R2 values of ≥0.999. The lower limit of quantification was the lowest concentration of the standards for each of the compounds. Accuracies were ≥90% for all compounds, and the intra- and interday variation did not exceed 10% for the range of concentrations tested.
Pharmacokinetic analysis.
After multiple flushes of the catheters with medium from the central compartment of the model, samples (500 μl) were obtained at serial time points for the determination of antifungal concentrations. Samples were stored at −70°C until analysis. The peak concentration (Cmax), trough concentration (Cmin), elimination half-life, and 24-h area under the concentration-time curve (AUC0-24) were calculated from the concentration time data with a noncompartmental method for intravenous bolus administration (WinNonLin version 3.0; Pharsight, Mountain View, Calif.).
Pharmacodynamic analysis.
The efficacy of the simulated antifungal regimens was determined by quantifying viable CFU in samples recovered both from the model and through experimentally infected catheters. Samples (500 μl) were removed from the central compartment of the model through the catheter lumen as well as a peripheral sampling port at predetermined time points after antifungal therapy was started. Samples were diluted 1:10 in sterile saline, vortexed, and plated (50 μl) in triplicate on 110-mm potato dextrose agar plates with an automated spiral plater (Autoplate 4000; Spiral Biotech, Bethesda, Md.). Plates were then incubated for 48 h at 35°C. Colony counts were then performed and analyzed with a plate scanner (CASBA-4 Colony Image Analysis; Spiral Biotech). This sampling method can accurately quantify 50 CFU/ml with minimal or no antifungal carryover (17). Repeat susceptibility testing was performed on isolates obtained from the model if >1 − log10 growth was noted between 24 and 48 h during an experiment.
To examine the activity of the antifungal regimens on adherent organisms, catheters were removed from the model at 24 and 48 h for culturing by the sonication method (20). The catheters were rinsed with sterile saline as previously described, cut 5 cm above the tip, and placed in a culture tube containing 10 ml of RPMI growth medium. The entire culture tube was sonicated (Mettler Electronics, Anaheim, Calif.) at 55,000 Hz for 10 min and then vortexed for 15 s. A sample (500 μl) of the culture medium was then removed and plated (50 μl) for CFU quantitation as previously described.
Scanning electron microscopy.
In selected experiments, catheters removed from the model were cut and rinsed as described previously but were then immediately immersed in a 4% aqueous solution of glutaraldehyde (Sigma, St Louis, Mo.). Catheter segments were then cut into 5-mm slices, fixed with osmium tetroxide, and dehydrated with a graded series of ethanol washes, followed by immersion in hexamethyldisilizane. Following removal from hexamethyldisilizane, samples were air dried, mounted on aluminum stubs, and sputter coated with Pd and Au. A Hitachi S4000 scanning electron microscope (Hitachi Scientific Instruments, Mountain View, Calif.) was used for visualization of the samples.
Data analysis.
Mean colony count data for samples removed from the model were plotted as a function of time for each isolate-drug regimen tested. Viable colony counts recovered from samples pulled through the catheter and peripheral ports as well as counts recovered from sonicated catheter cultures were compared by analysis of variance with Tukey's test for multiple comparisons. For all comparisons, a P value of ≤0.05 was considered significant. All statistical analyses were performed with the Sigmastat statistical software package (version 2.03; Jandel Scientific, San Rafael, Calif.).
RESULTS
Antifungal assay and pharmacokinetic analysis.
Actual drug concentrations measured in the model are presented in Table 1. The limits for detection were 0.1, 0.5, and 0.25 mg/liter for fluconazole, amphotericin B, and voriconazole, respectively. The results of HPLC assays were reproducible for all three antifungals, with median coefficients of variation of 1.5% (amphotericin B), 3.25% (fluconazole), and 4% (voriconazole). Although peak, trough, and elimination half-life were relatively close to target pharmacokinetic parameters in the model for azoles, the elimination of amphotericin B from the model was more rapid than expected. We suspected that this rapid elimination was an artifact of the poor stability of amphotericin B in warm RPMI. To evaluate this possibility, we performed HPLC studies on several amphotericin B concentrations (0.5, 1.0, and 2.0 mg/liter) after incubation in RPMI in the dark for 24 h. These studies demonstrated a 30 to 40% decrease from the original amphotericin B concentrations after incubation for 24 h at 35°C (data not shown). Therefore, the apparent rapid elimination of amphotericin B is probably explained by drug degradation in the model.
Susceptibility testing.
Median broth microdilution susceptibility testing results are presented in Table 2. MICs for amphotericin B and voriconazole were well within the range or below the drug concentrations achieved in the model. The C. glabrata isolates tested in the model were susceptible-dose dependent to fluconazole (16 to 32 mg/liter). MICs for organisms recovered from the model at 48 h did not differ by more than one dilution from the MIC of the starting inoculum used to infect the catheters.
TABLE 2.
Microdilution broth 48-h MICs for study isolates
| Isolate | Median MIC (mg/liter) (n = 8 tests)
|
||
|---|---|---|---|
| Amphotericin B | Fluconazole | Voriconazole | |
| C. albicans ATCC 90028 | 0.5 | 0.5 | 0.06 |
| C. glabrata ATCC 582 | 1 | 16 | 0.5 |
| C. parapsilosis OY8-68 | 0.5 | 0.25 | 0.015 |
Pharmacodynamic analysis.
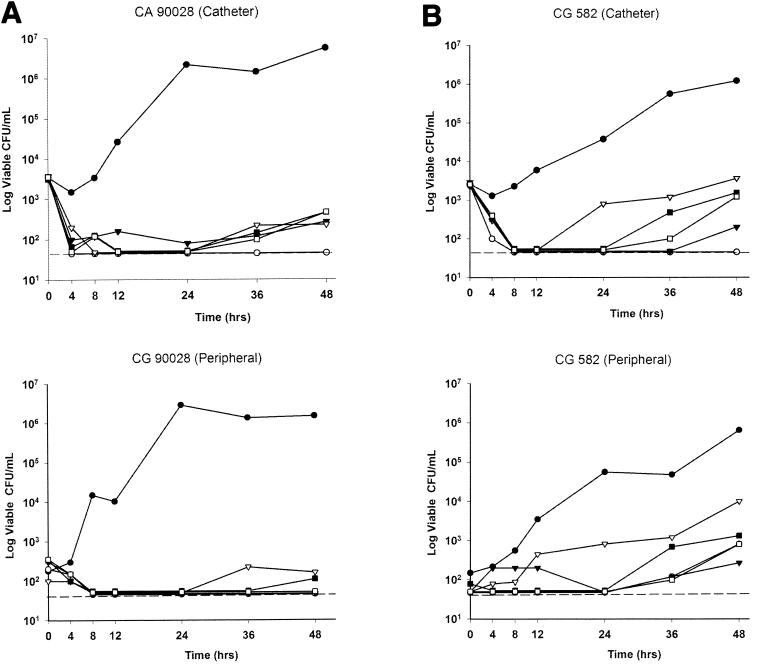
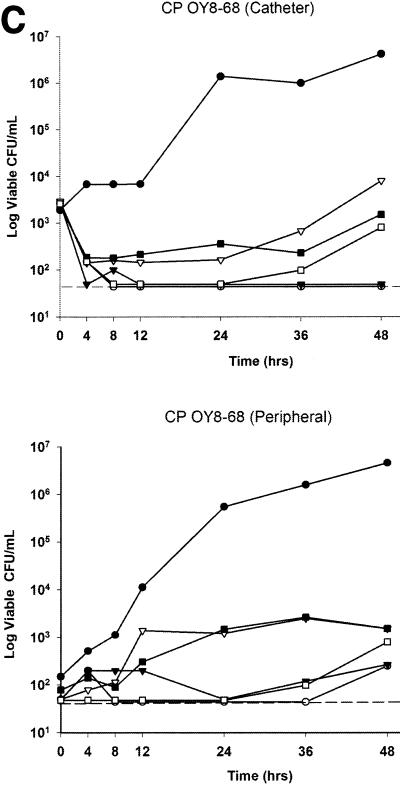
Plots of time versus viable CFU recovered from the catheter and peripheral draws are presented in Fig. 2. Fungal counts in samples pulled through the catheter exhibited a 2-log10 reduction over the first two sampling time points. This drop was seen with both fungistatic (fluconazole, voriconazole) and fungicidal (amphotericin B) regimens and likely represents an initial loss of loosely adherent fungal plaques from the inside of the catheter lumen. At 48 h, a nearly 4-log10 increase in CFU per milliliter was noted in the model for infected catheters that were not treated with antifungals. All antifungal regimens were effective in suppressing fungal counts in the model versus the control by either catheter or peripheral sampling (P = 0.005). No regimen, however, completely suppressed the recovery of viable CFU from the model. Amphotericin B at 1.0 mg/kg every 24 h was the most active regimen tested by catheter or peripheral sampling (P = 0.004), followed by amphotericin B (0.5 mg/kg) ≥ voriconazole (4 mg/kg every 12 h) > fluconazole (800 mg every 24 h) > fluconazole (400 mg every 24 h).
FIG. 2.
Time versus median CFU plots from catheter and peripheral samples. (A) Candida albicans ATCC 90028 (CA 90028). (B) Candida glabrata ATCC 582 (CG 582). (C) Candida parapsilosis OY8-68 (CP OY8-68). The dashed line shows the lower limit of quantitation. Symbols: •, control; ○, amphotericin B, 1 mg/kg every 24 h; ▴, amphotericin B, 0.5 mg/kg every 24 h; ▵, fluconazole, 400 mg every 24 h; ▪, fluconazole, 800 mg every 24 h; □, voriconazole, 4 mg/kg every 12 h.
Sonicated catheter cultures revealed that none of the colonized catheters was completely sterilized following exposure to antifungal therapy (Table 3). No significant differences in sonicated catheter cultures were noted for any of the antifungal regimens versus the control at 24 h (P = 0.108). However, at 48 h, amphotericin B at 1.0 mg/kg every 24 h appeared to significantly reduce (but not eliminate) the burden of organisms on catheters infected with C. albicans ATCC 90028 or C. glabrata ATCC 582 at 48 h (P = 0.037). Similarly, at 48 h, voriconazole-exposed catheters exhibited a significantly lower burden of organisms versus the control for isolate CA90028 (P = 0.037). For the heavy slime-producing isolate C. paraspilosis OY8-68, none of the antifungal regimens tested appeared to have a measurable effect on the burden of catheter colonization.
TABLE 3.
Median viable CFU recovered from sonicated catheter culturesa
| Regimen | Median log10 CFU (range)
|
|||||
|---|---|---|---|---|---|---|
|
C. albicans
|
C. glabrata
|
C. parapsilosis
|
||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| Control | 3.3 (2.7-3.9) | 4.2 (3.6-4.8) | 3.1 (2.7-3.5) | 4.3 (3.7-4.9) | 3.3 (2.8-3.8) | 3.1 (2.7-3.5) |
| Amphotericin B | ||||||
| 0.5 mg/kg | 2.7 (2.2-3.2) | 3.4 (2.3-4.7) | 3.0 (2.5-3.4) | 3.4 (3-3.9) | 3.1 (2.7-3.5) | 3.3 (2.8-3.8) |
| 1.0 mg/kg | 2.2 (1.8-2.6) | 2.3* (1.7-2.9) | 2.2 (1.9-2.3) | 2.3* (1.6-3.0) | 3.0 (2.5-3.6) | 4.3 (3.7-4.8) |
| Fluconazole | ||||||
| 400 mg | 3.3 (2.7-3.9) | 3.4 (2.8-3.9) | 3.0 (2.4-3.6) | 4.3 (3.9-4.7) | 3.3 (2.9-3.7) | 3.3 (2.8-3.8) |
| 800 mg | 3.4 (2.6-4.2) | 3.3 (2.8-4.1) | 3.0 (2.6-3.4) | 3.7 (3.2-4.2) | 3.4 (2.9-3.9) | 3.7 (3.1-4.3) |
| Voriconazole | ||||||
| 4.0 mg/kg | 2.3 (1.7-2.8) | 2.3* (1.8-2.8) | 3.0 (2.6-3.4) | 3.2 (2.7-3.7) | 3.1 (2.6-3.8) | 3.1 (2.8-3.4) |
*, significantly different from control at 48 h (P = 0.037). All other values not significant.
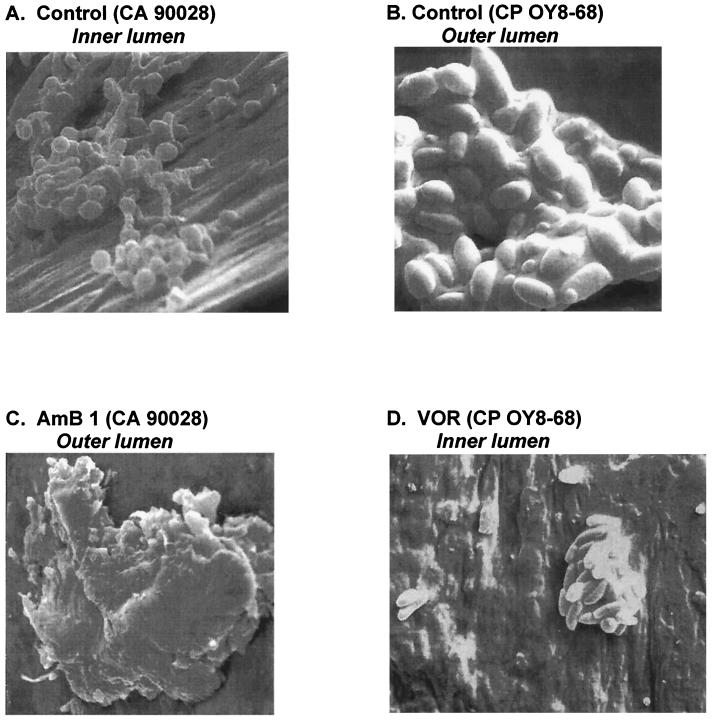
Electron microscopy of catheters removed from the model confirmed the presence of biofilm-encased plaques containing Candida species on both the inner and exterior lumen surfaces of the catheter (Fig. 3). No relationship was noted between the antifungal regimens and the pattern or density of biofilm plaques or fungal morphology observed by electron microscopy. However, copious biofilm production by the slime-producing isolate of C. parapsilosis could be easily appreciated in electron microscopy studies (Fig. 3B, 3D).
FIG. 3.
Representative scanning electron microscopy images of catheter segments recovered from the model at 48 h. (A) Control; C. albicans ATCC 90028 (CA 90028), inner lumen (1,000×). (B) Control; C. parapsilosis OY8-68 (CP OY8-68), outer lumen (5,000×). (C) Amphotericin B (AmB), 1.0 mg/kg every 24 h; C. albicans ATCC 90028, outer lumen (2,000×). (D) Voriconazole (VOR), 4 mg/kg every 12 h; C. parapsilosis OY8-68, outer lumen (2,000×).
Notably, fungal regrowth was present in our model between 36 and 48 h for nearly all of the regimen-isolate combinations tested and was especially prominent after 24 h for the azole-susceptible dose-dependent isolate (C. glabrata ATCC 582) and the heavy biofilm producing isolate of C. parapsilosis (OY8-68). When regrowth isolates recovered from the model were retested for antifungal susceptibility, MICs were unchanged.
DISCUSSION
Prompt catheter exchange or removal is an important component in the management of patients with catheter-related Candida bloodstream infection (23). Retention of catheters colonized with Candida species has been associated with prolonged fungemia, high failure rates for antifungal therapy, and increased risk of metastatic complications and death (6, 11, 24). Results from our model are consistent with the clinical observation that current antifungal therapy alone has poor activity against Candida species embedded in the biofilm matrix.
Biofilm-mediated antifungal resistance is a well-documented phenomenon among Candida species and likely contributes to its pathogenic niche in catheter-related bloodstream infection (8, 10). The formation of a biofilm layer on the catheter may impart resistance to antifungals by restricting their penetration, decreasing the growth rate (and hence susceptibility) of organisms in the biofilm matrix, and upregulating genes that are responsible for antifungal resistance (12). Several studies have described dramatic decreases in the in vitro antifungal susceptibility of C. albicans to azoles, flucytosine, and amphotericin B (100- to 1,000-fold increase in MIC) when cells are grown in biofilm versus planktonic conditions (1, 2, 22).
Using a microtiter-based method colorimetric assay, Ramage and colleagues examined the antifungal susceptibilities of seven C. albicans isolates grown under planktonic and sessile (biofilm-embedded) conditions (22). Under planktonic conditions, azole MICs ranged from 0.5 to 16 mg/liter. However, the median MIC that inhibited 50% and 90% of isolates for sessile cells was in excess of 1,024 mg/liter. Although differences were noted in the amphotericin B MICs for isolates tested in planktonic versus sessile conditions, the median MIC rarely increased by more than two to four dilutions. This discrepancy in antifungal susceptibility noted between planktonic and biofilm-embedded Candida species may partially explain why in vitro susceptibility testing with planktonic cells sometimes demonstrates poor correlation with in vivo response to antifungal therapy.
Recently, Baillie and Douglas used special culture techniques to examine the influence of C. albicans biofilm thickness on the extent of antifungal resistance (2). Interestingly, these authors found that biofilm thickness did not correlate with susceptibility to five antifungals (amphotericin B, fluconazole, itraconazole, ketoconazole, and flucytosine) with different physiochemical characteristics, suggesting that the biofilm matrix itself does not constitute a barrier to antifungal perfusion. Similar to our findings, these authors noted that amphotericin B at high concentrations was the only antifungal agent capable of modestly reducing (20 to 50%) the burden of biofilm-embedded Candida organisms (2).
We believe that the presence of regrowth in the model between 36 and 48 h is an important finding of our study. However, when the regrowth isolates were retested for antifungal susceptibility, MICs were either unchanged or within one dilution (plus or minus) of the original MIC for the test inoculum. Therefore, it appears that regrowth in the model was not due to selection of a less-susceptible subpopulation of organisms or induction of new resistance mechanisms. Regrowth was most likely the result of seeding of recalcitrant organisms that remained viable in the biofilm matrix surrounding the catheters. This seeding was most evident as antifungal levels in the model fell near or below the MIC for the organisms and occurred earlier for the less-susceptible isolates tested in the model.
Studies examining antibacterial activity in biofilms have noted a similar phenomenon, where the inoculum of biofilm-embedded organisms drops by two to three orders of magnitude only to leave an even more slowly growing subpopulation of biofilm cells that are insensitive to further increases in drug concentrations (5). These cells, called persister cells, are paradoxically preserved in the biofilm despite the presence of antimicrobial concentrations that inhibit their growth in vitro (12). Normally, these persister cells would be eliminated by the immune system; however, the biofilm matrix prevents their recognition and elimination. As drug concentrations drop both inside and outside the biofilm matrix, they resume their metabolic activity, divide, and spawn new planktonic cells.
The persister phenotype has not been extensively examined for Candida species, and it is not known which antifungal agents or dosing strategies would be most effective for eradicating them from colonized catheters. Fungicidal agents against Candida (polyenes and echinocandins, either alone or in combination) would seem to be the most promising agents. However, more data will be needed to better define the optimal agents and combinations for persistent Candida subpopulations.
Although we believe the model used in this study is useful for comparing antifungal regimens for catheter-related bloodstream Candida infection, it has several limitations. One of these limitations is that the efficacy of the simulated antifungal regimens was tested in an artificial medium (RPMI 1640) that does not necessarily reflect in vivo conditions or account for the potential contribution of the host immune response. Additionally, these experiments were only designed to compare antifungal activity against catheters that were already colonized with Candida species. The ability of various antifungal regimens to prevent catheter colonization or preemptively treat early colonization was not addressed in the present study. Moreover, the applicability of test variables selected for testing antifungal regimens in the model (isolates, growth medium, and inoculum) to actual in vivo catheter-related bloodstream Candida infection is not fully known.
In conclusion, although we found some differences among the ability of antifungal regimens to suppress growth of Candida species, none of the regimens demonstrated an ability to completely eradicate catheter-related Candida bloodstream colonization. The results of this in vitro study illustrate the inherent difficulty in sterilizing central venous catheters colonized with Candida species and support the practice of prompt catheter removal in candidemic patients. Lack of activity against biofilm-encased organisms appeared to be the primary reason for antifungal failure in this model.
Acknowledgments
Research support for this study was provided by Pfizer, Inc.
We thank Fred Clubb for help with the electron microscopy studies, Michael Pfaller and Sam Messer for providing some of the study isolates, and Jingduan Chi for help with the antifungal assays.
REFERENCES
- 1.Baillie, G. S., and L. J. Douglas. 1999. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 310:644-656. [DOI] [PubMed] [Google Scholar]
- 2.Baillie, G. S., and L. J. Douglas. 2000. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 46:397-403. [DOI] [PubMed] [Google Scholar]
- 3.Bayston, R. 1984. A model of catheter colonization in vitro and its relationship to clinical catheter infections. J. Infect. 9:271-276. [DOI] [PubMed] [Google Scholar]
- 4.Bayston, R., V. Zdroyewski, and S. Barsham. 1988. Use of an in vitro model for studying the eradication of catheter colonization by Staphylococcus epidermidis. J. Infect. 16:141-146. [DOI] [PubMed] [Google Scholar]
- 5.Brooun, A., S. Liu, and K. Lewis. 2000. A dose-response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 44:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dato, V. M., and A. S. Dajani. 1990. Candidemia in children with central venous catheters: role of catheter removal and amphotericin B therapy. Pediatr. Infect. Dis. J. 9:309-314. [DOI] [PubMed] [Google Scholar]
- 7.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 8.Fridkin, S. K., and W. R. Jarvis. 1996. Epidemiology of nosocomial fungal infections. Clin. Microbiol. Rev. 9:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guggenbichler, J. P., D. Berchtold, F. Allerberger, H. Bonatti, J. Hager, W. Pfaller, and M. P. Dierich. 1992. In vitro and in vivo effect of antibiotics on catheters colonized by staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 11:408-415. [DOI] [PubMed] [Google Scholar]
- 10.Hawser, S. P., G. S. Baillie, and L. J. Douglas. 1998. Production of extracellular matrix by Candida albicans biofilms. J. Med. Microbiol. 47:253-256. [DOI] [PubMed] [Google Scholar]
- 11.Lecciones, J. A., J. W. Lee, E. E. Navarro, F. G. Witebsky, D. Marshall, S. M. Steinberg, P. A. Pizzo, and T. J. Walsh. 1992. Vascular catheter-associated fungemia in patients with cancer: analysis of 155 episodes. Clin. Infect. Dis. 14:875-883. [DOI] [PubMed] [Google Scholar]
- 12.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis, R. E., B. C. Lund, M. E. Klepser, E. J. Ernst, and M. A. Pfaller. 1998. Assessment of antifungal activities of fluconazole and amphotericin B administered alone and in combination against Candida albicans by with a dynamic in vitro mycotic infection model. Antimicrob. Agents Chemother. 42:1382-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mermel, L. A., B. M. Farr, R. J. Sherertz, Raad, I. I., N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 32:1249-1272. [DOI] [PubMed] [Google Scholar]
- 14a.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard M27-A. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 15.Ng, T. K., R. C. Chan, F. A. Adeyemi-Doro, S. W. Cheung, and A. F. Cheng. 1996. Rapid high performance liquid chromatographic assay for antifungal agents in human sera. J. Antimicrob. Chemother. 37:465-472. [DOI] [PubMed] [Google Scholar]
- 16.Pearson, M. L. 1996. Guideline for prevention of intravascular device-related infections. I. Intravascular device-related infections: an overview. Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control 24:262-277. [DOI] [PubMed] [Google Scholar]
- 17.Pearson, R. D., R. T. Steigbigel, H. T. Davis, and S. W. Chapman. 1980. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob. Agents Chemother. 18:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perea, S., G. J. Pennick, A. Modak, A. W. Fothergill, D. A. Sutton, D. J. Sheehan, and M. G. Rinaldi. 2000. Comparison of high-performance liquid chromatographic and microbiological methods for determination of voriconazole levels in plasma. Antimicrob. Agents Chemother. 44:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller, M. A., S. A. Messer, and R. J. Hollis. 1995. Variations in DNA subtype, antifungal susceptibility, and slime production among clinical isolates of Candida parapsilosis. Diagn. Microbiol. Infect. Dis. 21:9-14. [DOI] [PubMed] [Google Scholar]
- 20.Raad, I. I., M. F. Sabbagh, K. H. Rand, and R. J. Sherertz. 1992. Quantitative tip culture methods and the diagnosis of central venous catheter-related infections. Diagn. Microbiol. Infect. Dis. 15:13-20. [DOI] [PubMed] [Google Scholar]
- 21.Raad, I. 1998. Intravascular-catheter-related infections. Lancet 351:893-898. [DOI] [PubMed] [Google Scholar]
- 22.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rex, J. H. 1996. Editorial response: catheters and candidemia. Clin. Infect. Dis. 22:467-470. [DOI] [PubMed] [Google Scholar]
- 24.Rex, J. H., J. E. Bennett, A. M. Sugar, P. G. Pappas, J. Serody, J. E. Edwards, and R. G. Washburn. 1995. Intravascular catheter exchange and duration of candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Clin. Infect. Dis. 21:994-996. [DOI] [PubMed] [Google Scholar]
- 25.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, and J. E. Edwards. 2000. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin. Infect. Dis. 30:662-678. [DOI] [PubMed] [Google Scholar]
- 26.Wenzel, R. P. 1995. Nosocomial candidemia: risk factors and attributable mortality. Clin. Infect. Dis. 20:1531-1534. [DOI] [PubMed] [Google Scholar]