Abstract
Real-time PCR was applied to quantify the abundance of human adenoviruses in two southern California urban rivers, the San Gabriel and Los Angeles. A total of 114 river samples from five different locations were collected over a 1-year period and analyzed for human adenoviruses, along with fecal indicator bacteria and coliphages. Adenoviruses were detected by real-time PCR in ∼16% of the samples, with concentrations ranging from 102 to 104 genomes per liter. However, a plaque assay using two human tissue culture cell lines, HEK-293A and A549, yielded negative results, suggesting that adenoviruses detected by real-time PCR are likely noninfectious. Enterovirus genome was detected in ∼7% of the samples by reverse transcription-PCR. Analysis by Spearman's rho rank order correlation showed significant correlations between fecal indicator bacteria and indicator virus (total coliform, fecal coliform, enterococcus, and coliphage values). However, no significant correlations were found between human adenoviruses quantified by real-time PCR and culturable coliphages or fecal indicator bacteria. Kruskal-Wallis chi-square analysis showed significant seasonal variability of all fecal indicator bacteria and coliphages, while no significant variability was observed for adenoviruses or enteroviruses. This study presents the first quantitative measurement of human adenovirus genomes in urban rivers and their statistical relationship to fecal indicator bacteria and coliphages. The uncoupling between high-number genome copies of adenoviruses detected by real-time PCR and the absence of infectivity detected by tissue culture suggests that genome-based detection methods are inadequate for direct assessment of human health risk.
Rapid population growth and urbanization occurring along coastal regions have made southern California one of the most densely populated areas in the United States. More than two-thirds of Californians visit local beaches at least once a year. Southern California beaches have unquestionably become one of the most desired beach vacation spots in the world, attracting over 100 million visitors to the shoreline annually and offering recreational activities such as swimming, diving, yachting, surfing, snorkeling, windsurfing, and fishing. Correspondingly, an increase in the volume of domestic sewage discharge and urban runoff to the coastal ocean has become a major public health concern in southern California (21, 30) and likewise nationwide.
Microbial contamination of our aquatic environments poses a potential public health risk when improperly managed (1, 8). The U.S. Environmental Protection Agency has established microbial pollution indicator standards recommending the use of bacterial indicators, including counts of total and fecal coliforms and enterococci, during routine monitoring (10). However, studies have shown that current bacterial indicator standards may not accurately indicate the viral quality of our waters (20, 41). Furthermore, previous studies of viral quality of coastal waters are mainly qualitative, rather than quantitative. Although a few recent investigations have reported developing real-time PCR methods for quantification of human viruses and their detection in sewage (26, 28, 29), little is known yet about the quantitative abundance of human viruses in the aquatic environment (13).
Proper monitoring of human viruses in our waters is of particular importance, because the Centers for Disease Control and Prevention suggest that the causative agent of nearly 50% of all acute gastrointestinal illnesses is suspected to be viral (6). With approximately 100 potentially water-transmissible human viruses associated with human waste, it is simply impossible to detect all viruses (2). However, certain human viruses and coliphages may beneficially serve as an index in determining viral contamination and the presence of human fecal waste (20, 31, 32). Adenovirus, a double-stranded DNA virus belonging to the family Adenoviridae, is a possible candidate as an index virus. Adenovirus is frequently found in urban rivers and polluted coastal waters (5, 7, 20, 32, 33, 39), and studies conducted in Europe suggest that adenovirus be used as an index of human viral pollution (32). Adenoviruses have also been shown to display greater resistance to UV treatments because they contain double-stranded DNA, which allows for repair of damaged DNA by using the enzymes of host cells (18, 23). Thurston-Enriquez et al. (40) showed that a UV dose of 103 mJ/cm2 was required to achieve a 99% inactivation of adenovirus serotype 40 (1 of 51 serotypes of adenovirus) in treated groundwater. Currently, the UV dose commonly applied for drinking water and wastewater treatment is between 30 and 40 mJ/cm2.
This study demonstrates the application of a newly developed quantitative PCR method (19) for assessing the adenovirus concentration in southern California urban rivers and investigates the relationship between adenoviruses detected by genomic amplification and by tissue culture infectivity assay. This study also provides insight into the relationships of current monitoring standards by determining if significant correlations exist between fecal indicator bacteria, coliphages, and human viruses.
MATERIALS AND METHODS
Sampling sites.
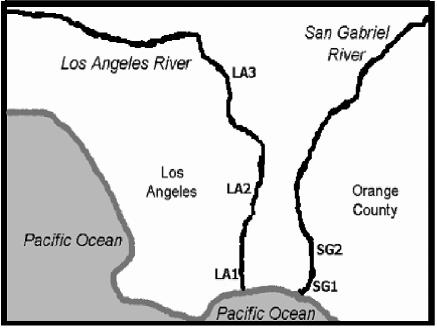
Between February 2002 and February 2003, water samples were collected from three different sampling sites along the Los Angeles River and two sites along the San Gabriel River (Fig. 1). The five sampling sites are designated LA1, LA2, and LA3 for the Los Angeles River and SG1 and SG2 for the San Gabriel River. Sites SG1 and LA1 are located at the mouth of each river, and LA2, LA3, and SG2 are located upstream approximately 5 to 35 miles apart. All sites are located in urban settings; the upstream sites are adjacent to major highways, while the two sites located at the mouths of the rivers are located in commercial settings. The water flow was fairly consistent for all sampling sites at the Los Angeles River, while water level and water turbidity fluctuated in the San Gabriel River throughout the study period, which may be associated with the discharge of tertiary treated sewage from the Los Angeles Sanitation District into the river. All sites of each river were sampled semimonthly using 10-liter containers disinfected with bleach. The samples were transported back to the laboratory at the University of California, Irvine, within 2 h of collection for immediate processing. Sample salinity was determined by refractometry (10), water temperature was measured on site, and rainfall events were recorded. During the study period, a total of 114 10-liter samples were collected from both rivers.
FIG. 1.
Sampling locations along Los Angeles and San Gabriel Rivers. LA1 is located at the mouth of the Los Angeles River, LA2 is located approximately 10 miles upstream, and LA3 is located approximately 35 miles upstream from the mouth. SG1 is located at the mouth of the San Gabriel River, and SG2 is located approximately 5 miles upstream.
Detection of indicator bacteria.
Analysis of fecal and total coliforms and enterococci was performed immediately after sample collection by membrane filtration, following the Standard Methods for the Examination of Water and Wastewater (10). Ten milliliters of serially diluted or undiluted environmental sample was filtered through a 0.45-μm-pore-size nitrocellulose filter. Fecal coliforms and total coliforms on the membrane were cultured using m-FC agar and m-Endo agar (Fisher Scientific, Inc., Tustin, CA) incubated overnight at 44.5°C and 37°C, respectively. For enterococcus analysis, mE agar (Fisher Scientific, Inc.) was used and incubated for 2 days at 41.5°C. Membranes were then transferred to Esculin Iron Agar plates (Becton Dickinson, Sparks, MD) and incubated for 20 min for confirmation and recorded as the number of CFU per 100 ml.
Concentration of water samples for viral analysis.
Ten-liter water samples were concentrated to ∼115 ml using a tangential flow filtration system (TFF) with a 30-kDa molecular-mass-cutoff Omega cartridge (Pall Life Science, Hauppauge, NY) at a flow rate of 350 to 450 ml/min under a constant pressure at <10 lb/in2. The samples were spiked with bacteriophage φHSIC, and the titers were determined with water samples prior to and after TFF concentration by plaque assay to determine the rate of viral recovery. φHSIC was isolated from the coastal water off Hawaii and was not found in urban river waters in southern California. Therefore, there are no interfering effects from the viral recovery assay (22). Immediately after concentration, 1 ml of the concentrated samples was used for coliphage plaque assay; the rest were stored at −80°C for later use.
Detection of coliphages.
Densities of coliphages and F-specific coliphages were determined by using both TFF-concentrated and nonconcentrated water samples. Both 100 μl and 1 ml of the concentrated and nonconcentrated samples were mixed with 1 ml of bacterial host in 3 ml of soft agar and poured over an LB agar bottom plate. Escherichia coli ATCC 15597 strain was used as the host for general coliphages (both somatic and F+ coliphage), while E. coli Famp was the specific host used for F-specific coliphages. Details of the plaque assay can be found in Jiang et al. (20). Plaques were enumerated after 12 to 24 h of incubation at 37°C.
Viral nucleic acid purification by GuSCN-silica bead extraction.
The method of Boom et al. (3) was used for viral nucleic acid purification and removal of PCR inhibitors for all environmental concentrates. Briefly, 900 μl of guanidinium thiocyanate lysis buffer (GuSCN) was added to a mixture of 40 μl of silica particle and 50 μl or 100 μl of viral concentrate at room temperature for 10 min. The mixture was then centrifuged, the supernatant was discarded, and the pellet was washed. The pellet was dried briefly, and nucleic acid was eluted from beads with 50 μl of 1× Tris-EDTA at 56°C.
Detection of human enterovirus by RT-PCR and probe hybridization.
The pan-enterovirus-specific primer sequences used for reverse transcription-PCR (RT-PCR) were 5′-CCTCCGGCCCCTGAATG-3′ and 5′-ACCGGATGGCCAATCCAA-3′ (11). This primer pair amplifies a target at the highly conserved 5′ untranslated region of enteroviruses. The PCR protocol followed that previously described by Tsai et al. (42). To confirm the amplicon, DNA in gel was transferred to positively charged MagnaGraph 0.45 Transfer Nylon membrane (Osmonics, Inc., Minnetonka, MN) via Southern transfer (36), and the membranes were prehybridized for 1 h at 37°C in solution containing 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 5× Denhardt solution, 0.05% sodium pyrophosphate, 100 mg of salmon sperm/ml, and 0.5% sodium dodecyl sulfate. Hybridization was carried out overnight at 45°C in solution containing 6× SSC, 1× Denhardt solution, 100 mg of salmon sperm/ml, 0.05% sodium pyrophosphate, and 25 ng of the [γ-32P]ATP-labeled oligonucleotide probe/ml. The sequence for the pan-enterovirus probe is 5′-TACTTTGGGTGTCCGTGT TTC-3′. After hybridization, the membrane was washed three times at room temperature (15 min each wash) and two more times at 55°C (1 h each time) with a solution containing 6× SSC, 1% sodium dodecyl sulfate, and 0.05% sodium pyrophosphate to remove any unbound probe. The membrane was sealed in a plastic bag and exposed to X-ray film for 24 to 48 h at −80°C.
Detection of human adenovirus by real-time PCR.
For adenovirus analysis by real-time PCR, samples were further concentrated by ultracentrifugation. In brief, 10 ml of TFF concentrate was centrifuged at 41,000 rpm with an SW41 Beckman rotor for 1.5 h at 10°C. The pellets were resuspended in ∼200 μl of the supernatant, and 100 μl was purified by GuSCN-silica bead extraction (as described above). Either 1 μl or 4 μl of purified viral concentrate (TFF ultracentrifugation) was used in triplicate reaction mixtures for real-time PCR. The standard curve used for quantification was plotted using Ct values of sixfold dilution of cloned adenovirus serotype 5 hexon gene (19). Quadruplicate samples were used for each dilution point, and a standard curve was run for each set of assay.
Real-time PCR primers and protocols were those developed by He and Jiang (19). Each real-time PCR mixture (25 μl) contained 1× TaqMan Universal PCR Master mix, 300 nM each primer, and 200 nM TaqMan probe. Degenerative primers specific to adenoviruses were 5′-GACTCYTCWGTSAGYTGGCC-3′ and 5′-CCCTGGTAKCCRATRTTGTA-3′, and the probe was FAM-AACCAGTCYTTGGTCATGTTRCATTG-TAMRA. (FAM is 6-carboxyfluorescein, and TAMRA is 6-carboxytetramethylrhodamine.) This real-time PCR assay specifically detects human adenovirus serotypes 1 to 7, 9, 17, 19, 21, 28, 37, 40, and 41 and simian serotype 25. The PCR cycles consist of holding samples at 50°C for 2 min and then 95°C for 10 min, followed by 45 cycles, each consisting of 95°C for 15 s, 56°C for 15 s, and 62°C for 30 s with 1-s increments between each cycle. An Applied Biosystems 7000 Sequence Detector system was used for real-time PCR, and the data was analyzed with ABI Prism 7000 software.
Tissue culture analysis for infectious human adenoviruses.
The infectivity of adenoviruses in environmental concentrates was analyzed using two cell lines, A549 and HEK-293A (19). The human embryonic kidney cell line (HEK-293A) was obtained from the University of Southern California (courtesy of Michael Lai) at 34 passages and was used in this study between passages 42 and 50. A human lung carcinoma cell line (A549) was obtained from the Los Angeles Sanitation District (courtesy of Shawn Thompson) at 108 passages and was used in this study between passages 115 and 123. The A549 human lung carcinoma cell line is currently the most commonly used cell line for adenovirus propagation and plaque titration while the 293 human kidney embryonic cells are mostly used as a package cell line for production of nonreplicative adenovirus vectors (15, 17, 37). HEK-293A is a recombinant cell line with an insertion of adenovirus E1 gene, so that E1 protein (early expression protein) is always expressed to enhance adenovirus replication (16). HEK-293A has a better ability to adhere to tissue culture plates than 293.
After TFF concentration and ultracentrifugation, approximately 200 μl of viral concentrate was purified by chloroform extraction, filtered through 0.2-μm low-protein binding and retention Whatman filters (Whatman International, Ltd., Clifton, NJ), and overlaid onto monolayers of HEK-293A and A549 cells. After being gently shaken for 60 min at 37°C, the monolayers were washed with prewarmed phosphate-buffered saline and overlaid with 1.25% agarose containing 5% Dulbecco's modified essential medium, 50-μg/ml gentamicin, and 2.5-μg/ml amphotericin B. The cell culture was incubated for 7 to 8 days in 5% CO2 at 37°C, and then a second overlay was carried out as described above and incubated for an additional 7 to 10 days. Cell cultures were examined under an Olympus microscope using a 10× magnification lens for plaque formation.
Statistical analysis.
Spearman's rho rank order correlation was used to determine correlations in seasonal distribution of fecal indicator bacteria, coliphages, and human adenoviruses detected by real-time PCR. In addition, a point-to-point correlation of each data point was viewed by scatter plots. Kruskal-Wallis chi-square analysis was used to determine significant variability in parameters during rainy and nonrainy seasons. The SPSS program (SPSS, Inc., Illinois) was used for all statistical analyses; correlations were considered significant at a 95% confidence level.
RESULTS
Indicator bacteria and coliphages.
Concentrations of fecal indicator bacteria and coliphages were log-normal distributed throughout the study period. Figure 2a to c report the seasonal geometric mean concentrations of all sampling sites for each month. All three fecal indicator bacterium counts were highest in concentration in November during a period of heavy rainstorm (Fig. 2a to c), while relatively lower concentrations were found in the months preceding the first rainfall of the season (November). Seasonal water temperatures varied within 4°C in all samples tested, and there was no apparent relationship between water temperature and the variability of indicator geometric means in each month. Figure 2d shows the spatial distribution of indicator bacteria; higher geometric means were detected in LA2 and SG2, where salinity was near 0 ppt, while bacterial contamination was approximately 1 order of magnitude lower at site LA1, where salinity averaged 28 ppt throughout the study period.
FIG. 2.
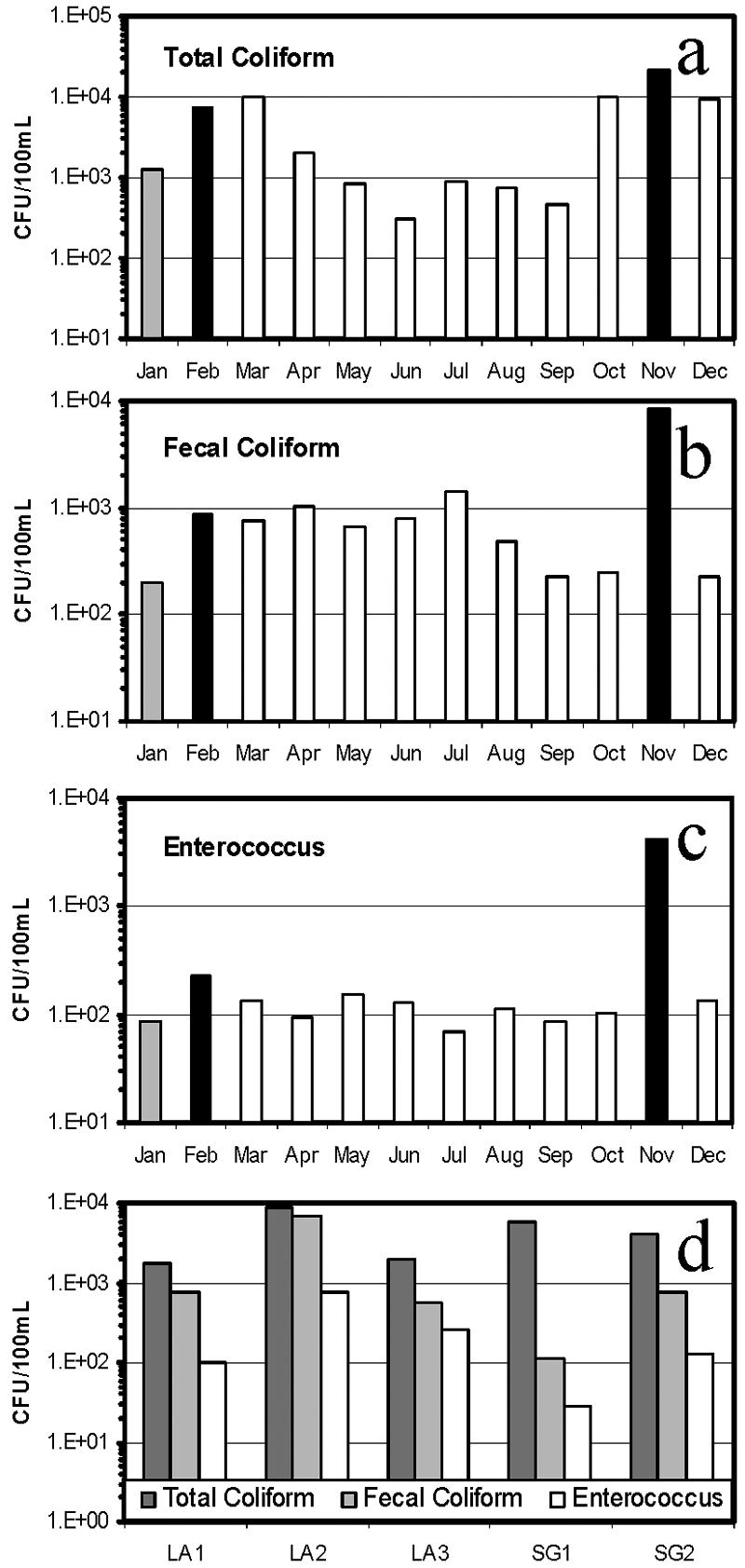
Monthly geometric mean distribution of total coliforms (a), fecal coliforms (b), and enterococci (c) at all sampling sites, and their annual geometric mean distribution at each sampling site (d). Dark bars indicate storm events within 48 h of sample collection, light gray bars indicate light rain (<0.05 in.), and open bars indicate no precipitation within 72 h of sample collection.
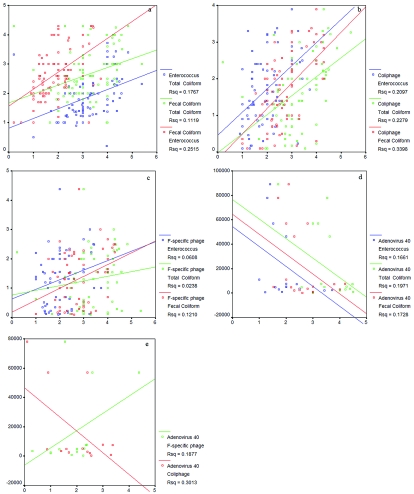
The seasonal geometric mean distribution of coliphages was similar to that of fecal indicator bacteria, with a major peak occurring within 48 h of a rainstorm during November (Fig. 3a and b). Increases in coliphage concentration surpassing 1 × 102 PFU/100 ml were also observed during the months of February, March, and June, while the lowest concentration was observed during the summer month of July. Similar to the trend of indicator bacteria spatial distribution pattern, higher concentrations of coliphage were also detected at LA2 and SG2 (Fig. 3c). Statistical analysis by Spearman's test showed significant correlations in seasonal distribution between coliphages and all three fecal indicator bacteria (P < 0.05). Statistical analysis by the Kruskal-Wallis test also showed a significant difference between rainy and nonrainy periods (P = 0.053) in coliphages, and all three fecal indicator bacterium concentrations. To visualize the point-by-point relationship between each parameter collected at each sample time and site, all data points were also plotted using the Scatter Plot tool in the SPSS software package. Positive correlations were identified among all fecal indicator bacteria and coliphages with an R2 coefficient of 0.024 to 0.340 (Fig. 4a to c).
FIG. 3.
Monthly geometric mean distribution of coliphages (a) and F-specific coliphages (b) at all sampling sites and their annual geometric mean distribution at each sampling site (c). Bar symbols are the same as described in the legend to Fig. 2.
FIG. 4.
Scatter plots of point-by-point relationships of all biological parameters collected during the study. (a) Best-fit lines represent the relationships between enterococci and fecal coliforms (blue), total coliforms and fecal coliforms (green), and total coliforms and enterococci (red). (b) Best-fit lines represent the relationships between coliphages and fecal coliforms (blue), coliphages and total coliforms (green), and coliphages and enterococci (red). (c) Best-fit lines represent the relationships between F-specific coliphages and enterococci (blue), F-specific coliphages and total coliforms (green), and F-specific coliphages and fecal coliforms (red). (d) Best-fit lines represent the relationships between adenoviruses and enterococci (blue), adenoviruses and total coliforms (green), and adenoviruses and fecal coliforms (red). (e) Best-fit lines represent the relationship between adenoviruses and coliphages (green) and adenoviruses and F-specific coliphages (red).
Human adenoviruses and enteroviruses.
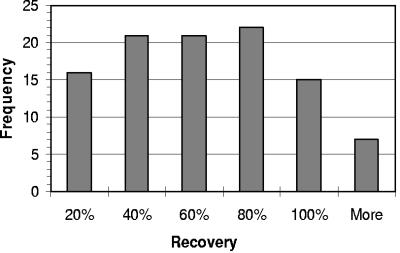
Viral concentrations with TFF recovered, on average, 54% of seeded bacteriophage φHSIC in the water samples, with a range of recovery rate from <10% to >100% (Fig. 5). φHSIC belongs to the Siphoviridae family, with an average head size of ∼47 nm and tail length of ∼147 nm (22). Human adenoviruses are medium-sized viruses with an average capsid size ranging from 90 to 100 nm, while enteroviruses are small viral particles with most members being <60 nm. Therefore, the recovery for adenoviruses may be slightly higher than that indicated by φHSIC seeding study, while enteroviruses may have equal or a slightly lower recovery rates. Due to the variability of viral recovery rates, the final viral concentration quantified by real-time PCR was not corrected by the loss during viral concentration. Therefore, the concentration presented below represents a conservative estimation of viruses in the environment.
FIG. 5.
Histogram of bacteriophage ΦHSIC concentration recovery rates with the Tangential Flow Filtration system with a 30-kDa molecular-mass-cutoff filtration cartridge.
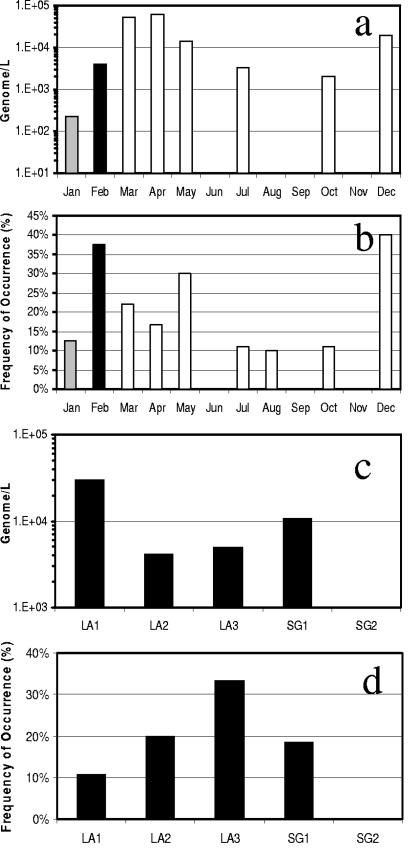
Adenoviruses were detected in approximately 16% of all samples analyzed by real-time PCR. The real-time PCR detection limit is approximately 1 × 102 to 2 × 102 genomes per liter. Therefore, the concentration of adenoviruses detected ranged from 102 to 104 genomes per liter of water for individual detectable samples. Approximately 5% (6 of 114 samples) of the samples exceeded 104 genomes per liter, 10-fold lower than those reported in primary sewage effluent by the same detection method (19). The concentrations of adenoviruses detected during the study period do not fit into normal or log-normal distribution patterns. Monthly median of all sampling sites showed the highest adenoviral genome concentrations during the months of March, May, and December (Fig. 6a). Adenoviruses were also most frequently detected in February, May, and December, where 30% or more of the samples collected were positive by real-time PCR (Fig. 6b). Spatial analysis showed that median adenovirus concentrations were higher at the mouth of Los Angeles and San Gabriel Rivers (Fig. 6c), while the frequency of occurrence was greater at the site most upstream in the Los Angeles River (Fig. 6d). Spearman's test showed no significant correlation in seasonal distribution of adenoviruses and fecal indicator bacteria or coliphages. Point-by-point correlations showed inverse relationships between indicator bacteria and adenoviruses with R2 coefficients ranging from 0.167 to 0.301 (Fig. 4d and e). The Kruskal-Wallis test showed no significant seasonal variability of adenovirus during rainy versus nonrainy periods.
FIG. 6.
Adenovirus monthly median concentrations (a), occurrence at all sampling sites (b), and annual median concentrations (c) and occurrence (d) at each sampling site. Bar symbols are the same as described in the legend to Fig. 2.
Approximately 7% of all samples analyzed were positive for enteroviruses by RT-PCR and confirmed by hybridization. The seasonal distribution of enteroviruses was sporadic and no trend or correlation with any other parameters measured could be established (Fig. 7a). Enteroviruses were slightly more prevalent in site LA2 (15% of samples were positive) (Fig. 7b), but they were detected in at least one sample collected from each site during the study period (Fig. 7b). Similar to adenovirus, no significant seasonal variability was observed for the occurrence of enteroviruses.
FIG. 7.
Monthly occurrence of enteroviruses (a) and the frequency of their detection at each sampling site (b). Bar symbols are the same as described in the legend to Fig. 2.
Adenovirus plaque assay using two different cell cultures showed no positive results in any of the 114 samples analyzed. A few samples were randomly selected and retested by a second round of infection to ensure that the results were true negative. Cytotoxicity was observed in <3% of samples tested after purification.
DISCUSSION
Storm water and urban runoff are now recognized as major sources of coastal water pollution, as numerous studies suggest that shorelines receiving runoff fail to meet water quality standards (20, 24, 27, 30, 34). For example, a 90% increase in the frequency of violation of recreational water quality standards has been previously reported for shorelines adjacent to urban runoff outlets following rainstorms in Southern California Bight (30). Reeves et al. (34) showed that, on a year-round basis, >99% of fecal indicator bacterial load from an urban watershed in southern California occurs during storms. Lipp et al. (27) demonstrated that the highest concentrations of fecal indicator bacteria were observed near areas of urban stream inputs in Charlotte Harbor, Florida. Most recently, human viral contamination was also found to be widespread in southern California urban rivers and streams, which negatively influence the coastal water quality (21). This current study further investigated the presence of human viral pollution in urban rivers by presenting the first quantitative assessment of human adenoviruses in urban rivers by real-time PCR and explored the relationship between PCR-detectable adenoviruses and infectious viruses.
In accordance with previous observations, our current investigation shows that the Los Angeles and San Gabriel Rivers are contaminated with fecal indicator bacteria and human viruses. The concentrations of both fecal indicator bacteria and coliphages were higher at the upstream locations of both rivers, while median concentrations of adenoviruses were higher at the mouth of each river. The decrease of fecal indicator bacteria and coliphage concentration from freshwater to saline water indicates the land source of these organisms and their decay and dilution at the mouth of rivers by ocean water. The higher concentrations of adenoviruses detected at the mouth of rivers could be technology artifacts, due to the presence of higher concentrations of PCR inhibitors in the upstream water. The investigation of real-time PCR efficiency using various environmental matrices has shown that the real-time PCR efficiency reduced to one-third when nutrient-rich creek water was used as the viral seeding matrix in comparison to ocean water as a seeding matrix (20a). To achieve the absolute quantification of viral genomes in environmental samples, a viral seeding study needs to be conducted for each individual sample to correct for nucleic acid extraction and PCR efficiencies. In spite of the higher concentration of adenoviruses detected at the mouth of the river, adenoviruses were found more frequently at upstream sites of the Los Angeles River. This indicates, as for the fecal indicator bacteria and coliphages, that the sources of adenoviruses are urban.
It is not a surprise that plaque assays for adenoviruses of river samples yielded negative results. The most likely explanation for the discrepancy in results obtained by real-time PCR and plaque assay is that most human viruses in the aquatic environment are noninfectious. Natural or sewage treatment processes such as solar radiation or chlorination can inactivate adenoviruses without degrading viral physical structure or genome integrity. A previous study addressed this concern by showing that environmental samples that contain only inactivated viruses tested positive by molecular methods such as RT-PCR (38). The discrepancy between viral particle counts and PFU is well known in clinical virology (25). This discrepancy, generally known as plaquing efficiency, varies by virus, virus serotype, type of tissue cultures used for plaque assay, etc. He and Jiang (19) showed that ∼0.1% of 105 adenovirus genomes per liter in primary treated sewage were infectious by plaque assay of HEK-293A tissue culture. Assuming that the contamination source in the urban rivers investigated in this study resembles primary sewage effluence, we expect <10 infectious adenoviruses per 104 adenovirus genome to be detected by real-time PCR. This theoretical calculation is near the borderline of our lower detection limit for plaque assay. Therefore, any environmental and sewage treatment processes that further reduce the ratio between genome copy and PFU will result in nondetectable results. Another explanation for this nondetectable result by tissue culture may be that these environmental adenoviruses did not possess any binding affinity to the cell lines used. For example, the A549 cell line is sensitive to adenoviruses serotypes 2 and 5, while the HEK-239A line is known to support the proliferation of serotypes 2, 5, 40, and 41. The diversity of human adenovirus serotypes in the aquatic environment is not yet known. Furthermore, adenoviruses in the river water may adsorb to suspended solids (9), which may impair their ability to bind to cell receptors, potentially causing false-negative results. Finally, the sensitivity of the plaque assay may be less than the infectivity assay based on cytopathic effect; we may have missed some infectious viruses by using only the plaque assay in this study.
When the seasonal distributions of fecal indicator bacteria and coliphages were compared, the highest concentrations of all parameters were detected in November during the first major storm of the rainy season. Similar trends were observed with high concentrations during the month of February during a period of heavy rainstorms. However, no significant increase of indicator concentration was observed during the month of January, suggesting that light localized rain showers may not have a significant impact on bacterial load in urban runoff. The similarity between indicator bacterium and coliphage seasonal distribution patterns suggests that both bacteria and coliphages are of similar origin. This is also supported by best-fit lines in scatter plots indicating positive relationships between fecal indicator bacteria and coliphages (Fig. 4a to c). In contrast, the seasonal distribution of adenoviruses detected by real-time PCR was very different and did not respond well to the rain events. No positive correlation between adenoviruses and fecal indicator bacteria or coliphages was observed, except for F-specific phages (Fig. 4d and e). Negative relationships between adenoviruses and fecal indicator bacteria or coliphages indicate that adenoviruses may be of different origin. Indicator bacteria and coliphages may be generated by natural sources such as birds, animal feces, and/or regrowth in nutrient-rich soil and/or riverbed sediments (4, 12, 14, 35). They are flushed out by rainwater and transported to rivers during storm events. Human adenoviruses and enteroviruses are only from human source contamination. The pollution of the river from human sources may be sporadic throughout the season, which is independent of rain events. On the other hand, our real-time PCR method may fail to detect adenoviruses after storm events, due to the higher concentrations of PCR inhibitors presented in storm water (C. Surbeck et al., unpublished data).
This study presents the first quantitative investigation of human adenoviruses in environmental samples with real-time PCR, and it represents a significant advancement in our ability to make a rapid assessment of the viral quality of waters. However, PCR results significantly overestimated the occurrence of infectious viruses in the environment. Therefore, caution should be taken in directly extrapolating positive PCR results of human viruses to human health risks. Furthermore, human adenoviruses were more frequently detected than enteroviruses, suggesting their potential to serve as a better index for human sewage.
Acknowledgments
Funding for this project was provided by grants from the Water Environmental Research Foundation, award 01-HHE-2a, and the Center for Water Resources, award P-00-38.
We thank Daniel Lee and Mike Vuong for assistance with sample collection, fecal indicator bacteria, and coliphage analysis. We thank Mandy Han for assistance with tissue culture analyses and Weiping Chu for technical assistance in the laboratory.
REFERENCES
- 1.Alexander, L. M., A. Heaven, A. Tennant, and R. Morris. 1992. Symptomatology of children in contact with sea water contaminated with sewage. J. Epidemiol. Community Health 46:340-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg, G. 1983. Viral pollution of the environment. CRC Press, Boca Raton, Fla.
- 3.Boom, R., C. Sol, M. Beld, J. Weel, J. Goudsmit, and P. Wertheim-Van Dillen. 1999. Improved silica-guanidiniumthiocyanate DNA isolation procedure based on selective binding of bovine alpha-casein to silica particles. J. Clin. Microbiol. 37:615-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byappanahalli, M. N., and R. S. Fujioka. 1998. Evidence that tropical soil environment can support the growth of Escherichia coli. Water Sci. Technol. 38:171-174. [Google Scholar]
- 5.Castignolles, N., F. Petit, I. Mendel, L. Simon, L. Cattolico, and C. Buffet-Janvresse. 1998. Detection of adenovirus in the waters of the Seine River estuary by nested-PCR. Mol. Cell. Probes 12:175-180. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1988. Water-related disease outbreaks, 1985. Morb. Mortal. Wkly. Rep. 37:15-24. [Google Scholar]
- 7.Chapron, C. D., N. A. Ballester, J. H. Fontaine, C. N. Frades, and A. B. Margolin. 2000. The detection of astrovirus, enteroviruses, adenovirus type 40 and 41, and rotavirus in surface water samples collected and evaluated by the Information Collection Rule and an integrated cell culture-nested PCR procedure, p. 547. Abstr. 100th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, D.C. [DOI] [PMC free article] [PubMed]
- 8.Cheung, W. H., K. C. Chang, R. P. Hung, and J. W. Kleevens. 1990. Health effects of beach water pollution in Hong Kong. Epidemiol. Infect. 105:139-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung, H., and M. D. Sobsey. 1993. Comparative survival of indicator viruses and enteric viruses in seawater and sediment. Water Sci. Technol. 27:425-428. [Google Scholar]
- 10.Clesceri, L. S., A. E. Greenberg, and A. D. Eaton (ed.). 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, D.C.
- 11.DeLeon, R., Y. S. C. Shieh, R. S. Baric, and M. D. Sobey. 1990. Presented at the Water Quality Conference, San Diego, Calif.
- 12.Desmarais, T. R., H. M. Solo-Gabriele, and C. J. Palmer. 2002. Influence of soil on fecal indicator organisms in a tidally influenced subtropical environment. Appl. Environ. Microbiol. 68:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donaldson, K. A., D. W. Griffin, and J. H. Paul. 2002. Detection, quantitation and identification of enteroviruses from surface waters and sponge tissue from the Florida Keys using real-time RT-PCR. Water Res. 36:2505-2514. [DOI] [PubMed] [Google Scholar]
- 14.Fujioka, R., C. Sian-Denton, M. Borja, J. Castro, and K. Morphew. 1999. Soil: the environmental source of Escherichia coli and enterococci in Guam's streams. J. Appl. Microbiol. 85:83s-89s. [DOI] [PubMed] [Google Scholar]
- 15.Giard, D. J. S., A. Aaronson, G. J. Todaro, P. Arnstein, J. H. Kersey, H. Dosik, and W. P. Parks. 1973. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 51:1417-1423. [DOI] [PubMed] [Google Scholar]
- 16.Goldsmith, K. T., L. D. Dion, D. T. Curiel, and R. I. Garver, Jr. 1998. Trans E1 component requirements for maximal replication of E1-defective recombinant adenovirus. Virology 248:406-419. [DOI] [PubMed] [Google Scholar]
- 17.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 18.Harm, W. 1980. Biological effects of ultraviolet radiation. Cambridge University Press, Cambridge, United Kingdom.
- 19.He, J. W., and S. Jiang. 2005. Quantification of enterococci and human adenoviruses in environmental samples by real-time PCR. Appl. Environ. Microbiol. 71:2250-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang, S., R. Noble, and W. Chu. 2001. Human adenoviruses and coliphages in urban runoff-impacted coastal waters of southern California. Appl. Environ. Microbiol. 67:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Jiang, S., H. Dezfulian, and W. Chu. 2005. Real time quantitative PCR for enteric adenovirus serotype 40 in environmental waters. Can. J. Microbiol. 51:393-398. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, S. C., and W. Chu. 2004. PCR detection of pathogenic viruses in southern California urban rivers. J. Appl. Microbiol. 97:17-28. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, S. C., C. A. Kellogg, and J. H. Paul. 1998. Characterization of marine temperate phage-host systems isolated from Mamala Bay, Oahu, Hawaii. Appl. Environ. Microbiol. 64:535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kallenbach, N. R., P. A. Cornelius, D. Negus, D. Montgomerie, and S. Englander. 1989. Inactivation of viruses by ultraviolet light, vol. 56. Karger, Basel, Switzerland. [DOI] [PubMed]
- 24.Kim, J. H., S. B. Grant, C. D. McGee, B. F. Sanders, and J. L. Largier. 2004. Locating sources of surf zone pollution: a mass budget analysis of fecal indicator bacteria at Huntington Beach, California. Environ. Sci. Technol. 38:2626-2636. [DOI] [PubMed] [Google Scholar]
- 25.Knipe, D. M., P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.). 2001. Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 26.Le Cann, P., S. Ranarijaona, S. Monpoeho, F. Le Guyader, and V. Ferre. 2004. Quantification of human astroviruses in sewage using real-time RT-PCR. Res. Microbiol. 155:11-15. [DOI] [PubMed] [Google Scholar]
- 27.Lipp, E. K., R. Kurz, R. Vincent, C. Rodriguez-Palacios, S. R. Farrah, and J. B. Rose. 2001. The effects of seasonal variability and weather on microbial fecal pollution and enteric pathogens in a subtropical estuary. Estuaries 24:266-276. [Google Scholar]
- 28.Monpoeho, S., M. Coste-Burel, M. Coste-Mattioli, B. Besse, J. J. Chomel, S. Billaudel, and V. Ferre. 2002. Application of a real-time polymerase chain reaction with internal positive control for detection and quantification of enterovirus in cerebrospinal fluid. Eur. J. Clin. Microbiol. Infect. Dis. 21:532-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monpoeho, S., A. Maul, C. Bonnin, L. Patria, S. Ranarijaona, S. Billaudel, and V. Ferre. 2004. Clearance of human-pathogenic viruses from sludge: study of four stabilization processes by real-time reverse transcription-PCR and cell culture. Appl. Environ. Microbiol. 70:5434-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noble, R. T., S. B. Weisberg, M. K. Leecaster, C. D. McGee, J. H. Dorsey, P. M. Vainik, and V. Orozco-Borbon. 2003. Storm effects on regional beach water quality along the southern California shoreline. J. Water Health 1:23-31. [PubMed] [Google Scholar]
- 31.Payment, P., and E. Franco. 1993. Clostridium perfringens and somatic coliphages as indicators of the efficiency of drinking water treatment for viruses and protozoan cysts. Appl. Environ. Microbiol. 59:2418-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pina, S., M. Puig, F. Lucena, J. Jofre, and R. Girones. 1998. Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl. Environ. Microbiol. 64:3376-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puig, M., J. Jofre, F. Lucena, A. Allard, G. Wadell, and R. Girones. 1994. Detection of adenoviruses and enteroviruses in polluted waters by nested PCR amplification. Appl. Environ. Microbiol. 60:2963-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves, R. L., S. B. Grant, R. D. Mrse, C. M. Copil Oancea, B. F. Sanders, and A. B. Boehm. 2004. Scaling and management of fecal indicator bacteria in runoff from a coastal urban watershed in southern California. Environ. Sci. Technol. 38:2637-2648. [DOI] [PubMed] [Google Scholar]
- 35.Roll, B. M., and R. S. Fujioka. 1997. Sources of faecal indicator bacteria in a brackish, tropical stream and their impact on recreational water quality. Water Sci. Technol. 35:179-186. [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Smith, C. D., D. W. Craft, R. S. Shiromoto, and P. O. Yan. 1986. Alternative cell-line for virus isolation. J. Clin. Microbiol. 24:265-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobsey, M. D., D. A. Battigelli, G. A. Shin, and S. Newland. 1998. RT-PCR amplification detects inactivated viruses in water and wastewater. Water Sci. Technol. 38:91-94. [Google Scholar]
- 39.Tani, N., Y. Dohi, N. Kurumatani, and K. Yonemasu. 1995. Seasonal distribution of adenoviruses, enteroviruses and reoviruses in urban river water. Microbiol. Immunol. 39:577-580. [DOI] [PubMed] [Google Scholar]
- 40.Thurston-Enriquez, J. A., C. N. Haas, J. Jacangelo, K. Riley, and C. P. Gerba. 2003. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl. Environ. Microbiol. 69:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toranzos, G. A., H. Hanssen, and C. P. Gerba. 1986. Occurrence of enteroviruses and rotaviruses in drinking-water in Colombia. Water Sci. Technol. 18:109-114. [Google Scholar]
- 42.Tsai, Y. L., M. D. Sobsey, L. R. Sangermano, and C. J. Palmer. 1993. Simple method of concentrating enteroviruses and hepatitis A virus from sewage and ocean water for rapid detection by reverse transcriptase-polymerase chain reaction. Appl. Environ. Microbiol. 59:3488-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]