Abstract
In mixed cultures, bacteriocin production by the sausage isolate Lactobacillus sakei CTC 494 rapidly inactivated sensitive Listeria innocua LMG 13568 cells, even at low bacteriocin activity levels. A small fraction of the listerial population was bacteriocin resistant. However, sausage fermentation conditions inhibited regrowth of resistant cells.
Lactobacillus sakei CTC 494, producer of the bacteriocin sakacin K, inhibits Listeria spp. during sausage fermentation (12). Bacteriocins are small peptides or proteins with antibacterial activity (5). The resistance of the target strain may compromise bacteriocin efficacy (6, 7, 11, 23). This study focuses on in vitro interaction kinetics between Lactobacillus sakei CTC 494 and Listeria innocua LMG 13568 and on listerial sakacin K resistance as a function of key sausage fermentation conditions (temperature, pH, salt, and nitrite) that may affect both bacteriocin production (13, 14, 17, 21, 22) and activity (9, 10). Listeria innocua, being more competitive (2) and resistant to sakacin K (12) than Listeria monocytogenes, offered a worst-case approach. An industrial, nonbacteriocinogenic Lactobacillus sakei I strain was used for comparison.
Fermentations were done in a Biostat C fermentor (B. Braun Biotech International, Melsungen, Germany) in 10 liters of customized MRS medium (3). Modifications involved the addition of 40 g of sodium chloride per liter (VWR International, Darmstadt, Germany) and 200 ppm of sodium nitrite (VWR International). Some experiments, being highly reproducible (13, 17, 21-23), were repeated. Culture preparation, fermentor setup, and on-line control were as described previously (13). Bacteriocin-free Lactobacillus sakei CTC 494 inocula (10%) were prepared in 100 ml MRS medium (Oxoid, Basingstoke, United Kingdom) at 37°C overnight, since no sakacin K is produced above 33°C (13).
Listeria innocua LMG 13568 inocula (1%) were prepared in 100 ml of brain heart infusion (Oxoid) at 30°C overnight. Lactobacilli were enumerated on MRS agar (Oxoid) after incubation at 30°C; Listeria innocua LMG 13568 did not grow on this medium during the first days. Listeriae were enumerated on Palcam agar (Oxoid) after incubation at 30°C. The standard deviation on both measurements equaled 0.07 log CFU ml−1. Bacteriocin activity in the cell-free culture supernatant was estimated by a twofold critical dilution method (4, 13). A variation coefficient of 20% on bacteriocin measurement occurs, but activity curves are reproducible (13, 14). Salt and nitrite did not interfere with the assay (14).
The bacterial population was regarded as an association of subpopulations (in CFU per ml) of Lactobacillus sakei CTC 494 (LAC) and both bacteriocin-sensitive (LISS) and bacteriocin-resistant (LISR) Listeria innocua LMG 13568. The equations used for the model shown in Table 1 (13-15) were solved by Euler integration in Microsoft Excel by minimizing residual sums of squares. The μmax, λ, and Kd of LISR were determined independently; the other parameters were set as for the Listeria monoculture and LISS. Upon repetition, the coefficients of variation of the parameters are generally lower than 10%, with the exception of kinact for which 25% can be observed (13, 17, 20-22).
TABLE 1.
Equations used for model developmenta
| Model | Equation | Conditions |
|---|---|---|
| Cell growth of Lactobacillus sakei CTC 494 | d[LAC]/dt = (μmax-LAC γi − Kd-LAC) [LAC] | t ≥ λLAC |
| γi = 1 | [LAC] < [LAC1] | |
| = 1 − I1 ([LAC] − [LAC1]) | [LAC1] ≤ [LAC] ≤ [LAC2] | |
| = (1 − I1 ([LAC2] − [LAC1]) − I2 ([LAC] − [LAC2]) | [LAC] > [LAC2] | |
| Cell growth of the resistant Listeria subpopulation | d[LISR]/dt = (μmax -LISR − Kd−LISR) [LISR] | t ≥ λLISR |
| Cell growth of the sensitive Listeria subpopulation | d[LISS]/dt = (μmax-LISS − Kd-LISS) [LISS] − ψ [B] [LISS] | t ≥ λLISS |
| Bacteriocin activity | d[B]/dt = kBd[LAC]/dt − kinact [LAC] [B] | [LAC] ≥ [LACB] |
[LAC] = cell concentration of Lactobacillus sakei CTC 494 (in CFU per ml), t = time (in hours), λ = lag phase (in hours), μmax = maximum specific growth rate (per hour), Kd = specific death rate (per hour), γi = self-inhibition function for Lactobacillus sakei CTC 494, [LAC1] and [LAC2] = critical cell concentrations (in CFU per ml) where the subscripts refer to inhibition phases, I1 and I2 = corresponding inhibition coefficients, [LIS]R = cell concentration of resistant Listeria innocua LMG 13568 (in CFU per ml), [LIS]S = cell concentration of sensitive Listeria innocua LMG 13568 (in CFU per ml), ψ = bacteriocidal coefficient (in ml hours per AU), [B] = bacteriocin activity (in arbitrary units ml−1), kB = specific bacteriocin production (in AU per CFU), kinact = apparent bacteriocin inactivation rate (in liters per CFU per hour), and [LACB] = minimum [LAC] for bacteriocin production, below which kB equals 0 (in CFU per ml).
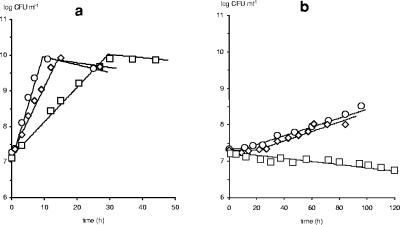
In monoculture, Listeria innocua LMG 13568 displayed good growth in MRS medium at a constant pH of 6.5 at all studied temperatures (Fig. 1a). Growth was slower at a constant pH of 5.2 (20°C), particularly in the presence of salt and nitrite (Fig. 1b). In association with Lactobacillus sakei CTC 494, Listeria counts sharply decreased to a residual, bacteriocin-resistant subpopulation (Fig. 2). The resistance frequency (averaging 4 × 10−4) was similar as for other antilisterial bacteriocins (11, 19, 23) and was not influenced by temperature, pH, or salt, which is in agreement with a former observation (11). Hence, if initial listerial counts are below 103 CFU per ml, as in Pleasants et al. (18), total inactivation is expected. At 20°C, bacteriocin activity was tripled compared to 30°C. Nevertheless, such differences did not affect the degree of listerial inactivation, typically observed at the onset of bacteriocin production (corresponding to merely 100 to 200 arbitrary units [AU] ml−1). Yet, high activities may be gainful in food matrices to overcome diffusion limitations and inactivation losses.
FIG. 1.
Influence of fermentation conditions on the cell growth (in log CFU ml−1) of a monoculture of Listeria innocua LMG 13568 in MRS medium at (a) constant pH 6.5 and 35°C (○), 30°C (⋄), and 20°C (□) and at (b) 20°C and constant pH 5.2 (○), with 40 g liter−1 of sodium chloride (⋄), and with 40 g liter−1 of sodium chloride and 200 ppm of sodium nitrite (□). Symbols represent experimental data, and lines are according to the model. Standard deviations (not shown) were below 5% of the mean value of the cell counts.
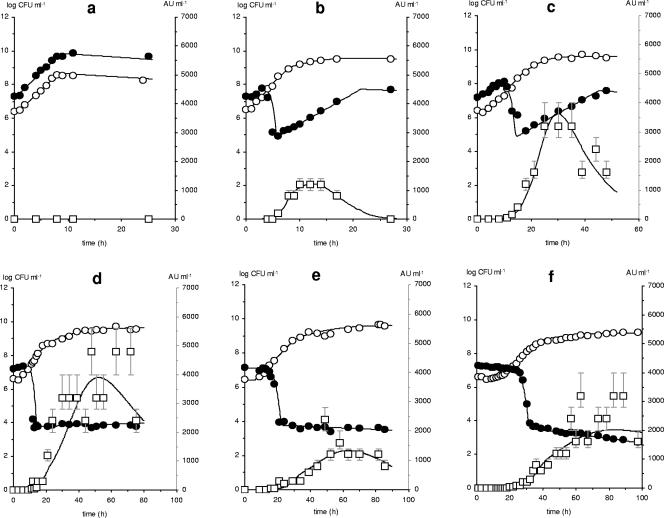
FIG. 2.
Influence of fermentation conditions on bacteriocin activity (□, in AU ml−1) and cell growth of a coculture of Listeria innocua LMG 13568 (•, in log CFU ml−1) and Lactobacillus sakei CTC 494 (○, in log CFU ml−1) in MRS medium at constant pH 6.5 and 35°C (a), 30°C (b), and 20°C (c), and at 20°C and constant pH 5.2 (d), with 40 g liter−1 of sodium chloride (e), and with 40 g liter−1 of sodium chloride and 200 ppm of sodium nitrite (f). Uncertainty ranges on bacteriocin activity (□, in AU ml−1) due to detection limitations of the bioassay method are indicated by flags. Symbols represent experimental data, and lines are according to the model. Standard deviations (not shown) were below 5% of the mean value of the cell counts.
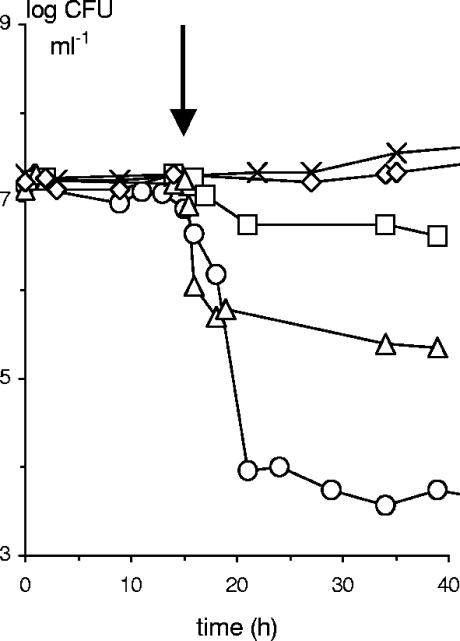
Similar growth kinetics were observed between Listeria innocua LMG 13568 in monoculture (Fig. 1a) and in association with Lactobacillus sakei CTC 494 at 35°C, when no bacteriocin was produced (Fig. 2a), or in the presence of the nonbacteriocinogenic Lactobacillus sakei I strain (Fig. 3). On the other hand, when external sakacin K-containing cell-free culture supernatant was added to a monoculture of Listeria innocua LMG 13568, listerial counts dropped. Initial activities of 20 and 100 AU ml−1 in the fermenter disappeared instantly, indicating rapid adsorption to bacterial cells (4), and resulted in a drop of 0.5 and 1.9 log CFU ml−1, respectively.
FIG. 3.
Cell counts of Listeria innocua LMG 13568 (in log CFU ml−1) growing as a monoculture (×), in the presence of the nonbacteriocinogenic Lactobacillus sakei I (⋄), in the presence of Lactobacillus sakei CTC 494 (○), and growing as a monoculture after addition of sakacin K-containing cell-free culture supernatant of Lactobacillus sakei CTC 494 leading to an initial bacteriocin activity of 20 AU ml−1 (□) or 100 AU ml−1 (▵) in MRS medium at 20°C and constant pH 5.2 in the presence of 40 g liter−1 of sodium chloride. The arrow indicates the addition of cell-free culture supernatant or the start of bacteriocin production. Standard deviations (not shown) were below 5% of the mean value of the cell counts.
At 20°C, resistant Listeria innocua LMG 13568 grew to high numbers at constant pH 6.5, whereas at constant pH 5.2 resistant levels stabilized (Fig. 2d). Outgrowth of resistant listeriae at pH 6.5 was observed previously (23). Salt and sodium nitrite contributed to the stabilization of the bacteriocin-resistant subpopulation (Fig. 2e,f). The μmax of resistant Listeria innocua LMG 13568 was slightly lower (6 to 8%) than that of the sensitive cells. Poor competitiveness of bacteriocin-resistant cells in nature is probably due to fitness costs associated with resistance development (6, 8, 11). Stresses impose selection for stress-resistant phenotypes, but a single phenotype will seldom be competitively superior in all environments (16). For instance, a bacteriocin-resistant phenotype of Listeria monocytogenes B73 was not able to invade the sensitive population, even at initial frequencies of 10−1 or higher (6). The resistance of listeriae can be stable or lost after a few generations in the absence of bacteriocin (6, 7, 11, 19). When a bacteriocin-resistant Listeria innocua LMG 13568 colony was picked up, cultivated in fresh brain heart infusion medium (30°C, 12 h), and exposed to a new cocultivation with Lactobacillus sakei CTC 494 (1 liter MRS medium, initial pH 6.5), resistance was maintained despite activity of 600 AU ml−1. This indicates that resistance is not due to reversible adaptation but linked to a resistant subpopulation.
Whereas listerial inhibition by bacteriocin-producing Carnobacterium piscicola was mainly due to nutrient competition (1), the present study demonstrates that bacteriocin production is the basis for inactivation of Listeria innocua LMG 13568 by Lactobacillus sakei CTC 494. A liquid system based on meat peptides was used as a model for the sausage water phase, because of the difficulty to quantify bacteriocin activity in meat. However, diffusion limitations and surface growth could be important too. Nevertheless, this study forms a basis for interpreting results on a practical scale, involving real sausage making.
Acknowledgments
We acknowledge financial support from the Research Council of the Vrije Universiteit Brussel, the Fund for Scientific Research-Flanders (FWO), and the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT), in particular the STWW project Functionality of Novel Starter Cultures in Traditional Fermentation Processes. F.L. was supported by a postdoctoral fellowship of the FWO.
Lactobacillus sakei CTC 494 was kindly provided by M. Hugas (Institut de Recerca i Tecnología Agroalimentáries, Centre de Tecnología de la Carn, Monells, Spain).
REFERENCES
- 1.Buchanan, R. L., and L. K. Bagi. 1997. Microbial competition: effect of culture conditions on the suppression of Listeria monocytogenes Scott A by Carnobacterium piscicola. J. Food Prot. 60:254-261. [DOI] [PubMed] [Google Scholar]
- 2.Cornu, M., M. Kalmokoff, and J.-P. Flandrois. 2002. Modelling the competitive growth of Listeria monocytogenes and Listeria innocua in enrichment broths. Int. J. Food Microbiol. 73:261-274. [DOI] [PubMed] [Google Scholar]
- 3.de Man, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 4.De Vuyst, L., Callewaert, R., and Pot, B. 1996. Characterisation and antagonistic activity of Lactobacillus amylovorus DCE 471 and large scale isolation of its bacteriocin amylovorin L471. Syst. Appl. Microbiol. 19:9-20. [Google Scholar]
- 5.De Vuyst, L., and E. J. Vandamme. 1994. Bacteriocins of lactic acid bacteria: microbiology, genetics and applications. Blackie Academic & Professional, London, United Kingdom.
- 6.Dykes, G. A., and J. W. Hastings. 1998. Fitness costs associated with class IIa bacteriocin resistance in Listeria monocytogenes B73. Lett. Appl. Microbiol. 26:5-8. [DOI] [PubMed] [Google Scholar]
- 7.Ennahar, S., N. Deschamps, and J. Richard. 2000. Natural variation in susceptibility of Listeria strains to class IIa bacteriocins. Curr. Microbiol. 41:1-4. [DOI] [PubMed] [Google Scholar]
- 8.Ennahar, S., T. Sashihara, K. Sonomoto, and A. Ishizaki. 2000. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol. Rev. 24:85-106. [DOI] [PubMed] [Google Scholar]
- 9.Gänzle, M. G., C. Hertel, and W. P. Hammes. 1996. Antimicrobial activity of bacteriocin-producing cultures in meat products: modelling of the effect of pH, NaCl, and nitrite concentrations on the antimicrobial activity of sakacin P against Listeria ivanovii DSM 20750. Fleischwirtschaft 76:409-412. [Google Scholar]
- 10.Gänzle, M. G., S. Weber, and W. P. Hammes. 1999. Effect of ecological factors on the inhibitory spectrum and activity of bacteriocins. Int. J. Food Microbiol. 46:207-212. [DOI] [PubMed] [Google Scholar]
- 11.Gravesen, A., A.-M. J. Axelsen, J. M. da Silva, T. B. Hansen, and S. Knøchel. 2002. Frequency of bacteriocin resistance development and associated fitness costs in Listeria monocytogenes. Appl. Environ. Microbiol. 68:756-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugas, M., M. Garriga, M. T. Aymerich, and J. M. Monfort. 1995. Inhibition of Listeria in dry fermented sausages by the bacteriocinogenic Lactobacillus sake CTC 494. J. Appl. Bacteriol. 79:322-330. [Google Scholar]
- 13.Leroy, F., and L. De Vuyst. 1999. Temperature and pH conditions that prevail during the fermentation of sausages are optimal for the production of the antilisterial bacteriocin sakacin K. Appl. Environ. Microbiol. 65:974-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leroy, F., and L. De Vuyst. 1999. The presence of salt and a curing agent reduces bacteriocin production by Lactobacillus sakei CTC 494, a potential starter culture for sausage fermentation. Appl. Environ. Microbiol. 65:5350-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leroy, F., and L. De Vuyst. 2001. Growth of the bacteriocin-producing Lactobacillus sakei strain CTC 494 in MRS broth is strongly reduced due to nutrient exhaustion: a nutrient depletion model for the growth of lactic acid bacteria. Appl. Environ. Microbiol. 67:4407-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massey, R. C., and A. Buckling. 2002. Environmental regulation of mutation rates at specific sites. Trends Microbiol. 10:580-584. [DOI] [PubMed] [Google Scholar]
- 17.Messens, W., J. Verluyten, F. Leroy, and L. De Vuyst. 2003. Modelling growth and bacteriocin production by Lactobacillus curvatus LTH 1174 in response to temperature and pH values used for European sausage fermentation processes. Int. J. Food Microbiol. 81:41-52. [DOI] [PubMed] [Google Scholar]
- 18.Pleasants, A. B., T. K. Soboleva, G. A. Dykes, R. J. Jones, and A. E. Filippov. 2001. Modelling of the growth of populations of Listeria monocytogenes and a bacteriocin-producing strain of Lactobacillus in pure and mixed cultures. Food Microbiol. 18:605-615. [Google Scholar]
- 19.Rekhif, N., A. Atrih, and G. Lefebvre. 1994. Selection and properties of spontaneous mutants of Listeria monocytogenes ATCC 15313 resistant to different bacteriocins by lactic acid bacteria strains. Curr. Microbiol. 28:237-241. [Google Scholar]
- 20.Verluyten, J., F. Leroy, and L. De Vuyst. 2004. Influence of complex nutrient source on growth of and curvacin A production by sausage isolate Lactobacillus curvatus LTH 1174. Appl. Environ. Microbiol. 70:5081-5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verluyten, J., W. Messens, and L. De Vuyst. 2003. The curing agent sodium nitrite, used in the production of fermented sausages, is less inhibiting to the bacteriocin-producing meat starter culture Lactobacillus curvatus LTH 1174 under anaerobic conditions. Appl. Environ. Microbiol. 69:3833-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verluyten, J., W. Messens, and L. De Vuyst. 2004. Sodium chloride reduces production of curvacin A, a bacteriocin produced by the Lactobacillus curvatus strain LTH 1174 originating from fermented sausage. Appl. Environ. Microbiol. 70:2271-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vignolo, G., J. Palacios, M. E. Farías, F. Sesma, U. Schillinger, W. Holzapfel, and G. Olliver. 2000. Combined effects of bacteriocins on the survival of various Listeria species in broth and meat system. Curr. Microbiol. 41:410-416. [DOI] [PubMed] [Google Scholar]