Abstract
The cytochrome P450 monooxygenase Ema1 from Streptomyces tubercidicus R-922 and its homologs from closely related Streptomyces strains are able to catalyze the regioselective oxidation of avermectin into 4"-oxo-avermectin, a key intermediate in the manufacture of the agriculturally important insecticide emamectin benzoate (V. Jungmann, I. Molnár, P. E. Hammer, D. S. Hill, R. Zirkle, T. G. Buckel, D. Buckel, J. M. Ligon, and J. P. Pachlatko, Appl. Environ. Microbiol. 71:6968-6976, 2005). The gene for Ema1 has been expressed in Streptomyces lividans, Streptomyces avermitilis, and solvent-tolerant Pseudomonas putida strains using different promoters and vectors to provide biocatalytically competent cells. Replacing the extremely rare TTA codon with the more frequent CTG codon to encode Leu4 in Ema1 increased the biocatalytic activities of S. lividans strains producing this enzyme. Ferredoxins and ferredoxin reductases were also cloned from Streptomyces coelicolor and biocatalytic Streptomyces strains and tested in ema1 coexpression systems to optimize the electron transport towards Ema1.
Emamectin benzoate (compound MK-244) is an extremely potent insecticide that controls many agriculturally important pests (7). The widespread commercial exploitation of emamectin benzoate has, however, been hindered by the high cost of its chemical synthesis from its natural product precursor, avermectin. The most costly step of this process is the oxidation of the 4"-carbinol group of avermectin to the corresponding ketone, a demanding step due to the presence of two other hydroxyl groups on the substrate molecule that must be chemically protected before and deprotected after oxidation.
We are developing a cost- and time-effective method for the regioselective oxidation of the 4"-carbinol group of avermectin that involves the utilization of resting microbial cells as a biocatalyst. The accompanying article (12) describes the identification of Streptomyces strains and the isolation and characterization of cytochrome P450 monooxygenase (CYP) enzymes from these strains that are able to carry out the regioselective oxidation of avermectin at the 4" position, as well as the cloning and sequencing of the corresponding genes (ema1 to ema17). As biocatalysts, CYPs are proficient in the oxidative catabolism of a wide range of xenobiotics; they can also be used to carry out highly enantio- and regiospecific biosynthetic reactions (23, 35). CYPs from or expressed in Streptomycetes have been shown to catalyze the regiospecific oxidation of secondary metabolites (34) and steroids (5) and the activation and catabolism of xenobiotics (22, 31). Investigations of Pseudomonas-derived CYPs and pseudomonads as whole-cell biocatalysts have also been described for the bioremediation of xenobiotics (8) and for the biotransformation of disparate chemicals including natural products (27).
Class II CYPs that are associated with the endoplasmic reticulum in eukaryotes use NADH-cytochrome P450 reductase as an electron donor. Soluble class I CYPs of bacteria receive electrons via the small iron-sulfur protein ferredoxin (FD) and the flavin adenine dinucleotide (FAD)-containing ferredoxin reductase (FRE). Specific CYPs have higher activity when they interact with specific FDs (21, 23). Similar to the case with FDs and CYPs, specific FREs are also known to be better electron donors for certain FDs than for others (16).
The electrons channeled to CYPs by their redox partners are ultimately derived from NAD(P)H; thus, the biotransformation reactions catalyzed by these enzymes require cofactor regeneration. Although cofactor regeneration can be achieved in vitro enzymatically (28, 32), directly (3), or bypassed altogether (10), metabolically active cells could provide an easier and more cost-effective solution. Thus, the present article describes engineered Streptomyces lividans and Pseudomonas putida strains that express the CYP enzyme Ema1 of Streptomyces tubercidicus R-922 for the oxidation of the 4"-carbinol of avermectins. We also cloned FD and FRE proteins that support an increased level of biocatalytic activity upon coexpression with Ema1 in these strains.
MATERIALS AND METHODS
Bacterial strains and plasmids.
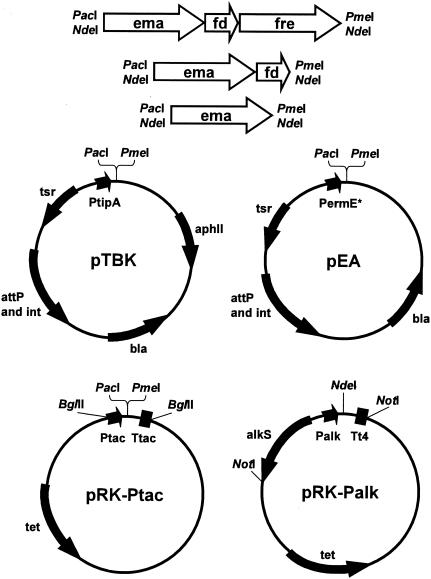
Bacterial strains and cloning and expression vectors used in this study are listed in Tables 1 and 2, respectively. The Streptomyces integrative expression vectors pTBK and pEA are based on the Escherichia coli cloning vector pNEB193 (New England Biolabs, Beverly, MA) and the φC31-derived integrase (14) and were developed in-house (37; I. Molnár, unpublished data) from published vector components (Fig. 1). The broad-host-range, transmissible plasmid pRK290 (9) was used for Pseudomonas with the alkane-inducible Palk promoter-alkS-positive regulator expression cassette (24) or the Ptac promoter derived from pUK21 (33) which is constitutive in pseudomonads (Fig. 1).
TABLE 1.
Bacterial strains used in this studya
| Species | Strain | Note | Source |
|---|---|---|---|
| S. lividans | ZX7 | Expression host | JIC |
| S. coelicolor | M145 | FRE donor | JIC |
| S. avermitilis | MOS-0001 | Expression host, avermectin producer | SYN |
| S. tubercidicus | R-922 | Ema1 producer | SYN |
| S. tubercidicus | I-1529 | Ema2 producer | SYN |
| P. putida | 17453 | Solvent-tolerant expression host | ATCC |
| P. putida | 700801 | Solvent-tolerant expression host | ATCC |
| E. coli | DH10B | Routine cloning host | INV |
Sources: JIC, John Innes Institute (Norwich, United Kingdom); SYN, Syngenta in-house strain collection (Basel, Switzerland); ATCC, American Type Culture Collection (Manassas, VA); INV, Invitrogen Corporation (Carlsbad, CA).
TABLE 2.
Vectors used in this studya
| Vector | Use | Promoter | Selectable marker(s) | Source or reference |
|---|---|---|---|---|
| pNEB193 | E. coli cloning vector | NA | bla | NEB |
| pTBK | Streptomyces expression vector | PtipA | tsr, aphII, bla | This work and reference 37 |
| pEA | Streptomyces expression vector | PermE* | tsr, bla | This work |
| pRK290 | Broad-host-range-transmissible cloning vector | NA | tet | 9 |
| pUK21 | E. coli cloning vector | Ptac | aphII | 33 |
| pSPZ2Not | Pseudomonas expression vector | Palk | bla | 24 |
| pRK-Ptac | Pseudomonas expression vector | Ptac | tet | This work |
| pRK-Palk | Pseudomonas expression vector | Palk | tet | This work |
Promoters: PtipA, thiostrepton-inducible promoter for Streptomyces (19); PermE*, constitutive promoter for Streptomyces (6); Palk, alkane-inducible promoter for Pseudomonas (24); Ptac, constitutive promoter in Pseudomonas, derived from pUK21 (33); NA, not applicable. Selectable markers: bla, β-lactamase gene for ampicilin resistance; aphII, aminoglycoside phosphotransferase gene for kanamycin resistance; tsr, 23S rRNA methyltransferase gene for thiostrepton resistance; tet, tetracycline efflux-type resistance gene. NEB, New England Biolabs (Beverley, MA).
FIG. 1.
Schematic representation of expression vectors and artificial operon cassettes used in this study. Details of the cloning procedures are provided in Materials and Methods. attP and int, φC31-derived integrase (14); see Table 2 for explanations of the other gene name abbreviations. The ema, ema-fd, and ema-fd-fre artificial operon cassettes were cloned either as PacI-PmeI or NdeI fragments.
Culture conditions and molecular genetic procedures.
E. coli and Streptomyces cultivation, transformation, recombinant DNA procedures, and DNA sequence analysis followed standard protocols (13, 26) as detailed in the accompanying article (12). Streptomyces avermitilis protoplasts were transformed as previously described (30). P. putida strains were cultured in TB broth (26) and transformed by interspecies conjugation from E. coli (9).
Agar-based biocatalytic assay.
Transformed Streptomyces strains were grown on ISP-2 agar plates supplemented with 50 μg/ml thiostrepton for selection (pEA derivatives) or 50 μg/ml kanamycin for selection and 5 μg/ml thiostrepton for induction of the PtipA promoter (pTBK derivatives). The ISP-2 agar media were also supplemented with 1 g/liter (final concentration) of technical-grade abamectin (stock solution, 20 g/liter abamectin in dimethyl sulfoxide:Tween 40 = 1:1. Abamectin is the natural mixture of 85% avermectin B1a and 15% avermectin B1b as produced by Streptomyces avermitilis). The cultures were incubated at 28°C for 7 days, two agar cylinders of 5-mm diameter each were cut out and extracted with 1 ml acetonitrile, and the extracts were analyzed as described in the accompanying article (12).
Resting-cell assays.
Transformed S. lividans and S. avermitilis strains were cultivated at 28°C with shaking at 200 rpm for 2 days in 50 ml peptone-yeast-glucose (PYG) medium, supplemented with 5 μg/ml thiostrepton (pEA-derived plasmids) or 10-μg/ml kanamycin (pTBK derivatives); 5 ml of this preculture was transferred to a 500-ml Erlenmeyer flask containing 100 ml PYG medium supplemented with the same antibiotics. Thiostrepton at 5 μg/ml was used to induce the PtipA promoter in the cultures that had been transformed with pTBK-derived plasmids. The main cultures were incubated at 28°C with shaking at 200 rpm for 2 days. Mycelia were harvested by centrifugation and washed once with 50 mM potassium phosphate buffer, pH 7.0. A total of 500 mg of the mycelium (wet cell weight) was transferred into a 50-ml Erlenmeyer flask and resuspended in 10 ml of 50 mM potassium phosphate buffer, pH 7.0. After the addition of 15 μl of a solution of avermectin B1a in isopropanol (30 mg/ml), the reaction proceeded at 28°C for 16 h with shaking at 160 rpm. Bioconversion rates measured in Streptomyces resting-cell assays followed the same tendencies but were substantially higher than those obtained in the agar-based biocatalytic assays.
Transformed P. putida strains were grown overnight in 3 ml of TB medium supplemented with 20 μg/ml tetracycline at 28°C with shaking at 200 rpm. A total of 0.1 ml of these cultures was used to inoculate 100 ml TB supplemented with 20 μg/ml tetracycline and incubated overnight at 28°C with shaking at 200 rpm. Heptane at 0.1% (vol/vol) was used in the final cultures (but not in the starter cultures) to induce the Palk promoter for strains carrying pRK-Palk expression vectors. Cells were harvested by centrifugation and washed in 50 mM potassium phosphate buffer, pH 7.0. A total of 500 mg cells (wet weight) were transferred into a 50-ml Erlenmeyer flask and resuspended in 10 ml of 50 mM potassium phosphate buffer, pH 7.0. After the addition of 200 μl of 20% glucose and 15 μl of a solution of avermectin B1a in isopropanol (30 mg/ml), the mixture was incubated at 28°C for 16 h with shaking at 160 rpm.
To produce solvent-conditioned cells, transformed P. putida strains were grown overnight in 3 ml of TB medium supplemented with 20 μg/ml tetracycline at 28°C with shaking at 200 rpm. One milliliter of these cultures was used to inoculate 25 ml TB supplemented with 20 μg/ml tetracycline and incubated for 3 h at 28°C with shaking at 200 rpm. The cultures were supplemented at this point with 0.5% (vol/vol) dioctyl phthalate and the cultivation was continued for 2 days at 28°C with shaking at 200 rpm. To condition the cells to higher solvent concentrations, 1 ml of this culture was used to inoculate 25 ml TB, incubated for 3 h at 28°C with shaking at 200 rpm, supplemented with 2.5%, 5.0%, or 10% dioctyl phthalate; the incubation was then continued for 2 days as described above. One milliliter of the culture with 5.0% solvent was used to inoculate 25 ml TB supplemented with 25%, 37.5%, or 50% dioctyl phthalate and incubated for 3 h as before; the incubation was continued for 2 days. Tetracycline selection (20 μg/ml) was maintained throughout the fermentation train. Heptane at 0.1% (vol/vol) was used to induce the Palk promoter in the cultures that were used directly for resting-cell assays (but not in the starter or intermediate cultures) in strains carrying pRK-Palk expression vectors. Cells from solvent-conditioned and -induced cultures were collected by centrifugation and washed in 50 mM potassium phosphate buffer, pH 7.0, and 500 mg (wet weight) of cells was used for the biotransformation reaction as described above.
Resting-cell assay mixtures were extracted with 30 ml methyl-t-butyl-ether (MTBE). The ether phase was collected and evaporated in vacuo, and the residue was redissolved in 1.2 ml acetonitrile. Formation of 4"-oxo-avermectin was followed by high-performance liquid chromatography as described in the accompanying article (12).
Cloning ema1 and ema2 into expression vectors.
The ema1 coding sequence was amplified by PCR using Pfu DNA Polymerase (Stratagene, La Jolla, CA) with the following primers: 5′-AGATTAATTAATGTCGGAATTAATGAACTGTCCGTT-3′ (where the underlined sequence is a PacI recognition sequence and the sequence in boldface type is the start of the coding sequence of ema1), and 5′-AAACTCACCCCAACCGCACCGGCAGCGAGTTC-3′ (where the underlined sequence is half of a PmeI recognition sequence; the boldface type sequence is the reverse complement of the ema1 translation stop codon followed by the 3′ end of the ema1 gene). The amplified ema1 gene was cloned into the Streptomyces expression vectors as a PacI-PmeI fragment (Fig. 1).
The ema2 coding sequence was cloned into the Streptomyces expression vectors using a similar strategy, using the primers 5′-AGATTAATTAATGTCGGCATTATCCAGCTCTCC-3′ and 5′-AAACTCACCCCAGCCGCAACGGCAGGGAAT-3′ (see the paragraph above for an explanation of the typefaces used).
For expression in Pseudomonas hosts using the Ptac promoter, the ema genes were cloned as PacI-PmeI fragments into the plasmid pUK21 (33) between the Ptac promoter and Ttac terminator. The Ptac-ema-Ttac expression cassettes were excised from pUK21 as BglII fragments and cloned into the broad-host-range transmissible plasmid, pRK290 (9). For expression in Pseudomonas hosts using the Palk promoter, the ema genes were PCR amplified as NdeI fragments and cloned into the NdeI site of plasmid pSPZ2Not (24) between the Palk promoter and the T4 transcriptional terminator Tt4. The alkS-Palk-ema-Tt4 expression cassettes were then excised from the resulting plasmids by NotI and cloned into the NotI site of a pRK290 derivative where the unique BglII site was replaced by a BamHI-NotI-BamHI adapter.
Cloning of ferredoxins.
The FD-encoding genes fd233 and fdEA (from S. tubercidicus I-1529; GenBank accession no. AY549200 and AY549197, respectively), and fd230 (from S. tubercidicus R-922; GenBank accession no. AY549204), were found to be clustered with the CYP-encoding genes cyp233, cypEA, and cyp230 cloned from the corresponding strains (12). The FD-encoding gene fd232 (GenBank accession no. AY552101) was identified using the fd233 gene as a hybridization probe against a cosmid library of strain S. tubercidicus R-922 prepared in SuperCos I (Stratagene, La Jolla, CA). A strongly hybridizing cosmid, pPEH232, was identified, and the hybridizing DNA was subcloned and sequenced. To test the biological activity of each of these ferredoxins in combination with the Ema CYPs, each individual FD-encoding gene was amplified by PCR to produce a gene fragment that included a blunt 5′ end, the native ribosome-binding site, the FD coding sequence, and a PmeI restriction site on the 3′ end. Each such FD gene fragment was cloned into the PmeI site located 3′ to the ema genes that had already been inserted into the Streptomyces expression plasmids. In this way, artificial operons consisting of one of the ema genes and one of the FD-encoding genes functionally linked to the same promoter were created (Fig. 1). The artificial operons consisting of one of the ema genes and one of the FD-encoding genes were also subcloned into the Pseudomonas expression vectors as described above for the ema genes.
Cloning of ferredoxin reductases.
Bacterial FRE protein sequences (GenBank accession no. 9929802, 3059213, 3059191, 9885215, 7619822, 3411185, 3251295, 1813616, and 146002) were retrieved from GenBank and aligned with the program CLUSTALW. Two conserved regions, approximately 266 amino acid residues apart, were used to design degenerate oligonucleotides for PCR. The FRE forward primer 5′-CGSCCSCCSCTSWSSAAS (where S is C or G and W is A or G) and the FRE reverse primer 5′-SASSGCSTTSBCCCARTGYTC (where S is C or G; B is C, G, or T; R is A or G; and Y is C or T) were used to amplify 800-bp products from both S. tubercidicus R-922 and I-1529. These amplicon pools were cloned into TOPO TA cloning vectors (Invitrogen, Inc., Carlsbad, CA), and 20 clones (each) from strains R-922 and I-1529 were sequenced. Sequencing revealed that four unique fre gene fragments were isolated: three from R-922 (fre3, fre12, and fre14; GenBank accession numbers AY549205, AY549206, and AY549207), and one from I-1529 (fre16; GenBank accession number AY549208). The fre3, fre12, fre14, and fre16 gene fragments were used as probes to identify full-length FRE genes from cosmid libraries of strains R-922 and I-1529, both prepared in SuperCos I (Stratagene, La Jolla, CA).
The gene freEA (AY549197), encoding a FRE-homologous protein, was found to be clustered with the CYP-encoding gene cypEA, cloned from S. tubercidicus I-1529 (12). The FRE genes for SCO 7117, SCO 0681, and the oxidoreductase/FRE-homologous genes for SCO 0158 and SCO 4595 were identified in the Streptomyces coelicolor genome database (http://streptomyces.org.uk/S.coelicolor/index.html). All FRE-encoding genes were amplified by PCR using Pfu DNA polymerase (Stratagene, La Jolla, CA), using the appropriate cosmids as templates for freEA, fre3, fre12, fre14, and fre16 or S. coelicolor M145 genomic DNA as the template for the SCO-encoding genes. Each amplicon included a blunt 5′ end, the native ribosome-binding site, the FRE coding sequence, and a PmeI restriction site on the 3′ end. Each FRE gene fragment was cloned into the PmeI site located 3′ to the ema-fd233 operons that had already been inserted into the Streptomyces expression plasmids (Fig. 1). In this way, artificial operons consisting of one of the ema genes, the ferredoxin fd233, and one of the FRE genes functionally linked to the same promoter were created. Some of these artificial operons were also subcloned into the Pseudomonas expression vectors as described above for the ema genes.
RESULTS
Expression of ema1 and ema2 in S. lividans and P. putida.
The accompanying article (12) describes CYP enzymes that are able to catalyze the bioconversion of avermectin B1a and B1b to 4"-oxo-avermectin B1a and B1b, respectively. The genes encoding these enzymes were isolated, sequenced, and expressed in Escherichia coli to produce active enzymes for further biochemical characterization (12). To facilitate the genetic manipulation of the biocatalytic enzymes and their electron supply partners, we set out to develop heterologous expression systems for the Ema proteins. We chose the CYPs Ema1 and Ema2 as our initial targets based on their favorable enzymatic and expression characteristics in their native hosts (12). For primary expression hosts, we chose S. lividans based on its ease of manipulation and widespread use as a Streptomyces host for gene expression and later P. putida based on its extensive use in industrial biocatalysis and relative ease of manipulation. Both of these host strains were tested and found to be unable to carry out the biocatalytic reaction in the absence of the cloned ema genes.
The coding sequence of ema1 was cloned into Streptomyces expression vectors harboring the inducible PtipA (19) or the constitutive PermE* (6) promoters (Table 2; Fig. 1) as described in Materials and Methods. These constructs were transformed into S. lividans ZX7, where they integrated into the host chromosome via the φC31-derived integrase (14). Formation of 4"-oxo-avermectins was detected with cultures grown on avermectin-containing agar medium as described in Materials and Methods. The biocatalytic conversion was found to be more effective with the expression vector pEA that harbored the constitutive PermE* promoter (Fig. 2).
FIG. 2.
Agar-based biocatalytic conversion assays with S. lividans ZX7 strains. Avermectin to 4"-oxo-avermectin end-point bioconversion assays were performed in triplicate with strains growing on avermectin-containing agar plates as described in Materials and Methods. S. lividans ZX7 carried different expression vector-ema gene-fd (ferredoxin) gene combinations as shown below the bars. Gene versions for ema: 1, ema1; 1A, ema1A; 1CTG, ema1CTG; 2, ema2; 2CTG, ema2CTG. Fd-encoding genes: 232, fd232; 233, fd233; none, no FD-encoding gene was included in the construct.
In a similar experiment, the coding sequence of ema2 was also subcloned into the same expression vectors and tested in the bioconversion reaction in S. lividans ZX7 as a host. Unexpectedly, these strains displayed barely detectable biocatalytic activities (Fig. 2).
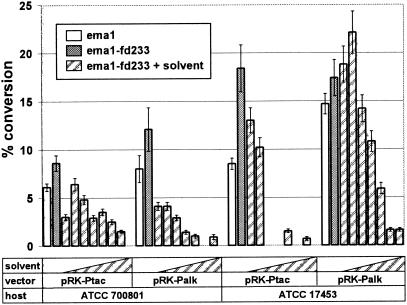
Next, the ema1 gene was cloned into the broad-host-range, transmissible plasmid pRK290 (9) and introduced by conjugal transfer from E. coli into P. putida ATCC 700801 and ATCC 17453. The alkane-inducible Palk promoter (24) and the Ptac promoter (33), which is constitutive in pseudomonads, were used to drive the expression of ema1. P. putida ATCC 700801 and ATCC 17453 containing plasmids pRK-Ptac-ema1 and pRK-Palk-ema1 were tested for their ability to catalyze the oxidation of avermectin in resting-cell assays as described in Materials and Methods. Biocatalytic conversion of avermectin to 4"-oxo-avermectin was detected with each recombinant strain (Fig. 3), reaching 15% with hexane-induced P. putida ATCC 17453 (pRK-Palk-ema1). In a similar manner, a pRK-Ptac-ema2 construct was created and expressed in P. putida ATCC 700801. The biocatalytic conversion rate of P. putida ATCC 700801 expressing Ema2 was similar to that of the strain carrying Ema1 (results not shown).
FIG. 3.
Biocatalytic conversion with resting cells of P. putida strains. Avermectin to 4"-oxo-avermectin conversion assays with resting cells of P. putida strains ATCC 700801 or ATCC 17453, carrying different expression vector-ema gene-ferredoxin gene combinations were performed in triplicate as described in Materials and Methods. White bars, ema1-expressing strains grown with 0% solvent; gray bars, ema1-fd233-expressing strains grown in the presence of 0% solvent; bars with diagonal stripes, ema1-fd233-expressing strains grown in the presence of increasing concentrations of solvent (0.5%, 2.5%, 5.0%, 10%, 25%, 37.5%, and 50% dioctyl phthalate, respectively). Missing bars in the solvent concentration series indicate that experiments were not done.
Expression of altered ema1 and ema2 genes in S. lividans.
Both ema1 and ema2 contain the leucine codon TTA, encoding Leu4 in the corresponding CYPs. The TTA(Leu) codon is extremely rare in Streptomyces and has been implicated as a posttranscriptional regulatory device (17, 18). During the cloning of ema1 into expression vectors, we identified a clone where this CYP gene was serendipitously truncated with a new start at Met5 (Ema1, MSELMNS…; Ema1A, MNS…) (the new start is indicated by boldface type), thereby omitting the TTA-encoded Leu4. We subcloned ema1A into the expression vectors pTBK and pEA, introduced these constructs into S. lividans ZX7, and tested the resulting strains for biocatalytic activity upon growth on avermectin-containing agar medium. A significant increase in the biocatalytic activity of the strain carrying ema1A was seen in the pTBK, but not in the pEA, background compared to similar strains carrying ema1 (Fig. 2).
To further investigate the effect of the presence of the TTA(Leu) codon in Ema1, we changed this codon to CTG(Leu) by site-directed mutagenesis and measured the biocatalytic activity of S. lividans ZX7 (pTBK-ema1CTG). The strain carrying this mutated gene converted 4.6 times more avermectin to 4"-oxo-avermectin than the strain with pTBK-ema1 and 1.4 times more than the strain with pTBK-ema1A (Fig. 2).
Since Leu4 is also encoded by a TTA codon in the ema2 gene, we were interested to see whether a similar change would improve the biocatalytic capacity of S. lividans strains expressing this gene. The TTA(Leu4) codon was changed to CTG(Leu4) via site-directed mutagenesis, and S. lividans ZX7 strains carrying this ema2CTG gene were assayed on agar medium. No improvement in the bioconversion was seen compared to S. lividans ZX7 carrying ema2 (Fig. 2).
Identification of ferredoxins that are active with Ema1.
None of the 17 ema genes described in the accompanying article (12) was found to be clustered with FD- or FRE-encoding genes. During our initial attempts to clone the biocatalytic CYP, however, we isolated and sequenced several additional CYP-encoding genes from strains R-922 and I-1529. Although these CYPs did not have a role in the biocatalytic reaction, some of them were clustered with genes encoding FDs. Thus, we have identified FD-encoding genes fd230 from strain R-922 and fd233 and fdEA from strain I-1529. To test the biological activity of each of these FDs in combination with Ema1, artificial operons consisting of the ema1A gene and one of the fd genes were created and functionally linked to the PtipA promoter in the Streptomyces expression vector pTBK (see Materials and Methods). Fd233, but not the other two FDs, provided for a significantly increased biocatalytic activity in S. lividans ZX7, compared to that of ema1A alone in the same plasmid and host background (Fig. 2 and results not shown).
Since fd233 is derived from strain I-1529 and ema1 is from strain R-922, the proteins encoded by the two genes cannot interact with each other in nature. Thus, we cloned an fd233-homologous gene from strain R-922, as described in Materials and Methods. Comparison of the deduced amino acid sequences of Fd233 (from strain I-1529) and its R-922 homolog, Fd232, revealed that they differ in only a single amino acid (S in Fd232 versus T in Fd233 at position 60). Fd233 and Fd232 were tested in pTBK- and pEA-based coexpression systems with Ema1A and separately with full-length Ema1 in S. lividans ZX7. Both FDs increased the biocatalytic conversion rate in both expression vectors carrying ema1A or ema1, with Fd232 being slightly less effective than Fd233 (Fig. 2).
In a similar manner, an artificial operon was created from ema2 and fd233, cloned into pTBK and pEA, and assayed in S. lividans ZX7 as the host for the biocatalytic reaction. Dramatic increases in the biocatalytic ability of these strains were recorded compared to the strains carrying ema2 in the same vector background (Fig. 2).
The ema1-fd233 artificial operon cassettes were also subcloned into the Pseudomonas vectors pRK-Ptac and pRK-Palk and introduced into P. putida strains ATCC 700801 and ATCC 17453. The resulting strains showed elevated levels of biocatalytic activity in resting-cell assays, compared to strains harboring the ema1 gene alone in the corresponding plasmids (Fig. 3).
A BLAST analysis of the amino acid sequence of Fd233 revealed that the closest match in the databanks was to a ferredoxin from S. coelicolor (GenBank accession number 21219296; 79% identity at the amino acid level). All FDs described here belong to the low-potential monocluster 3Fe-4S ferredoxin group (29) where the iron-sulfur cluster is covalently attached to the protein at Cys10, Cys16, and Cys54 (Fd233 numbering). The Cys that would coordinate the fourth iron in 4Fe-4S clusters was replaced by Ala13 in Fd233 (Fig. 4A).
FIG. 4.
Sequence alignments of ferredoxins and ferredoxin reductases. (A) Multiple sequence alignment of the ferredoxins described in this study with their nearest homologs from the databanks. Residues conserved in all 10 proteins are shown in white letters on a black background. Residues conserved in at least 7 of 10 proteins are highlighted in light gray. Numbers above the sequences denote the position of the cysteine residues that coordinate the irons of the iron-sulfur clusters in FDs. Fd_ave, ferredoxin from S. avermitilis (GenBank accession number 5824147); Fd_coe, ferredoxin from S. coelicolor (GenBank accession number 21219296); Fd_liv, ferredoxin from S. lividans (GenBank accession number 3293541); Fd_GrhO4, ferredoxin GrhO4 from the griseorhodin biosynthetic gene cluster of Streptomyces sp. JP95 (GenBank accession number 21039506); Fd_NysM, ferredoxin NysM from the nystatin biosynthetic gene cluster of S. noursei (GenBank accession number 8050844); Fd_SoyB, soy flour-inducible ferredoxin SoyB from S. griseus (GenBank accession number 119970). (B) Multiple sequence alignment of conserved regions in ferredoxin reductases. Consensus sequences for the FAD ADP-binding motifs, the NAD ADP-binding motifs, and the FAD ribytil-binding motifs (2) are shown above the alignment. Fre12, Fre14, Fre16, Fre3, and FreEA are the ferredoxin reductases described in this study. Three FREs (SCO 7117, SCO 0681, and SCO 2469) and three probable oxidoreductases that are homologous to FRE enzymes (SCO 0158, SCO 2106, and SCO 4595) are from the completely sequenced genome of S. coelicolor A3 (2,4).
Bioconversion with Streptomyces resting cells.
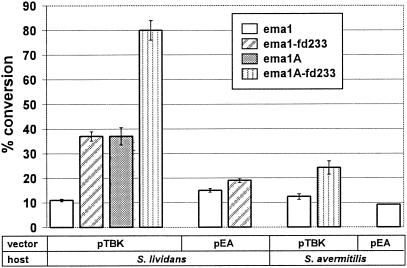
Selected S. lividans ZX7 transformants were cultivated in liquid PYG medium, and the mycelia were used in resting-cell bioconversion assays as described in Materials and Methods. Resting cells of S. lividans ZX7 transformants were more effective biocatalysts than agar surface cultures but converted avermectin to 4"-oxo-avermectin with the biotransformation rates following the same trend as was seen in the agar-based bioconversion assays (ema1A-fd233 > ema1A ∼ema1-fd233 > ema1). The highest level of conversion was attained with S. lividans ZX7 (pTBK-ema1A-fd233), with the product yield exceeding 80% after 16 h of incubation (Fig. 5).
FIG. 5.
Biocatalytic conversion with resting cells of Streptomyces strains. S. lividans ZX7 and S. avermitilis MOS-0001 strains carrying different expression vector-ema gene-fd gene combinations were cultivated in triplicate in liquid media, and the cells were harvested and used in resting-cell bioconversion assays as described in Materials and Methods.
In addition, some of the constructs were introduced into Streptomyces avermitilis MOS-0001 by protoplast-mediated transformation (30), and the resulting strains were evaluated for the bioconversion reaction by the resting-cell assay as described in Materials and Methods. S. avermitilis MOS-0001 carrying the cloned ema1 gene was able to transform avermectin to 4"-oxo-avermectin, with 24% product yield in a 16-h incubation in the case of pTBK-ema1A-fd233 (Fig. 5). S. avermitilis MOS-0001 is a producer of avermectin, so we also evaluated the transformed strains in avermectin fermentations for the direct production of 4"-oxo-avermectin. No formation of 4"-oxo-avermectin was detected (results not shown). Sampling the producer cultures on each day of the fermentation followed by resting-cell bioconversion assays revealed that the biocatalytic activity of the MOS-0001 transformants declined rapidly during the fermentation, with no biocatalytic activity measurable after day 5, while avermectin production starts around day 8 (results not shown).
Resting-cell assays with P. putida transformants grown in the presence of solvents.
Since avermectins are barely soluble in water, we were interested in delivering higher concentrations of substrate to the biocatalyst by employing a biphasic reaction system. The P. putida strains used in this study are described in the literature as resistant to different solvents (36). We tested the growth of P. putida strains ATCC 17453 and ATCC 700801 in different organic solvents that were also able to solubilize an excess of 50 g/liter avermectin in water-saturated biphasic systems. Dioctyl phthalate was found to be well tolerated by both Pseudomonas strains used and served as an excellent solvent for avermectins (solubility, 300 g/liter). We grew P. putida ATCC 17453 and ATCC 700801, carrying one of the plasmids pRK-Ptac-ema1-fd233 or pRK-Palk-ema1-fd233, in the presence of various concentrations of dioctyl phthalate and used these solvent-conditioned cells in resting-cell assays as described in Materials and Methods. As shown in Fig. 3, the biocatalytic performance of the biocatalyst rapidly declined with increasing concentrations of solvent, possibly as a result of a decreased expression of the ema1-fd233 expression cassette (results not shown).
Identification and cloning of genes encoding ferredoxin reductases.
The electron transport chain that supports class I CYPs includes an FRE that channels electrons from NAD(P)H to FD. To further optimize the biocatalytic activity of our engineered strains, a number of FRE genes from Streptomyces strains were cloned and evaluated for their impacts on the biocatalytic potential of the recombinant strains. To do this, degenerate oligonucleotides were designed for two conserved regions in bacterial FRE sequences and were used for PCR against S. tubercidicus R-922 and I-1529 genomic DNA. Unique PCR products were used as probes to identify full-length FREs from R-922 and I-1529 genomic clone banks, as described in Materials and Methods. Three unique FRE genes from R-922 (fre3, fre12, and fre14), and one from I-1529 (fre16) were cloned and sequenced in this manner.
Three FRE genes (genes for SCO 7117, SCO 0681, and SCO 2469) have been identified in the completely sequenced genome of S. coelicolor A3 (2, 15). Two additional probable oxidoreductases were also annotated in the S. coelicolor genome project as close homologs of FRE enzymes (SCO 0158 and SCO 2106), while another oxidoreductase, SCO 4595, has a Pfam match to flavodoxin/ferredoxin oxidoreductases (4). We amplified the genes encoding SCO 7117, SCO 0681, SCO 0158, and SCO 4595 from the genomic DNA of S. coelicolor M145 (13), as described in Materials and Methods. Finally, an additional FRE gene, freEA, was found adjacent to the fdEA gene on the chromosome of S. tubercidicus I-1529.
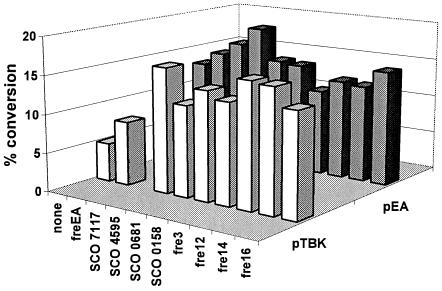
To assess the potential of the cloned FREs in increasing the biocatalytic activity of Ema1, each gene was amplified by PCR and used to create artificial operons consisting of ema1, fd233, and the individual FRE-homologous genes. Each of these artificial operon cassettes was subcloned into the Streptomyces expression vectors pTBK and pEA and transformed into S. lividans ZX7, and the resulting strains were evaluated by the agar-based bioconversion assay. Significant increases in 4"-oxo-avermectin formation were seen with some of the FRE-homologous genes tested (Fig. 6). The improvements provided by the different FRE-homologous genes, however, varied with the vector background: the oxidoreductase/FRE-homologous SCO 4595, for example, yielded a threefold-higher rate of conversion in the pTBK background compared with the 1.5-fold enhancement in the pEA background.
FIG. 6.
Agar-based biocatalytic conversion assays with S. lividans ZX7 strains carrying different ema1-fd233-fre operons. Avermectin to 4"-oxo-avermectin end-point bioconversion assays were performed in triplicate with strains growing on avermectin-containing agar plates as described in Materials and Methods. S. lividans ZX7 carried different ema1-fd233-fre (ferredoxin reductase) artificial operons in pTBK (white bars) or pEA (gray bars). None, the expression cassette contained ema1 and fd233 but no fre genes.
The S. coelicolor ferredoxin reductases SCO 7117, SCO 4595, and SCO 0158 and the I-1529-derived FreEA were also used to create artificial operons with ema1A-fd233 in the pTBK and pEA vectors. The FRE genes, however, did not provide significant increases in the bioconversion yields in the agar-based bioassays (results not shown).
In a similar approach, the R-922 and I-1529-derived fre genes were inserted into the Pseudomonas expression plasmid pRK-Ptac-ema1-fd233. These constructs were introduced into P. putida strains ATCC 17453 and ATCC 700801, and the transformants were analyzed for their biocatalytic activity by the resting-cell assay. The effect of the FRE genes were found to be host strain dependent (results not shown): while the FREs did not increase the biocatalytic activity of ATCC 700801-derived strains, Fre12 and Fre16 provided for a nearly twofold-higher 4"-oxo-avermectin yield in P. putida ATCC 17453.
The FRE from S. tubercidicus R-922 and I-1529 all contained the three conserved regions implicated in FAD and NAD(P)H binding. Thus, the putative ADP-binding site of NAD(P)H (GXGX2GX3AX6GX6E), and the ADP-binding site (GXGX2GX3AX6G) and ribityl-binding site (TX6AXGD) of FAD (2) are well conserved (Fig. 4B). Fre3 (from strain R-922) and Fre16 (from I-1529) are 95.5% identical at the amino acid level, while FreEA shows <35% identity with the other four FRE proteins identified in this study. Fre3, Fre14, and Fre16 were most similar to FRE from S. coelicolor (SCO 7117 and SCO 2469), with identities in the 50 to 65% range. Fre12 and FreEA showed the highest identities (57% and 59%, respectively) to FRE from S. avermitilis (GenBank accession number 29833498) and Myxococcus xanthus (GenBank accession number 4633137), respectively.
DISCUSSION
In this work, we expressed CYPs Ema1 and Ema2, derived from S. tubercidicus R-922 and I-1529, respectively, in Pseudomonas and Streptomyces host strains and cloned ferredoxins and ferredoxin reductases from different Streptomyces strains that were able to increase the biocatalytic activity of these enzymes when coexpressed in the same host strains.
The gene for Ema1 was expressed from different promoters to provide easily measurable biocatalytic activities in the S. lividans host in the agar-based test system and in the P. putida hosts in resting-cell assays. The successful expression of ema1 in Streptomyces and Pseudomonas strains to produce biocatalytically active cells indicates that Ema1 is able to partner with FD and FRE electron transport proteins from these strains. CYP enzymes do not require exclusive FD partners but are able to work with a range of FDs, and this relative promiscuity seems to apply to the FD-FRE partners too (15). Thus, 18 CYPs have been identified in the S. coelicolor genome project, but these enzymes apparently rely on six FDs and three to five FREs for electron transport (4). S. avermitilis has been reported to command 33 CYPs, served by six FDs and nine FRE enzymes (11). The daunorubicin-producer S. peucetius ATCC 27952 harbors 19 CYPs supported by only two FDs and four FRE (25). The completely sequenced genome of P. putida KT2440 contains two CYP-encoding genes, with five deduced proteins each annotated as FD and FRE (20). Some heterologous CYP enzymes expressed in surrogate Streptomyces hosts were able to function well (34), while others were nonfunctional unless a suitable FD was coexpressed (21). In this study, we have cloned four different 3Fe-4S FD genes from S. tubercidicus strains R-922 and I-1529 and tested these by creating artificial operons with ema1 for expression in S. lividans. Coexpression of Fd233 with Ema1 led to an approximately twofold increase in the bioconversion rate in agar-based biocatalytic assays (Fig. 2), while FdEA and Fd230 did not support improvements in this system. In spite of the near identity of Fd232 (derived from R-922) and Fd233 (derived from I-1529), Fd232 was found to be a less effective electron donor partner for Ema1 (itself derived from R-922) in the S. lividans surrogate host. Improved biocatalytic performances with ema1-fd233 or ema1A-fd233 operons (compared to ema1 or ema1A alone) were also recorded in resting-cell assays in the S. lividans host (Fig. 5). The increase in the biocatalytic activity with ema1-fd233 artificial operons, compared to ema1 alone, was also manifested in other hosts like P. putida ATCC 17453, but this was less evident with P. putida ATCC 700801 (Fig. 3). Further testing of FDs from strain R-922, possibly those with 2Fe-2S or 4Fe-4S clusters, in coexpression systems with Ema1 can lead to the identification of the cognate FD partner for this CYP and might further improve the efficiency of the recombinant biocatalyst.
In contrast to ema1, S. lividans ZX7 strains carrying ema2 displayed barely detectable biocatalytic activities. Indeed, our initial attempts to clone the biocatalytic enzyme from strain I-1529 that had relied on expression libraries constructed in S. lividans ZX7 have proven futile (12). On the other hand, S. tubercidicus I-1529, the native producer of Ema2, shows vigorous biocatalytic activity, and purified Ema2 enzyme from strain I-1529 or recombinant Ema2 from E. coli has been shown to be competent in biocatalysis, with Km and Vmax values similar to those of Ema1 (12). When expressed in P. putida host strains, Ema2 provided for a similar biocatalytic performance, as did Ema1 (results not shown). The biocatalytic activity of Ema2 in S. lividans could not be improved by site-directed mutagenesis that replaced the rare TTA codon by CTG encoding Leu4. Creating artificial operons of ema2 with fd233, however, dramatically improved the biocatalytic performance in S. lividans ZX7 strains (Fig. 2), although the biocatalytic conversion still did not reach the same level as with the ema1-fd233 cassette in the same strain-vector combinations. We speculate that the CYP Ema2 is not receptive to the FD and FRE partners found in S. lividans, but the electron flow and concomitant activity of the enzyme are restored with a cloned FD mediator that is acceptable to both the cloned Ema2 and the native FRE from this host.
Both ema1 and ema2 contain a TTA codon near their 5′ end, encoding Leu4 in the corresponding CYP proteins. This codon is extremely rare in Streptomyces, featured in only 260 out of 7,825 genes in the completely sequenced S. coelicolor genome (4). TTA codons are translated by the bldA-encoded Leu tRNA that becomes available only in the late phases of growth, thereby providing for a growth phase-dependent regulation of protein expression at the posttranscriptional level (17, 18). Releasing Ema1 from bldA regulation by either truncating the ema1 gene or mutating the TTA codon to CTG supported significant increases in the biocatalytic activities of S. lividans strains carrying these genes functionally linked to the inducible PtipA promoter but not with strains where the ema genes were under the control of the constitutive PermE* promoter (Fig. 2). In these experiments, the PtipA promoter was induced with thiostrepton from the moment of inoculation onto the avermectin-containing agar medium. In detailed investigations of the PtipA promoter, maximum levels of expression were achieved when S. lividans cultures were induced at the early stages of growth, with the expression level peaking at the exponential phase and declining rapidly in later stages (1). Inducibility also seemed to be restricted to the early growth phase, with little induction (and overall expression) achieved when thiostrepton was added to the cultures in the stationary phase (1). We hypothesize that the initial absence of the bldA-encoded tRNA at the early stages of growth equally restricted the translation of the ema1-derived mRNA with both PtipA and PermE* as promoters. By the time the bldA-encoded tRNA became available in the stationary phase, the activity of the PtipA promoter had already declined, while the PermE* promoter still actively transcribed the ema1 gene. Since the translation of the ema1A or the ema1CTG mRNA does not require the bldA-encoded tRNA, the strains expressing these genes can utilize the high early stage activity levels of the PtipA promoter, leading to a significant increase in the biocatalytic performance of these strains compared with those strains that express ema1.
To further optimize the biocatalyst, we cloned three FREs from S. tubercidicus R-922, two from S. tubercidicus I-1529, and four FRE homologs from S. coelicolor and used these to create artificial operons with ema1-fd233 or ema1A-fd233. These operons were cloned into expression vectors and tested with S. lividans and P. putida. Fre12 and Fre16 provided for a nearly twofold-higher 4"-oxo-avermectin yield in P. putida ATCC 17453, but none of the FRE tested increased the biocatalytic activity of the P. putida ATCC 700801-derived strains (results not shown). In S. lividans, significant increases in the biocatalytic performance were seen when FRE-encoding genes were coexpressed with Ema1 and Fd233 with the PtipA promoter (Fig. 6), while the improvements were less pronounced with the PermE* promoter and absent when Ema1A was used instead of Ema1, irrespective of the promoter choice (results not shown). We propose that FRE availability might have been the limiting factor in S. lividans only in those cases where the translation of the CYP-encoding mRNA was reduced in the early stages of the culture by the absence of the bldA-encoded tRNA. This limitation was accentuated by the expected reduction in the expression of CYP from the PtipA promoter in the later stages of growth. Indeed, while the expression of the S. coelicolor CYP complement could be detected even in early stage cultures, FRE expression was found to be restricted to older cultures, representing a novel mechanism for the regulation of CYP activity in this close relative of the S. lividans host used in our experiments (16). It is possible, however, that none of the R-922-derived FREs cloned in this study represented the cognate FRE for the Ema1 CYP, and identification of the physiological FRE partner, in conjunction with the cognate FD, would yield more substantial improvements in the performance of our biocatalysts.
We have planned to use solvent-water biphasic systems for the biocatalytic reaction to facilitate the conversion of larger amounts of the water-immiscible substrate; to this end, we cultivated solvent-tolerant P. putida strains ATCC 17453 and ATCC 700801, carrying different Ema expression constructs, in the presence of dioctyl phthalate. In spite of acceptable growth rates, bioconversion activities of cells grown in the presence of higher concentrations of solvent declined rapidly (Fig. 3), apparently due to a rapid decrease of the expression of the Ema1 protein (results not shown).
We also attempted to create a bacterial strain that would produce 4"-oxo-avermectins de novo. To this end, the ema1 gene or the ema1A-fd233 operon was engineered into S. avermitilis MOS-0001, a producer of avermectins. Although young mycelia from the transformed cultures were able to carry out the biocatalytic conversion (Fig. 5), stationary-phase cultures were not biocatalytically active, and no direct production of 4"-oxo-avermectin was observed (results not shown). Optimization of the expression pattern of ema1, using promoters that are active in the stationary phase, might help to harmonize avermectin production and biocatalytic enzyme expression.
In conclusion, we engineered S. lividans, S. avermitilis, and P. putida strains to express the CYP Ema1 and improved the biocatalytic performance of these strains by using different vectors and promoters, replacing an expression-limiting codon and coexpressing newly identified ferredoxins and ferredoxin reductases. Further development of these biocatalysts by fermentation optimization could lead to the replacement of the costly chemical process of avermectin 4" oxidation with a more environmentally friendly and cheaper biological route for the crop protection industry.
Acknowledgments
We acknowledge the excellent technical assistance of Amber Gaudreau, Sandy Emhart, and Keri Cavanaugh; the analytical support provided by Belle Abrera, James Pomes, Alex Frank, and Mario Jörg; and DNA sequencing by Ann Hu and Makoto Ono.
This work was supported by Syngenta Biotechnology, Inc. (Research Triangle Park, NC), and Syngenta Crop Protection AG (Basel, Switzerland).
REFERENCES
- 1.Ali, N., P. R. Herron, M. C. Evans, and P. J. Dyson. 2002. Osmotic regulation of the Streptomyces lividans thiostrepton-inducible promoter, ptipA. Microbiology 148:381-390. [DOI] [PubMed] [Google Scholar]
- 2.Asturias, J. A., E. Diaz, and K. N. Timmis. 1995. The evolutionary relationship of biphenyl dioxygenase from gram-positive Rhodococcus globerulus P6 to multicomponent dioxygenases from gram-negative bacteria. Gene 156: 11-18. [DOI] [PubMed] [Google Scholar]
- 3.Baik, S. H., C. Kang, C. Jeon II, and S. E. Yun. 1999. Direct electrochemical regeneration of NADH from NAD+ using cholesterol-modified gold amalgam electrode. Biotechnol. Tech. 13:1-5. [Google Scholar]
- 4.Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 5.Berrie, J. R., R. A. Williams, and K. E. Smith. 1999. Microbial transformations of steroids. XI. Progesterone transformation by Streptomyces roseochromogenes—purification and characterisation of the 16α-hydroxylase system. J. Steroid Biochem. Mol. Biol. 71:153-165. [DOI] [PubMed] [Google Scholar]
- 6.Bibb, M. J., G. R. Janssen, and J. M. Ward. 1985. Cloning and analysis of the promoter region of the erythromycin-resistance gene (ermE) of Streptomyces erythraeus. Gene 38:E357-E368. [DOI] [PubMed] [Google Scholar]
- 7.Cvetovich, R. J., D. H. Kelly, L. M. DiMichele, R. F. Shuman, and E. J. J. Grabowski. 1994. Syntheses of 4"-epi-amino-4"-deoxyavermectins B1. J. Org. Chem. 59:7704-7708. [Google Scholar]
- 8.Dejonghe, W., N. Boon, D. Seghers, E. M. Top, and W. Verstraete. 2001. Bioaugmentation of soils by increasing microbial richness: missing links. Environ. Microbiol. 3:649-657. [DOI] [PubMed] [Google Scholar]
- 9.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faulkner, K. M., M. S. Shet, C. W. Fisher, and R. W. Estabrook. 1995. Electrocatalytically driven omega-hydroxylation of fatty acids using cytochrome P450 4A1. Proc. Natl. Acad. Sci. USA 92:7705-7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda, H., J. Ishikawa, A. Hanamoto, M. Shinose, H. Kikuchi, T. Shiba, Y. Sakaki, M. Hattori, and S. Omura. 2003. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 21:526-531. [DOI] [PubMed] [Google Scholar]
- 12.Jungmann, V., I. Molnár, P. E. Hammer, D. S. Hill, R. Zirkle, T. G. Buckel, J. M. Ligon, and J. P. Pachlatko. 2005. Biocatalytic conversion of avermectin to 4"-oxo-avermectin: characterization of biocatalytically active bacterial strains and of cytochrome P450 monooxygenase enzymes and their genes. Appl. Environ. Microbiol. 71:6968-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom.
- 14.Kuhstoss, S., and R. N. Rao. 1991. Analysis of the integration function of the streptomycete bacteriophage φC31. J. Mol. Biol. 222:897-908. [DOI] [PubMed] [Google Scholar]
- 15.Lamb, D. C., T. Skaug, H.-L. Song, C. J. Jackson, L. M. Podust, M. R. Waterman, D. B. Kell, D. E. Kelly, and S. L. Kelly. 2002. The cytochrome P450 complement (CYPome) of Streptomyces coelicolor A3(2). J. Biol. Chem. 277:24000-24005. [DOI] [PubMed] [Google Scholar]
- 16.Lei, L., M. R. Waterman, A. J. Fulco, S. L. Kelly, and D. C. Lamb. 2004. Availability of specific reductases controls the temporal activity of the cytochrome P450 complement of Streptomyces coelicolor A3(2). Proc. Natl. Acad. Sci. USA 101:494-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leskiw, B. K., M. J. Bibb, and K. F. Chater. 1991. The use of a rare codon specifically during development? Mol. Microbiol. 5:2861-2867. [DOI] [PubMed] [Google Scholar]
- 18.Leskiw, B. K., E. J. Lawlor, J. M. Fernandez-Abalos, and K. F. Chater. 1991. TTA codons in some genes prevent their expression in a class of developmental, antibiotic-negative, Streptomyces mutants. Proc. Natl. Acad. Sci. USA 88:2461-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami, T., T. G. Holt, and C. J. Thompson. 1989. Thiostrepton-induced gene expression in Streptomyces lividans. J. Bacteriol. 171:1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson, K. E., C. Weinel, I. T. Paulsen, R. J. Dodson, H. Hilbert, V. A. Martins dos Santos, D. E. Fouts, S. R. Gill, M. Pop, M. Holmes, L. Brinkac, M. Beanan, R. T. DeBoy, S. Daugherty, J. Kolonay, R. Madupu, W. Nelson, O. White, J. Peterson, H. Khouri, I. Hance, P. Chris Lee, E. Holtzapple, D. Scanlan, K. Tran, A. Moazzez, T. Utterback, M. Rizzo, K. Lee, D. Kosack, D. Moestl, H. Wedler, J. Lauber, D. Stjepandic, J. Hoheisel, M. Straetz, S. Heim, C. Kiewitz, J. Eisen, K. N. Timmis, A. Dusterhoft, B. Tummler, and C. M. Fraser. 2002. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 4:799-808. [DOI] [PubMed] [Google Scholar]
- 21.O'Keefe, D. P., K. J. Gibson, M. H. Emptage, R. Lenstra, J. A. Romesser, P. J. Litle, and C. A. Omer. 1991. Ferredoxins from two sulfonylurea herbicide monooxygenase systems in Streptomyces griseolus. Biochemistry 30:447-455. [DOI] [PubMed] [Google Scholar]
- 22.Omer, C. A., R. Lenstra, P. J. Little, C. Dean, J. M. Tepperman, K. J. Leto, J. A. Romesser, and D. P. O'Keefe. 1990. Genes for two herbicide-inducible cytochromes P-450 from Streptomyces griseolus. J. Bacteriol. 172:3335-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz de Montellano, P. R. 1995. Cytochrome P450: structure, mechanism, and biochemistry, 2nd ed. Plenum Press, New York, N.Y.
- 24.Panke, S., A. Meyer, C. M. Huber, B. Witholt, and M. G. Wubbolts. 1999. An alkane-responsive expression system for the production of fine chemicals. Appl. Environ. Microbiol. 65:2324-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parajuli, N., D. B. Basnet, H. C. Lee, J. K. Sohng, and K. Liou. 2004. Genome analyses of Streptomyces peucetius ATCC 27952 for the identification and comparison of cytochrome P450 complement with other Streptomyces. Arch. Biochem. Biophys. 425:233-241. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Schmid, A., J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts, and B. Witholt. 2001. Industrial biocatalysis today and tomorrow. Nature 409:258-268. [DOI] [PubMed] [Google Scholar]
- 28.Seelbach, K., B. Riebel, W. Hummel, M.-R. Kula, V. I. Tishkov, A. M. Egorov, C. Wandrey, and U. Kragl. 1996. A novel, efficient regenerating method of NADPH using a new formate dehydrogenase. Tetrahedron Lett. 37:1377-1380. [Google Scholar]
- 29.Sticht, H. 1999. The structure of small electron-transfer proteins. Recent Res. Dev. Biochem. 1:1-27. [Google Scholar]
- 30.Stutzman-Engwall, K., Y. Katoh, and H. McArthur. 1999. Streptomyces avermitilis gene directing the ratio of B2:B1 avermectins. WO patent 99/41389.
- 31.Taylor, M., D. C. Lamb, R. Cannell, M. Dawson, and S. L. Kelly. 1999. Cytochrome P450105D1 (CYP105D1) from Streptomyces griseus: heterologous expression, activity, and activation effects of multiple xenobiotics. Biochem. Biophys. Res. Commun. 263:838-842. [DOI] [PubMed] [Google Scholar]
- 32.Taylor, M., D. C. Lamb, R. J. P. Cannell, M. J. Dawson, and S. L. Kelly. 2000. Cofactor recycling with immobilized heterologous cytochrome P450 105D1 (CYP105D1). Biochem. Biophys. Res. Commun. 279:708-711. [DOI] [PubMed] [Google Scholar]
- 33.Vieira, J., and J. Messing. 1991. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene 100: 189-194. [DOI] [PubMed] [Google Scholar]
- 34.Walczak, R. J., J. V. Hines, W. R. Strohl, and N. D. Priestley. 2001. Bioconversion of the anthracycline analogue desacetyladriamycin by recombinant DoxA, a P450-monooxygenase from Streptomyces sp. strain C5. Org. Lett. 3:2277-2279. [DOI] [PubMed] [Google Scholar]
- 35.Waterman, M. R., and E. F. Johnson (ed.). 1991. Methods in enzymology, vol. 206. Academic Press, Inc., San Diego, CA.
- 36.Weber, F. J., L. P. Ooijkaas, R. M. W. Schemen, S. Hartmans, and J. A. M. de Bont. 1993. Adaptation of Pseudomonas putida S12 to high concentrations of styrene and other organic solvents. Appl. Environ. Microbiol. 59:3502-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zirkle, R., J. M. Ligon, and I. Molnár. 2004. Heterologous production of the antifungal polyketide antibiotic soraphen A of Sorangium cellulosum So ce26 in Streptomyces lividans. Microbiology 150:2761-2774. [DOI] [PubMed] [Google Scholar]