Abstract
Natural populations thriving in heavy-metal-contaminated ecosystems are often subjected to selective pressures for increased resistance to toxic metals. In the present study we describe a population of the ectomycorrhizal fungus Suillus luteus that colonized a toxic Cu mine spoil in Norway. We hypothesized that this population had developed adaptive Cu tolerance and was able to protect pine trees against Cu toxicity. We also tested for the existence of cotolerance to Cu and Zn in S. luteus. Isolates from Cu-polluted, Zn-polluted, and nonpolluted sites were grown in vitro on Cu- or Zn-supplemented medium. The Cu mine isolates exhibited high Cu tolerance, whereas the Zn-tolerant isolates were shown to be Cu sensitive, and vice versa. This indicates the evolution of metal-specific tolerance mechanisms is strongly triggered by the pollution in the local environment. Cotolerance does not occur in the S. luteus isolates studied. In a dose-response experiment, the Cu sensitivity of nonmycorrhizal Pinus sylvestris seedlings was compared to the sensitivity of mycorrhizal seedlings colonized either by a Cu-sensitive or Cu-tolerant S. luteus isolate. In nonmycorrhizal plants and plants colonized by the Cu-sensitive isolate, root growth and nutrient uptake were strongly inhibited under Cu stress conditions. In contrast, plants colonized by the Cu-tolerant isolate were hardly affected. The Cu-adapted S. luteus isolate provided excellent insurance against Cu toxicity in pine seedlings exposed to elevated Cu levels. Such a metal-adapted Suillus-Pinus combination might be suitable for large-scale land reclamation at phytotoxic metalliferous and industrial sites.
Severe anthropogenic enrichment with heavy metals can cause a rapid change in the biotic communities living in and on a soil. Very few plant species are able to survive on or colonize highly polluted soils through fast selection of metal-tolerant ecotypes (2, 6). For most plants a rapid unprecedented change in the amount of available metals in a soil is likely to exceed their adaptive potential; this is especially true for tree species, which have long reproductive cycles (23). Since plants are sessile organisms with limited mechanisms for stress avoidance, they need flexible means for acclimation to changing environmental conditions. It has been suggested that trees can resist extreme environments through their great phenotypic plasticity and through their association with well-adapted ectomycorrhizal (ECM) fungi (30). Plant adaptation to selective pressures is often considered to be regulated by the plant genome, but it is likely that mutualistic microorganisms also can alleviate heavy metal toxicity in plants (1, 12).
Under natural conditions the majority of woody plants in temperate and boreal forests are associated with ECM fungi, and it seems that this mutualistic symbiosis persists on strongly metal-contaminated sites, which are slowly colonized by mycotrophic tree species, such as birches, pines, and willows (17, 29). Although soil fungi (at least saprotrophs) seem to be more resistant to heavy metal contamination than soil bacteria (21), extreme conditions impose selection pressure that may trigger additional evolutionary adaptation in the fungal populations toward even greater resistance. Such genetic adaptation to toxic Zn concentrations has been described previously for ECM fungal populations of suilloid fungi thriving in pioneer pine forests that colonize sites severely contaminated by Zn smelters in northeast Belgium (7). In a dose-response experiment with pines as a host (1), we previously showed that a Zn-sensitive Suillus bovinus isolate developed poorly and failed to acquire sufficient nutrients when the mycorrhizal root system was exposed to Zn concentrations representative of the soluble Zn fraction present in the pore water of soils close to the Zn smelters. In contrast, a Zn-adapted fungal genotype hardly suffered from severe Zn stress. As a consequence, the better performance of this fungus significantly improved the nutrient status of its host.
In 2001, we discovered a Suillus luteus population which was associated with small pine trees that colonized mine waste heaps from a small abandoned Cu mine in central Norway. We hypothesized that this S. luteus population had developed adaptive Cu tolerance. In a first experiment, we compared the in vitro Cu tolerance of S. luteus isolates collected from the Cu-contaminated site, from a Zn-contaminated site, and from nonpolluted sites. The objective was to identify differential Cu sensitivity in the populations and to test whether there is any cotolerance for Cu and Zn in S. luteus. In a second experiment, the Cu sensitivity of nonmycorrhizal (NM) Pinus sylvestris seedlings was compared to the Cu sensitivity of mycorrhizal seedlings colonized by either a Cu-sensitive S. luteus isolate or a Cu-tolerant S. luteus isolate. This experiment was performed in order to explore (i) whether mycorrhizal pine seedlings performed better than NM seedlings when they were exposed to toxic Cu concentrations and (ii) whether a Cu-tolerant isolate offered better host plant protection than a Cu-sensitive isolate.
MATERIALS AND METHODS
Fungal material.
In September 2001 fungal cultures were prepared from seven S. luteus (L.: Fr.) Roussel sporocarps collected in pioneer Scots pine forests in Norway at Hedmark, Folldal, Geitryggen on a Cu mine spoil. In September 2002 additional cultures were prepared from seven sporocarps collected from uncontaminated sites in Norway, including four sporocarps from Hedmark, Folldal, Vassaga, one sporocarp from Telemark, Kragerø, Kurdøla, and two sporocarps from Oslo, Østmarka. For the in vitro metal screening tests, we included 14 S. luteus isolates collected in Belgium. One half of these isolates were Zn tolerant and were collected in pioneer forests at severely Zn-contaminated sites in Lommel, Maatheide, and Lommel, Sahara; the other half were Zn sensitive and originated from an uncontaminated site in Paal (7, 9). To ensure that genetically different individuals were obtained, the minimum distance between sporocarps collected for isolation was 10 m. Genetic fingerprints confirmed that this distance is sufficient to avoid sampling of the same individuals of S. luteus in pioneer conditions (19).
Soil characteristics.
Soil samples (∼1 kg) from the mine spoil and from the Belgian collection sites were transferred to a greenhouse, and pore water samples were obtained with Rhizon soil moisture samplers (Eijkelkamp Agrisearch Equipment, Giesbeek, The Netherlands) as described by Knight et al. (15). Pore water was analyzed to determine the pH and the Cu and Zn concentrations. The total metal in a 0.5-g soil sample was extracted in aqua regia using microwave digestion (Milestone 1200 MEGA).
Fungal metal tolerance analysis.
The fungi were isolated, grown, and screened for Cu and Zn tolerance in vitro as previously described (7). The Cu tolerance was tested on solid modified Fries medium (11). The final basic solution contained 28 mM glucose, 5.4 mM ammonium tartrate, 1.5 mM KH2PO4, 0.4 mM MgSO4 · 7H2O, 0.3 mM NaCl, 0.2 mM CaCl2 · 2H2O, 0.1 mM FeCl3 · 6H2O, 50 μM MnSO4 · H2O, 5 μM CuSO4 · 5H2O, 0.15 mM ZnSO4 · 7H2O, 0.1 μM biotin, 0.5 μM pyridoxine, 0.3 μM riboflavin, 0.8 μM nicotinamide, 0.7 μM p-aminobenzoic acid, 0.3 μM thiamine, 0.2 μM Ca pantothenate, and 0.8% agar. Six Cu treatments were performed by adding CuSO4 · 5H2O to the nutrient medium. Cu2+ was added at concentrations of 0.005, 0.3, 0.8, 1.6, 2.4, and 4.8 mM. Most Cu2+ in the medium (>90%) was chelated with tartrate, and at a Cu concentration of 4.8 mM a proportion of the Cu was precipitated with phosphate. The free Cu2+ concentration in the medium ranged from 3.3% to 6%, as calculated with Geochem 2.0. Six Zn treatments were performed by adding ZnSO4 · 7H2O to the nutrient medium. Zn2+ was added at concentrations of 0.15, 3, 6, 9, 12, and 15 mM. Over this gradient the free Zn2+ concentration in the medium increased from 20 to 58%. Zn2+ was partially complexed by tartrate (77 to 30%,) and at the highest concentrations, phosphate precipitates were formed. The pH of the final media was adjusted to 4.5.
The mycelia were harvested after 10 days of incubation. Mycelia were frozen at −80°C and subsequently freeze-dried before weighing. The increase in dry weight during the 10-day test period was determined. Tolerance indices were calculated for each isolate by determining the percentages of biomass retained on the metal-enriched media compared to growth on the control Fries medium. The 50% effective concentration (EC50) (Cu or Zn concentration which reduced the fungal biomass by 50%) was determined for each isolate whenever possible.
Plant growth and Cu treatments.
Two S. luteus isolates (UH-Slu-Lsk10 and UH-Slu-Fg1.6) that had similar growth rates on basic medium but contrasting Cu sensitivities were selected for the plant experiment. Surface-sterilized seeds of P. sylvestris (provenance, Groenendaal B) were sown in a perlite-vermiculite mixture moistened with Ingestad nutrient solution for pine (13). A sandwich technique was used to inoculate 6-week-old seedlings with vigorously growing mycelia of either the Cu-tolerant or Cu-sensitive isolate of S. luteus (8). The mycorrhizal fungi were grown in 10-cm-diameter plastic petri dishes on modified Fries medium (see above) covered with sterile cellophane sheets. Once the mycelia had covered most of the cellophane surface, each cellophane sheet was removed from the agar and put in a new petri dish on thick filter paper that was soaked in Ingestad solution supplemented with glucose (28 mM). Subsequently, the root systems of two selected seedlings were spread over the young mycelia, and again a thick filter paper that was soaked in Ingestad solution was used to cover the roots and mycelia. For the noninoculated control plants included in the experiment we used the same procedure but did not add the fungal inoculum. After 3 days, plants were transferred to 140-ml containers filled with pure perlite. Perlite has a low nutrient-buffering capacity, which ensured that the plants were growing in a semihydroponic environment. The plants were watered three times a week with a balanced Ingestad nutrient solution (13). The nutrient solution contained 56 μM K2SO4, 77 μM KNO3, 38 μM KH2PO4, 35 μM K2HPO4 · 4H2O, 586 μM NH4NO3, 29 μM Ca(NO3)2 · 4H2O, 50 μM Mg(NO3)2 · 6H2O, 4 μM H3BO3, 1.6 μM Mn(NO3)2 · 4H2O, 2.4 μM FeCl3 · 2H2O, 0.1 μM Zn(NO3)2 · 4H2O, 0.1 μM CuCl2 · 2H2O, and 0.02 μM Na2MoO4 · 2H2O. Phosphorus was the growth limiting element, and the pH was adjusted to 4.5.
Cu treatments were started 5 weeks after inoculation. At this time, five plants from each inoculation treatment were harvested to determine the initial nutrient status and biomass of plants and fungi. Inoculated and noninoculated plants were divided at random into four Cu treatments to create a factorial setup with the factors Cu treatment (0.1, 15, 30, and 60 μM Cu2+) and fungal inoculation. This Cu gradient was selected because it results in severe disturbance of tree root functioning in (semi-)hydroponic conditions (26, 28). For each combined treatment there were five replicates. Extra Cu was added to the nutrient solution as CuSO4, · 5H2O. The pH and the Cu concentrations in the substrate solutions were restored every 2 weeks by flushing the plant containers with excess nutrient solutions (200 ml) containing the appropriate Cu concentrations. The experiment was performed in a growth chamber with 300 μmol m−2 s−1 of photosynthetic active radiation, a relative air humidity of 70%, and a day-night cycle consisting of 18 h of light at 22°C and 6 h of darkness at15°C.
Pre- and postharvest analyses.
Four and ten weeks after the Cu treatments were started, short-term net uptake of inorganic phosphate (Pi) and ammonium (NH4+) was analyzed with intact seedlings using a nondestructive method (8). Depletion of the nutrients was determined simultaneously with a nutrient solution that was supplemented with the appropriate Cu concentrations and was circulated through the plant containers for 5 h. Controls without plants were run in parallel. At regular times the Pi and NH4+ concentrations in the circulating solution were assessed colorimetrically with a flow injection analyzer (Lachat; QuickChemMethod 10-115-01-1-A, 10-107-06-1-C). From the depletion curves, net rates of uptake of Pi and NH4+ were calculated for each individual plant at external concentrations of 30 μM Pi and 300 μM NH4+. The uptake rate was calculated from the tangent lines touching the depletion curve. For plants that were harvested, specific uptake rates were expressed per unit of root dry weight.
The day after the last nutrient uptake measurement (i.e., 10 weeks after the Cu treatments were started), the plants were harvested. Shoots were oven dried (70°C, 120 h), and roots and perlite (containing external mycelia) were freeze-dried. Plant material and perlite were milled with a ball mill to a fine powder for analyses of elements and ergosterol. For element analyses the powdered mycelium was wet digested in Pyrex tubes in a heating block by using two cycles with 1 ml HNO3 (65%), followed by one cycle with 1 ml HCl (37%) at 120°C for about 5 h. Samples were eventually dissolved in HCl and diluted to obtain a final volume of 5 ml (2% HCl). Most analyses were performed in duplicate, and certified reference material was included as an external standard for element analyses; this material included Virginia tobacco leaves (CTA-VTL-2; Institute of Nuclear Chemistry and Technology, Warszawa, Poland) and spinach leaves (standard reference material 1570a; National Institute of Standards and Technology, Gaithersburg, Md.). Phosphate was analyzed colorimetrically, and Cu, Zn, Mg, and Fe concentrations were measured by atomic absorption spectroscopy.
Ergosterol was extracted and analyzed by high-performance liquid chromatography as described by Nylund and Wallander (20). The ergosterol assay is a sensitive method for determining active fungal biomass in mixed plant-fungus tissues exposed to elevated Cu levels (24, 28). The ergosterol data were converted to fungal biomass by using a conversion factor of 4.2 mg ergosterol g (dry weight) mycelium−1. This conversion factor was calculated from ergosterol levels determined for the freeze-dried fungal mats used in the in vitro Cu screening test. The ergosterol content in the Cu-treated mycelia was not significantly affected by the Cu treatments.
All data from the plant experiment were analyzed by nonparametric statistics using the Kruskal-Wallis test, followed by a multiple-comparison procedure tested for α = 0.05.
RESULTS
Metal contamination.
The metal loads of the soil and pore water are shown in Table 1. High levels of heterogeneity were observed for the metal concentrations and pH in the pore water retained in the mine substrate. Soils from the Zn-contaminated and uncontaminated sites in Belgium were less variable and on average had a higher pH.
TABLE 1.
Zn and Cu concentrations in soil samples collected at the study sitesa
| Site | Total
|
Pore water
|
pH | ||
|---|---|---|---|---|---|
| Cu concn (mg kg−1) | Zn concn (mg kg−1) | Cu concn (μM) | Zn concn (μM) | ||
| Folldal | 1,692 ± 446b | 273 ± 52 | 226 ± 198 | 18 ± 16 | 3.5 ± 1.0 |
| Lommel | 54 ± 11 | 1,529 ± 297 | <1 | 120 ± 44 | 4.7 ± 1.0 |
| Paal | 11 ± 4 | 26 ± 4 | <1 | 1.8 ± 0.4 | 4.1 ± 0.2 |
Total contents (aqua regia extraction) and concentrations in soil pore water, as well as pH values, were determined.
The values are means ± standard errors of the means (n = 3).
In vitro fungal growth.
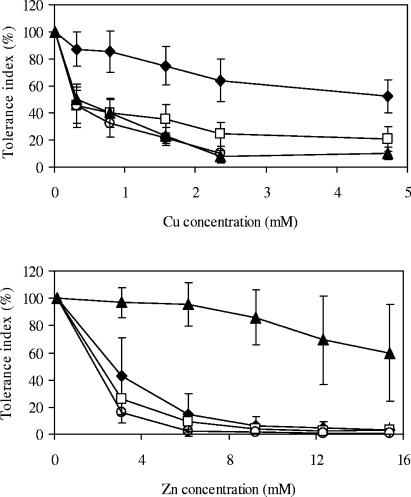
The average tolerance indices at the different Cu levels for each S. luteus population are shown in Fig. 1, upper panel. The in vitro EC50s of the Belgian isolates and the isolates from the uncontaminated sites in Norway were reached at 0.3 mM Cu, whereas the average EC50 for the Cu mine spoil isolates was more than 4.8 mM Cu. On Zn-supplemented media, only the S. luteus population from the Zn-contaminated area in Belgium showed a high degree of Zn tolerance (Fig. 1, lower panel). An average EC50 of 19.5 mM Zn was determined for these isolates (7). The average EC50s of Zn were 2.8 mM for the Norwegian isolates from the Cu mine spoil and 2 mM for the Norwegian isolates from the uncontaminated sites.
FIG. 1.
Copper (upper panel) and zinc (lower panel) tolerance indices for S. luteus isolates. Symbols: ⧫, Cu mine spoil isolate from Norway; □, isolate from unpolluted site in Norway; ▴, isolate from Zn-contaminated soil in Belgium; ○, isolate from unpolluted site in Belgium. The error bars indicate standard errors of the means (n = 7).
Two S. luteus isolates were selected for the plant experiment. The EC50 of Cu for the Cu-sensitive S. luteus isolate (UH-Slu-Lsk10) was 0.25 mM, whereas the in vitro EC50 of Cu for the Cu-tolerant isolate (UH-Slu-Fg1.6) from the Folldal copper mine spoil was 4.6 mM. The EC50 of Zn was 2.2 mM for isolate UH-Slu-Fg1.6 but was more than 15 mM for the UH-Slu-Lsk10 isolate. Hence, the latter isolate originating from Lommel was highly Zn tolerant.
Plant growth.
Copper toxicity gradually resulted in a severe reddish discoloration of needles in NM plants. This symptom was less pronounced in pines colonized by UH-Slu-Lsk10 and was not observed in pines colonized by UH-Slu-Fg1.6 (Fig. 2). In the absence of an elevated Cu concentration, there were no significant differences in shoot biomass between the inoculation treatments (Table 2), whereas the root biomass of NM plants was significantly higher than that of mycorrhizal plants. With increasing Cu concentrations, a significant decrease in aboveground biomass was observed in NM plants and in plants colonized by UH-Slu-Lsk10. Root growth was affected by Cu toxicity more than shoot growth (Table 2); in all inoculation treatments the root biomass was significantly reduced by 30 μM Cu. The toxic effect of Cu on root growth was greater in NM plants. This was also illustrated by the significant increase in the shoot/root ratio in NM plants with 30 and 60 μM Cu, whereas there was no such effect on the shoot/root ratio of mycorrhizal plants. Compared to the control Cu treatment, the total biomass of the NM plants was reduced by 76% with 60 μM Cu, the biomass of pines colonized by UH-Slu-Lsk10 was reduced by 41%, and the biomass of seedlings colonized by UH-Slu-Fg1.6 was reduced by only 23%.
FIG. 2.
Mycorrhizal and nonmycorrhizal pine seedlings treated with elevated copper concentrations for 10 weeks. (A) Normal conditions (0.1 μM Cu). (B) Exposure to sublethal Cu concentration (60 μM Cu). T, Cu-tolerant isolate of S. luteus; NT, Cu-sensitive isolate of S. luteus; NM, nonmycorrhizal.
TABLE 2.
Plant and fungal biomasses of mycorrhizal and nonmycorrhizal P. sylvestris seedlings treated with elevated Cu concentrations for 10 weeksa
| Fungal treatment | Copper treatment (μM) | Shoot wt (mg) | Root wt (mg) | Wt of mycelium in roots (mg) | Wt of extraradical mycelium (mg) |
|---|---|---|---|---|---|
| Cu-tolerant S. luteus UH-Slu-Fg1.6 | 0.1 | 165 ± 12 A | 286 ± 13 A | 78 ± 2 BC | 81 ± 5 A |
| 15 | 143 ± 9 A | 265 ± 10 A | 90 ± 3 A | 83 ± 6 A | |
| 30 | 156 ± 8 A | 219 ± 13 B | 82 ± 6 AB | 109 ± 13 A | |
| 60 | 149 ± 15 A | 199 ± 17 B | 64 ± 6 C | 82 ± 11 A | |
| Cu-sensitive S. luteus UH-Slu-Lsk10 | 0.1 | 179 ± 9 A | 272 ± 20 A | 160 ± 11 A | 54 ± 8 A |
| 15 | 136 ± 8 B | 256 ± 7 A | 97 ± 7 A | 22 ± 8 AB | |
| 30 | 128 ± 3 B | 217 ± 12 B | 80 ± 5 B | 8 ± 1 BC | |
| 60 | 119 ± 11 B | 185 ± 12 B | 58 ± 6 C | 5 ± 1 C | |
| Nonmycorrhizal | 0.1 | 199 ± 27 A | 799 ± 50 A | ||
| 15 | 152 ± 24 AB | 366 ± 41 A | |||
| 30 | 171 ± 15 A | 191 ± 27 B | |||
| 60 | 127 ± 10 B | 116 ± 18 C |
The values are means ± standard errors of the means (n = 5). Values followed by the same letter within an inoculation treatment are not significantly different (as determined by the Kruskal-Wallis test followed by a multiple-comparison procedure tested for α = 0.05).
Fungal growth in symbiosis.
At the start of the Cu treatments, 5 weeks after inoculation, at least 50% of the short roots were mycorrhizal in the inoculated plants. Noninoculated plants remained nonmycorrhizal throughout the experiment. Excess Cu caused minor changes in the active fungal biomass in roots colonized by UH-Slu-Fg1.6 (Table 2). However, the UH-Slu-Lsk10 isolate clearly suffered more from the Cu stress. The toxic effect of Cu was most obvious in the amount of active extraradical mycelium produced by this sensitive isolate. At 30 μM Cu there was a significant decrease, and at the highest Cu concentration the UH-Slu-Lsk10 isolate almost failed to form active extraradical mycelium. In contrast, the development of extraradical hyphae by UH-Slu-Fg1.6 was not affected by the Cu treatments (Table 2).
Nutrient uptake capacities.
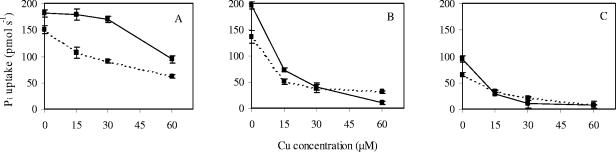
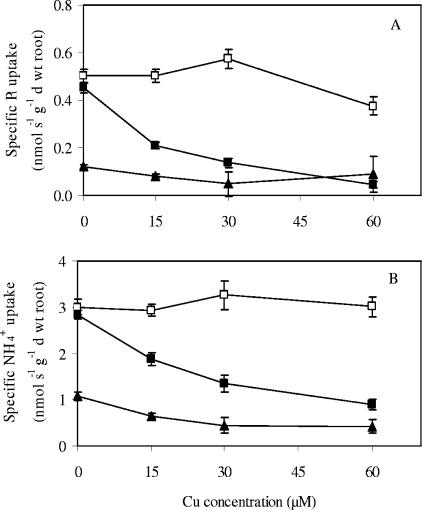
After 4 and 10 weeks of Cu treatment, Pi and NH4+ uptake rates were calculated from the depletion of Pi and NH4+ in the nutrient solution circulated through the plant containers for 5 h. Pi uptake rates are shown in Fig. 3. In the absence of an elevated Cu concentration, there was a significant increase in Pi uptake potential between weeks 4 and 10 as a result of the expanding (mycorrhizal) root system. The Cu treatments had a striking differential impact on the nutrient uptake of the plants. The uptake of Pi was maintained much better in seedlings associated with the UH-Slu-Fg1.6 isolate (Fig. 3A). In contrast, relatively low Cu concentrations (15 μM) disturbed Pi uptake in roots colonized by the UH-Slu-Lsk10 isolate (Fig. 3B). In any case, ECM seedlings took up Pi more efficiently than NM seedlings (Fig. 3C). There was no significant difference between the sensitive plant-fungus association and NM plants only at the highest Cu concentration tested (60 μM). Pi uptake in NM plants and plants inoculated with UH-Slu-Lsk10 decreased to almost zero by the end of the experiment, illustrating the ultimate failure of the nutrient uptake system (Fig. 3B and 3C). Similar data were obtained for NH4+ uptake (data not shown). Taking into account root biomass, specific uptake rates for Pi and NH4+ could be calculated when plants were harvested after 10 weeks of Cu treatment (Fig. 4). The data obtained largely confirmed the different nutrient uptake capacities and thus Cu sensitivities of the S. luteus isolates.
FIG. 3.
Pi uptake rates at different Cu levels for pine seedlings that either were inoculated with a Cu-tolerant isolate (A) or a Cu-sensitive isolate (B) of S. luteus or were nonmycorrhizal (C). The Pi uptake measurements were obtained 4 weeks (dotted lines) and 10 weeks (solid lines) after Cu addition was initiated. The error bars indicate standard errors of the means (n = 5).
FIG. 4.
Specific Pi (A) and NH4+ (B) uptake rates at different Cu levels for pine seedlings that either were inoculated with a Cu-tolerant isolate (□) or a Cu-sensitive isolate (▪) of S. luteus or were nonmycorrhizal (▴). The error bars indicate standard errors of the means (n = 5).
Cu accumulation in the plants.
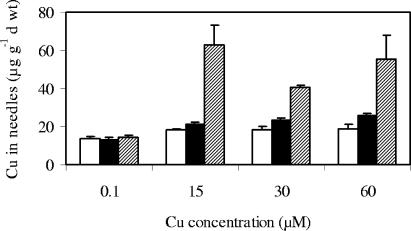
Exposure to elevated Cu concentrations significantly increased the Cu accumulation in the aboveground parts of the plants (Fig. 5). Pine seedlings colonized with UH-Slu-Fg1.6 seemed to control Cu influx better than NM plants and plants colonized with UH-Slu-Lsk10, resulting in transfer of less Cu to the pine needles. For all treatments, the Cu concentrations in roots were considerably higher than the Cu concentrations measured in shoots. In plants exposed to 60 μM Cu, the percentages of Cu translocated to the shoots were 0.8, 0.7, and 1.2% of the total Cu content for the mycorrhizal plants with the tolerant and sensitive isolates and the NM plants, respectively.
FIG. 5.
Cu concentrations in needles of pine seedlings treated with elevated Cu concentrations for 10 weeks. Open bars, plants inoculated with a Cu-tolerant S. luteus isolate; solid bars, plants inoculated with a Cu-sensitive S. luteus isolate; cross-hatched bars, nonmycorrhizal plants. The error bars indicate standard errors of the means (n = 5).
DISCUSSION
The pH and Cu concentration in the pore water of the Folldal mine spoil confirmed that this substrate is highly acidic and strongly contaminated with Cu (Table 1). The high acidity was probably due to oxidation of metal sulfides. Despite the low pH and high Cu toxicity, trees, shrubs, and grasses sparsely colonize the mine spoil (29). Especially Cu-resistant ecotypes of soil microorganisms and plants colonize such habitats, but these extreme conditions also impose great selection pressure that may trigger adaptive Cu tolerance. Relatively few plants seem to be able to evolve Cu-tolerant ecotypes (16, 22, 31). In tree species such genetic modifications are even less likely because of the long generation times (23, 30). Nevertheless, trees do colonize severely metal-contaminated sites. In the present study we demonstrated that the S. luteus population of the Folldal mine spoil has evolved adaptive Cu tolerance (Fig. 1, upper panel) that results in highly significant protection of pine seedlings against Cu toxicity. It is therefore likely that pine trees colonizing the Cu mine spoil are strongly assisted by their ECM associations with Cu-tolerant fungi inhabiting this toxic soil. S. luteus is a pioneer ECM bolete that specifically colonizes roots of Pinus spp. Zn-tolerant populations of this fungus have been discovered previously in Zn-contaminated soils (7).
In our in vitro screening experiment, all seven S. luteus isolates collected from the mine spoil exhibited high Cu tolerance (Fig. 1, upper panel), whereas Cu tolerance was not detected in populations from nonpolluted and Zn-polluted soils. However, the possibility that Cu tolerance was present at a low frequency in nonexposed populations could not be excluded. In previous reports, considerable inter- and intraspecific variation in response to excess Cu was observed in ECM fungi, but adaptive Cu tolerance was never discovered (5, 9, 14). It should be clear that the EC50s obtained in the in vitro study cannot be directly extrapolated to the natural soil environment. We assume that the rich nutrient composition of most in vitro media used to cultivate and test microorganisms, including ECM fungi, is a major factor that determines the upward shift of the toxicity range. The isolates grow much faster in vitro than in symbiosis (7).
The tests on Zn-supplemented media showed that the Cu mine isolates do not possess increased Zn tolerance (Fig. 1, lower panel). Similarly, Zn-tolerant isolates originating from Zn-contaminated sites do not exhibit any Cu tolerance (Fig. 1, upper panel), indicating that there was evolution of metal-specific tolerance mechanisms and that strong cotolerance was absent. The absence of cotolerance for Cu and Zn is supported by studies on the genetic basis of metal tolerance in bacteria and higher plants, which have shown that Cu tolerance and Zn tolerance are under the control of different genes (18, 22). The in vitro metal screening test allowed us to select two S. luteus isolates with contrasting Cu tolerance characteristics, and these isolates were subsequently used to inoculate pine seedlings. The copper sensitivity of myco- and phytobionts was determined in a dose-response experiment by analyzing several responses, including growth of the symbiotic partners, Cu accumulation, and the nutrient uptake potential of the seedlings. NM pines are very sensitive to elevated Cu concentrations, which result in fast inhibition of root growth (Table 2) and reduced nutrient uptake (Fig. 3C and 4), as well as a greater influx of Cu (Fig. 5). The average shoot concentration of Cu was 55 μg g (dry weight)−1 at the highest Cu concentration applied, a value that greatly exceeds the critical level for Cu toxicity in pine needles, which is estimated to be 20 μg g (dry weight)−1 (4). Nevertheless, most Cu was retained in the nonmycorrhizal root system. The strong belowground Cu retention and the very poor growth of nonmycorrhizal roots led us to believe that the root cells that are in direct contact with the Cu solution are the first target of excess Cu. Severe membrane damage in these cells results in an immediate disturbance in nutrient uptake. At the highest Cu concentration, the Cu/P ratio in NM plants (1.4) was considerably higher than the ratios in mycorrhizal plants (0.4 for the UH-Slu-Fg1.6 isolate and 0.7 for the UH-Slu-Lsk10 isolate), indicating that the fungi are able to maintain a more favorable nutritional status for their pine hosts. In a previous study we found evidence that the ECM fungi S. bovinus and Thelephora terrestris could protect pines from Cu stress (28). In both studies, the ameliorating effect of the ECM fungi on host fitness could be ascribed to better plant nutrition and reduced uptake of Cu. Ectomycorrhizal fungi largely control the pathway of nutrients, whether in excess or at trace levels, from the soil to the plant. The fungal sheath may act as a barrier, either through its ability to transform soluble heavy metal forms into insoluble forms (17) or by preventing apoplastic radial transport of water and ions to the root surface and thus reducing metal influx (3, 10). A biofiltering pattern has been observed in S. luteus-P. sylvestris mycorrhizas by the use of elemental imaging techniques (25).
However, there is considerable variation in the extent of the fungal protective effect among the ECM fungi. In the present study, seedlings colonized by UH-Slu-Lsk10 were affected in nutrient uptake capacity more quickly than plants colonized by UH-Slu-Fg1.6 (Fig. 3 and 4), and this effect was apparent even before there was clear growth inhibition of UH-Slu-Lsk10. At the highest Cu concentrations development of the external mycelium in particular was very poor and was in sharp contrast to the good growth of UH-Slu-Fg1.6 (Table 2).
We concluded that the better growth and nutrient status of the plants associated with the tolerant fungal isolate strongly suggest that metal-adapted isolates of ECM fungi provide excellent insurance against Cu toxicity in pine seedlings exposed to elevated Cu concentrations. The existence of tolerance to different metals in S. luteus provides opportunities to breed isolates with combined metal tolerances. Such a heavy-metal-adapted Suillus-Pinus combination might be most suited for large-scale land reclamation at metalliferous and industrial sites that have little plant cover due to their phytotoxicity. Phytostabilization of vast areas of metal-contaminated soils or wastes that resulted from previous mining and smelting activities is urgently needed to reduce further dispersion of heavy metals. Phytostabilization, combined with in situ immobilization of heavy metals, is a low-cost option that can deal with this problem (27). Reforestation would be especially attractive for industrial and mining sites since forests reduce erosion, restrict the dispersal of metals, and are sinks for atmospheric carbon dioxide.
Acknowledgments
We thank S. Poorters, C. Put, A. Wijgaerts, and F. Van Belleghem for experimental and technical assistance.
This research was supported by FWO-Vlaanderen (grant G0001.01), by the EU (MYCOREM grant QLK3-1999-00097), by a postdoctoral grant to T.V. from the Norwegian Research Council (grant NFR-145324/V40), and by a grant from the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen) to K.A.
REFERENCES
- 1.Adriaensen, K., D. van der Lelie, A. Van Laere, J. Vangronsveld, and J. V. Colpaert. 2004. A zinc-adapted fungus protects pines from zinc stress. New Phytol. 161:549-555. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hiyaly, S. A. K., T. McNeilly, and A. D. Bradshaw. 1990. The effect of zinc contamination from electricity pylons—contrasting patterns of evolution in 5 grass species. New Phytol. 114:183-190. [Google Scholar]
- 3.Ashford, A. E., W. G. Allaway, C. A. Peterson, and J. W. G. Cairney. 1989. Nutrient transfer and the fungus root interface. Aust. J. Plant Physiol. 16:85-97. [Google Scholar]
- 4.Balsberg-Påhlsson, A. M. 1989. Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants—a literature review. Water Air Soil Pollut. 47:287-319. [Google Scholar]
- 5.Blaudez, D., C. Jacob, K. Turnau, J. V. Colpaert, U. Ahonen-Jonnarth, R. Finlay, B. Botton, and M. Chalot. 2000. Differential responses of ectomycorrhizal fungi to heavy metals in vitro. Mycol. Res. 104:1366-1371. [Google Scholar]
- 6.Bradshaw, A. D., and T. McNeilly. 1981. Evolution and pollution. Stud. Biol. 130:1-76. [Google Scholar]
- 7.Colpaert, J. V., L. A. H. Muller, M. Lambaerts, K. Adriaensen, and J. Vangronsveld. 2004. Evolutionary adaptation to zinc toxicity in populations of suilloid fungi. New Phytol. 162:549-559. [Google Scholar]
- 8.Colpaert, J. V., K. K. Van Tichelen, J. A. Van Assche, and A. Van Laere. 1999. Short-term phosphorus uptake rates in mycorrhizal and non-mycorrhizal roots of intact Pinus sylvestris seedlings. New Phytol. 143:589-597. [DOI] [PubMed] [Google Scholar]
- 9.Colpaert, J. V., P. Vandenkoornhuyse, K. Adriaensen, and J. Vangronsveld. 2000. Genetic variation and heavy metal tolerance in the ectomycorrhizal basidiomycete Suillus luteus. New Phytol. 147:367-379. [Google Scholar]
- 10.Egerton-Warburton, L., and B. Griffin. 1995. Differential responses of Pisolithus tinctorius isolates to aluminium in vitro. Can. J. Bot. 73:1229-1233. [Google Scholar]
- 11.Fries, N. 1978. Basidiospore germination in some mycorrhiza-forming Hymenomycetes. Trans. Br. Mycol. Soc. 70:319-324. [Google Scholar]
- 12.Hall, J. L. 2002. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 53:1-11. [PubMed] [Google Scholar]
- 13.Ingestad, T., and M. Kähr. 1985. Nutrition and growth of coniferous seedlings at varied relative nitrogen addition rate. Physiol. Plant. 65:109-116. [Google Scholar]
- 14.Jones, M. D., and T. C. Hutchinson. 1988. The effects of nickel and copper on the axenic growth of ectomycorrhizal fungi. Can. J. Bot. 66:119-124. [Google Scholar]
- 15.Knight, B. P., A. M. Chaudri, S. P. McGrath, and K. E. Giller. 1998. Determination of chemical availability of cadmium and zinc in soils using inert soil moisture samplers. Environ. Pollut. 99:293-298. [DOI] [PubMed] [Google Scholar]
- 16.Lepp, N. W., J. Hartley, M. Toti, and N. M. Dickinson. 1997. Patterns of soil copper contamination and temporal changes in vegetation in the vicinity of a copper rod rolling factory. Environ. Pollut. 95:363-369. [DOI] [PubMed] [Google Scholar]
- 17.Leyval, C., K. Turnau, and K. Haselwandter. 1997. Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological and applied aspects. Mycorrhiza 7:139-153. [Google Scholar]
- 18.Mergeay, M., S. Monchy, T. Vallaeys, V. Auquier, A. Benotmane, P. Bertin, S. Taghavi, J. Dunn, D. van der Lelie, and R. Wattiez. 2003. Ralstonia metallidurans, a bacterium specifically adapted to toxic metals: towards a catalogue of metal-responsive genes. FEMS Microbiol. Rev. 27:385-410. [DOI] [PubMed] [Google Scholar]
- 19.Muller, L. A. H., M. Lambaerts, J. Vangronsveld, and J. V. Colpaert. 2004. AFLP-based assessment of the effects of environmental heavy metal pollution on the genetic structure of pioneer populations of Suillus luteus. New Phytol. 164:297-303. [DOI] [PubMed] [Google Scholar]
- 20.Nylund, J. E., and H. Wallander. 1992. Ergosterol analysis as a means of quantifying mycorrhizal biomass. Methods Microbiol. 24:77-88. [Google Scholar]
- 21.Rajapaksha, R. M. C. P., M. A. Tobor-Kaplon, and E. Bååth. 2004. Metal toxicity affects fungal and bacterial activities in soil differently. Appl. Environ. Microbiol. 70:2966-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schat, H., and R. Vooijs. 1997. Multiple tolerance and co-tolerance to heavy metals in Silene vulgaris: a co-segregation analysis. New Phytol. 136:489-496. [DOI] [PubMed] [Google Scholar]
- 23.Schützendübel, A., and A. Polle. 2002. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 53:1351-1365. [PubMed] [Google Scholar]
- 24.Tarhanen, S., S. Metsärinne, T. Holopainen, and J. Oksanen. 1999. Membrane permeability response of lichen Bryoria fuscescens to wet deposited heavy metals and acid rain. Environ. Pollut. 104:121-129. [Google Scholar]
- 25.Turnau, K., W. J. Przybylowicz, and J. Mesjasz-Przybyowicz. 2001. Heavy metal distribution in Suillus luteus mycorrhizas—as revealed by micro-PIXE analysis. Nucl. Instrum. Methods Phys. Res. Sect. B. 181:649-658. [Google Scholar]
- 26.Utriainen, M. A., L. V. Kärenlampi, S. O. Kärenlampi, and H. Schat. 1997. Differential tolerance to copper and zinc of micropropagated birches tested in hydroponics. New Phytol. 137:543-549. [DOI] [PubMed] [Google Scholar]
- 27.van der Lelie, D., J. P. Schwitzguébel, D. J. Glass, J. Vangronsveld, and A. Baker. 2001. Assessing phytoremediation's progress in the United States and Europe. Environ. Sci. Technol. 35:446a-452a. [DOI] [PubMed] [Google Scholar]
- 28.Van Tichelen, K. K., J. V. Colpaert, and J. Vangronsveld. 2001. Ectomycorrhizal protection of Pinus sylvestris against copper toxicity. New Phytol. 150:203-213. [Google Scholar]
- 29.Vrålstad, T., E. Myhre, and T. Schumacher. 2002. Molecular diversity and phylogenetic affinities of symbiotic root-associated ascomycetes of the Helotiales in burnt and metal polluted habitats. New Phytol. 155:131-148. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson, D. M., and N. M. Dickinson. 1995. Metal resistance in trees—the role of mycorrhizae. Oikos 72:298-300. [Google Scholar]
- 31.Wu, L., and S. L. Lin. 1990. Copper tolerance and copper uptake of Lotus purshianus (Benth.) Clem. & Clem. and its symbiotic Rhizobium loti derived from a copper mine waste population. New Phytol. 116:531-539. [DOI] [PubMed] [Google Scholar]