Abstract
The strain Pseudomonas putida DOT-T1E was tested for its ability to tolerate second phases of different alkanols for their use as solvents in two-liquid-phase biotransformations. Although 1-decanol showed an about 10-fold higher toxicity to the cells than 1-octanol, the cells were able to adapt completely to 1-decanol only and could not be adapted in order to grow stably in the presence of a second phase of 1-octanol. The main explanation for this observation can be seen in the higher water and membrane solubility of 1-octanol. The hydrophobicity (log P) of a substance correlates with a certain partitioning of that compound into the membrane. Combining the log P value with the water solubility, the maximum membrane concentration of a compound can be calculated. With this simple calculation, it is possible to predict the property of an organic chemical for its potential applicability as a solvent for two-liquid-phase biotransformations with solvent-tolerant P. putida strains. Only compounds that show a maximum membrane concentration of less than 400 mM, such as 1-decanol, seem to be tolerated by these bacterial strains when applied in supersaturating concentrations to the medium. Taking into consideration that a solvent for a two-liquid-phase system should possess partitioning properties for potential substrates and products of a fine chemical synthesis, it can be seen that 1-decanol is a suitable solvent for such biotransformation processes. This was also demonstrated in shake cultures, where increasing amounts of a second phase of 1-decanol led to bacteria tolerating higher concentrations of the model substrate 3-nitrotoluene. Transferring this example to a 5-liter-scale bioreactor with 10% (vol/vol) 1-decanol, the amount of 3-nitrotoluene tolerated by the cells is up to 200-fold higher than in pure aqueous medium. The system demonstrates the usefulness of two-phase biotransformations utilizing solvent-tolerant bacteria.
Biocatalysis using whole cells promised to play an important role in the industrial synthesis of fine chemicals, pharmaceuticals, and precursors for chemical syntheses. However, the number of successful processes using whole-cell biotransformations is very small so far because several factors limit the number of applications (29, 30). One important limitation of a successful application of whole-cell biotechnological processes toward classical chemical synthesis is that many reactions of interest involve substrates or products that are extremely toxic for the bacteria (35). This problem can be solved by the application of an organic solvent phase that functions as a source and a sink for toxic organic substrates and products, respectively (36). For biotransformations with whole cells, the major advantage of an addition of a second organic phase lies within its ability to act as a sink for the substrates as well as in the continuous removal of products (19, 21, 36). In both cases, the presence of a solvent phase keeps the concentrations of substrates and toxins at a level that does not lead to toxic effects on the cells, which would decrease the activity of the biocatalyst. Additionally, the higher solubility of the product in the organic phase allows much higher product yield and volume productivities. That will lead to a massive reduction of the costs of downstream processing and product recovery (3, 21).
To fulfill the needs for a certain process, the choice of an applicable organic solvent for a two-liquid-phase system depends on the substrates, the products, and the biocatalyst. The ideal solvent should be completely tolerated by the microorganism carrying out the biotransformation and should show high partitioning properties for the chosen substrates and resulting products. To allow an economically sound biotransformation process, both the substrates and the products should preferentially dissolve into the organic solvent phase. Each biotransformation process requires a series of experiments to test the behavior of the biocatalyst in the system.
Organic solvents with a log P (the logarithm of the partition coefficient of a solvent in a two-phase water-octanol standard system) between 1.5 and 4 are extremely toxic for most microorganisms (34). Therefore, the ideal solvents for two-liquid-phase fermentations were not applicable because they themselves were toxic to the biocatalysts. In 1989, Inoue and Horikoshi discovered a bacterial strain that was able to thrive in the presence of the toxic organic solvent toluene (15). The isolation and identification of several solvent-tolerant bacteria able to grow in the presence of organic solvents with toxic log P values like toluene (2.5) followed (13). Those discoveries were scientific breakthroughs that may help in overcoming limitations in industrial biotransformations and in expanding the applications of biocatalysts. The usage of solvent-tolerant bacteria as whole-cell biocatalysts in two-phase systems now allows the application of solvents with chemical properties needed for a successful implementation of whole-cell two-phase biotransformations.
One of the highly solvent-tolerant bacteria isolated is Pseudomonas putida DOT-T1E. This bacterium has been the subject of many investigations concerning the mechanisms responsible for these solvent tolerance properties (24, 31). The bacterium is well described regarding its degradation properties. Molecular biological tools for its genetic transformation have also been developed (23, 26). Thus, P. putida DOT-T1E represents an ideal biocatalyst for biotransformations.
The aim of this study was to investigate the tolerance and growth of P. putida DOT-T1E in a two-liquid-phase system of aliphatic alcohols such as 1-octanol (log P = 2.9) or 1-decanol (log P = 4). These solvents were chosen because they are not harmful to humans like toluene and are thus better applicable for industrial processes. Additionally, they both are suitable as the second phase in a two-liquid-phase system in order to realize the extraction of fine chemicals of interest. This process is an example for the use of desirable agents in biotechnological productions of fine chemicals in a bioreactor with two liquid phases.
MATERIALS AND METHODS
Strains and chemicals.
Pseudomonas putida DOT-T1E has previously been described (25). All chemicals were reagent grade and obtained from commercial sources.
Culture conditions.
Pseudomonas putida DOT-T1E was cultivated in a mineral medium as described by Hartmans et al. (7), with Na2-succinate as the carbon and energy source. Adaptation of the cells to solvents was achieved by growing the cells semicontinuously in batch cultures with increasing concentrations of the solvents up to concentrations above saturation. Cells were grown in 50-ml shake cultures at 30°C in a horizontally shaking water bath. Growth was monitored by measuring the turbidity (optical density) at 560 nm (OD560). A 1-ml inoculum from an overnight culture was transferred to 50 ml fresh medium, and cells were grown exponentially for 3 to 4 h (until an OD560 of 0.6 was reached).
Incubation with toxins.
For the measurements of the toxic effects of the investigated compounds, toxins were added to exponentially growing, nonadapted cultures as described by Heipieper et al. (10). Cultures were incubated in the presence of the toxins for 2 h in a shaking water bath at 30°C. Then the cells were harvested, washed two times with potassium phosphate buffer (50 mM, pH 7.0), and stored at −20°C prior to their use for the lipid extraction.
Lipid extraction and transesterification.
The lipids were extracted with chloroform-methanol-water as described by Bligh and Dyer (1). Fatty acid methyl esters (FAME) were prepared by incubation for 15 min at 95°C in boron trifluoride-methanol by applying the method of Morrison and Smith (22). FAME were extracted with hexane.
Analysis of fatty acid composition by GC-flame ionization detection.
Analysis of FAME in hexane was performed using a quadruple gas chromatography (GC) system (HP5890; Hewlett-Packard, Palo Alto, Calif.) equipped with a split/splitless injector. A CP-Sil 88 capillary column (length, 50 m; inner diameter, 0.25 mm; 0.25-μm-thick film; Chrompack, Middelburg, The Netherlands) was used for the separation of the FAME. GC conditions were as follows: injector temperature was held at 240°C, and detector temperature was held at 270°C. The injection was splitless, and carrier gas was He at a flow of 2 ml/min. The temperature program was 40°C, 2 min isothermal, 8°C/min up to 220°C, and 5 min isothermal at 220°C. The pressure program was 27.7 lb/in2 (equal to 186.15 kPa), 2 min isobaric; 0.82 lb/in2/min (equal to 5.65 kPa/min) to the final pressure 45.7 lb/in2; and 5.55 min isobaric at 45.7 lb/in2 (equal to 310.26 kPa). The peak areas of the carboxylic acids in total ion chromatograms (TIC) were used to determine their relative amounts. The fatty acids were identified by GC and coinjection of authentic reference compounds obtained from Supelco (Bellefonte). The mean data of three independent experiments are shown in Fig. 1. The standard deviation was less than 5%.
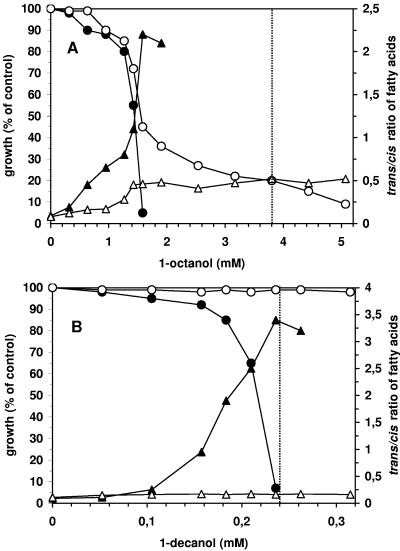
FIG. 1.
Effect of 1-octanol (A) and 1-decanol (B) on growth (•, ○) and trans/cis ratio of unsaturated membrane fatty acids (▴, ▵) of adapted (open symbols) and nonadapted (closed symbols) cells of P. putida DOT-T1E. The dashed lines mark the water solubility of the two solvents under the given conditions.
Preparation of resting cells.
Next, 50 ml of exponentially growing cells was harvested by centrifugation and suspended in the same volume of sodium phosphate buffer (50 mM, pH 7.0). Experiments were started 45 min after suspension of cells, by which time growth had stopped completely.
Cellular K+ content.
One-milliliter samples from cells suspended in sodium phosphate buffer were harvested at regular intervals before and after the addition of solutes. Separation of cells from the supernatant was carried out by rapid centrifugation (Eppendorf table centrifuge, 13,000 rpm, 5 min). The cell pellet was disrupted by boiling in 5% trichloroacetic acid, and debris was removed by centrifugation. The K+ of the supernatant was measured by inductively coupled plasma-optical emission spectroscopy (ICP-OES) using Spectroflame P/M (SPECTRO Analytical Instruments, Kleve, Germany). All experiments were carried out three times; the average data of these results are shown in Fig. 2. The standard deviation was less than 10%.
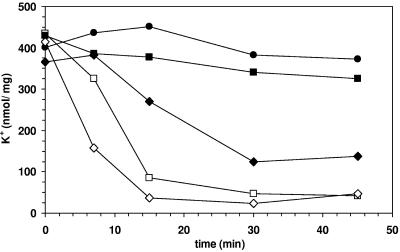
FIG. 2.
Effect of 1-octanol and 1-decanol on the intracellular K+ concentration of nonadapted resting cells of P. putida DOT-T1E. To allow a comparison of the effects of the alkanols on membrane permeabilization, both compounds were added in concentrations that caused a 50% (1-octanol, 0.0225 [□], and 1-decanol, 0.004 [▪] [vol/vol]) and 100% (1-octanol, 0.03 [⋄], and 1-decanol, 0.0045 [⧫]) (vol/vol) growth inhibition of P. putida DOT-T1E. •, control.
Analysis of 3-nitrotoluene.
The quantification of 3-nitrotoluene was done using high-performance liquid chromatography (HPLC). A C18 column (CC250/4 Nucleosil 100-5 C18 HD) from Macherey-Nagel (Düren, Germany) with the following attributes was used: pore size, 100 Å; particle size, 5 μm; inner diameter, 4 mm; length, 25 cm.
Fermentations.
The fermentations were carried out in a 5-liter fermentor (ISF-205; INFORS GmbH, Einsbach, Germany). Parameters such as temperature (°C), pH, CO2 output (%), O2 output (%), pO2 (%), airflow (liters/min), stirring (rpm), and total weight (kg) of the fermentor were monitored online. The used IRIS software (INFORS CONTROL AG, Bottmingen, Switzerland) allowed the control of the measured parameters. Data were obtained and recorded with the Servomex Analyzer Series 1400 (East Sussex, England). Standard parameters for fermentations were as follows: 30°C, pH 7.2, and 1,500 rpm. The fermentor was inoculated with an overnight culture in such a way that a starting OD560 of 0.08 was reached. Due to difficulties measuring the optical density in a dispersed two-phase system, we obtained growth data via measuring the protein content of the cells (2). In the computer-controlled fermentation unit, growth was continuously determined as CO2 production. For the fermentations carried out in this study, a direct correlation between CO2 production and protein content was observed.
RESULTS AND DISCUSSION
Adaptation of Pseudomonas putida DOT-T1E to the presence of 1-octanol and 1-decanol.
In a set of first experiments, the effect of the four chosen alkanols, 1-hexanol, 1-octanol, 1-decanol and 1-dodecanol, on the growth of Pseudomonas putida DOT-T1E was investigated. From all investigated compounds, 1-hexanol featured the lowest toxicity, with a MIC (concentration leading to a complete inhibition of cell growth) of 16 mM (data not shown). This concentration was comparable to previous data with other bacteria (17). In contrast, 1-dodecanol was not toxic to the cells at all (data not shown). As it was impossible to adapt cells to supersaturating concentrations of 1-hexanol and as the solvent properties of a very hydrophobic compound such as 1-dodecanol were not favorable for our purposes, further experiments were focused on 1-octanol and 1-decanol as solvents. For this reason, adapted and nonadapted cells of P. putida were grown in a mineral medium with Na2-succinate as the energy and carbon source. The solvents were added in different concentrations during the exponential growth phase. The organisms continued to grow exponentially but at reduced growth rates, allowing a quantification of growth inhibition by comparing the growth rates as described by Heipieper et al. (10). The results are summarized in Fig. 1. For nonadapted cells of P. putida DOT-T1E, 1-octanol showed an MIC of 2.2 mM. The MIC for 1-decanol was 0.23 mM and thus about 10-fold lower than that for 1-octanol. Despite this higher toxicity of 1-decanol, it was very easy to adapt the cells to a second phase of this solvent whereas this was not possible for 1-octanol.
Microorganisms are able to adapt to the presence of toxic organic compounds using a whole cascade of adaptive mechanisms (for a review, see references 24 and 31). One adaptive mechanism enabling several Vibrio and Pseudomonas strains to grow in the presence of membrane-disrupting compounds is the isomerization of cis- to trans-unsaturated fatty acids (6, 9, 11). This mechanism could also be found in Pseudomonas putida DOT-T1E (16). The extent of the isomerization, usually expressed as the trans/cis ratio of unsaturated fatty acids, apparently correlates with the toxicity of organic compounds (8, 12). Therefore, we used the trans/cis ratio of unsaturated fatty acids as an indicator for stress and stress adaptation. In nonadapted cells, both solvents caused a strong dose-dependent increase in the trans/cis ratio of unsaturated fatty acids (Fig. 1). The cis-trans isomerase is thought to be a kind of urgent response mechanism for the bacteria to adapt to stress (11), which is then substituted by other long-term adaptive mechanisms (24). When these adaptive steps have taken place, the trans/cis ratio is reduced to nearly zero (6). Therefore, low trans/cis ratios are an indicator for a complete adaptation of the bacteria to the environmental condition. Adapted cells showed a very low trans/cis ratio in the presence of 1-decanol, which proves that the cells were completely adjusted and thus no longer stressed by the solvent. Contrary to that, 1-octanol also caused a reaction of the adapted cells in the form of a slight increase in the trans/cis ratio of their unsaturated fatty acids. This indicated an incomplete adaptation to 1-octanol, which was confirmed by a reduced and unstable growth in the presence of a second phase of this solvent (data not shown).
Effect of 1-octanol and 1-decanol on the cell membranes.
Organic solvents are known to be toxic to cells, mainly due to their effect on the membranes as so-called “membrane-active compounds” (13, 34). One of the first indications of membrane damage in bacteria caused by organic solvents is the efflux of potassium ions (9, 20). Different concentrations of 1-octanol and 1-decanol were added to resting cells. For a better comparison of the effects of the alkanols on membrane permeabilization, both compounds were added in concentrations that had caused similar growth inhibition effects on nonadapted cells of P. putida DOT-T1E (Fig. 1). Figure 2 shows the time scale of cellular potassium concentrations of resting cells after the addition of both alkanols. It is obvious that 1-octanol caused a much more drastic permeability increase, seen as a loss of cellular K+ content, than those concentrations of 1-decanol that had similar effects on growth of the cells. Obviously, 1-decanol had a less dramatic effect on membrane fluidity than comparable concentrations of 1-octanol. Although 1-decanol shows a much higher dose-dependent toxicity, the cell membranes seem to be less affected by this compound. This is another explanation for the easier adaptability of the cells to 1-decanol compared to 1-octanol.
Solvents in membranes.
A relationship has been established between the toxicity of a solvent for an organism and the partitioning of that solvent to octanol from the water phase in a standard system (37). The logarithm of the octanol-water partition coefficient is designated log P. This parameter was used to be an indicator of the solvent's partitioning from the aqueous medium into the membranes of microorganisms (33). It is generally accepted that it is the solvent's effects on the cytoplasmic membrane, where it will preferentially accumulate, that result in the destruction of the organism (34, 37). As a consequence of the high solvent concentrations in this compartment, the cell is no longer able to perform essential biochemical reactions and eventually loses its integrity. Various analytical techniques (13, 33) can be employed to determine the dose-response relationship of a solvent in a membrane (4). The different techniques reveal similar patterns, supporting the view that the actual membrane concentration of a solvent is an important parameter.
The solvent's membrane concentration depends on the concentration of the solvent in the water phase, the partitioning of the solvent from the water phase into the membrane, and the ratio of the volumes of the two liquid phases. Thereby, a correlation has been found between the log P value of a solvent and its partitioning between membrane and buffer (33): logmembrane/buffer = 0.97 × logoctanol/water − 0.64.
Using this equation, it is possible to calculate the actual concentration of a solvent in a membrane if its concentration in the water phase is known (4, 33). We performed these calculations that had originally been done for toluene now with all even-numbered aliphatic alkanols. Odd-numbered aliphatic alcohols were not chosen because of their much higher price, which would not allow an economic application. The results also present the data of de Bont (4) for toluene as a reference compound and are summarized in Table 1.
TABLE 1.
Relation between partitioning of several organic compounds in octanol/water (log P), membrane/buffer, water solubility, and the maximum concentration in the membrane
| Organic compound | log Po/wa | log Pm/bb | Pm/bb | Solubilityc (mM) | MMCd (mM) | Adaptability |
|---|---|---|---|---|---|---|
| 1-Butanol | 0.89 | 0.21 | 1.6 | 970.0 | 1,586 | − |
| 1-Hexanol | 1.87 | 1.17 | 14.8 | 56.9 | 841 | − |
| Toluene | 2.48 | 1.76 | 57.5 | 6.3 | 368 | + |
| 1-Octanol | 2.92 | 2.19 | 154.9 | 3.8 | 588 | +/− |
| 1-Decanol | 3.97 | 3.21 | 1,621.8 | 0.23 | 379 | + |
| 1-Dodecanol | 5.02 | 4.23 | 16,982.4 | 0.015 | 254 | + |
| 1-Tetradecanol | 6.07 | 5.25 | 177,827.9 | 0.0008 | 142 | + |
A direct relation between the concentrations of organic compounds in the membrane and their EC50s (effective concentrations inhibiting 50% of cell growth) was already described by Heipieper et al. (10). In this study, it was found that for every compound, the actual concentration in the membrane necessary for an EC50 effect was nearly the same and ranged from around 200 mM (10). So, for all compounds tested, nearly the same concentration in the membrane is necessary to reach a comparable toxic impact. These results support the hypothesis of Sikkema et al. (33), who argued that the toxic effects of organic solvents are independent of the structural features of the molecules but are strongly related to their ability to accumulate in the membrane. On the other hand, the specific chemical toxicity of a compound has to be taken into consideration. For example, the nature and the degree of the substitution of N-aromatic substances were observed to have a profound effect on the toxicity of a compound (5).
Calculating actual solvent concentrations in membranes helps us to understand the behavior of solvents in biological membranes. Apparently, P. putida DOT-T1E can easily adapt to a second phase of those compounds which show a maximum membrane concentration (MMC) of around 400 mM or lower, such as toluene, 1-decanol, or 1-dodecanol. However, toluene was not tested as a second liquid phase in this study. In the case of 1-octanol, the MMC seems to be too high to allow a complete adaptation to the presence of a second phase. This compound is tolerated by the bacteria but does not allow nonaffected stable growth in its presence. Similar results have already been reported by Rojas et al. (28). Alkanols with short chain lengths (such as 1-hexanol and 1-butanol) show very high water solubility. Their MMC is so high that no bacterium should be able to adapt to such a second phase. The lower MMC of 1-decanol compared to that of 1-octanol is not in contradiction with the higher toxicity of this compound toward nonadapted cells. The major parameter that determines toxicity is the hydrophobicity, at least in a range up to a log P of around 4. Indeed, the MMC allows only a prediction of the toxicity of a compound when added in the form of supersaturated concentrations.
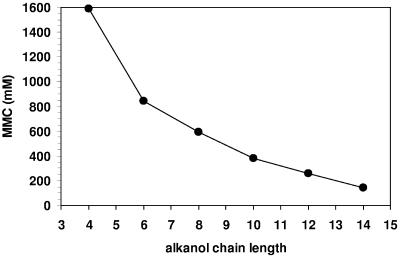
The higher the chain length of an alkanol, the higher is its hydrophobicity and thus its tendency to accumulate preferentially in membranes (Fig. 3). However, as water solubility decreases with increasing chain length, a compound such as 1-dodecanol will not reach a high membrane concentration and is therefore not toxic to an organism. This is the reason why solvents in a log P range of 1.5 to 4 are so extremely toxic: because they are relatively water soluble and still partition well into the membrane. As a result, the actual membrane concentration of these solvents will be too high (33, 34, 37). Thus, this calculation method is a useful tool to predict whether a solvent-tolerant bacterium is able to adapt to a second phase of a certain compound in order to use it as solvent for two-liquid-phase biotransformations.
FIG. 3.
Relation between the chain length of 1-alkanols and their MMC calculated according to de Bont (4) and as described in the text.
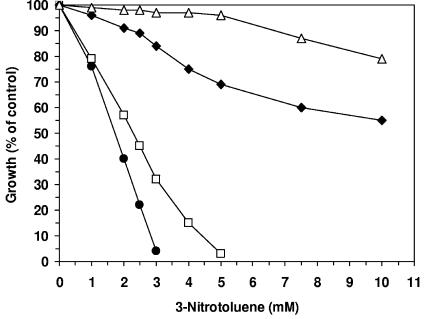
Effect of a second phase of 1-decanol on the toxicity of 3-nitrotoluene.
In shake cultures, the toxicity of 3-nitrotoluene as a model substrate for biotransformations was estimated. The EC50 value of 3-nitrotoluene in cells cultivated in mineral medium without a 1-decanol phase was 1.75 mM, and the MIC was 3 mM. These values drastically increased with the application of a second phase of 1-decanol. Even in the presence of only a low percentage (vol/vol) of 1-decanol, the bacteria showed a drastically increased tolerance toward 3-nitrotoluene (Fig. 4); the tolerance of the cells increased with the volume percentage of 1-decanol and thus with the amount of a second phase of the solvent. Contrary to that, the toxicity of 3-nitrotoluene was not affected by the presence of a second phase of 1-octanol (data not shown). This observed increase in tolerance is surely not only the effect of an adaptive process of the cells but mainly the result of a reduced bioavailability of the compound. For a detailed explanation of this effect of the solvent, we measured the partition coefficient of 3-nitrotoluene in a 1-decanol-water system (log Pd/w). The measured partition coefficient was 500 (log Pd/w = 2.72), which was very similar to the log Po/w value of 3-nitrotoluene (log Po/w = 2.46). Because of this preferential partitioning of 3-nitrotoluene in 1-decanol, a second phase drastically reduces the concentration of the substrate within the medium phase so that much higher concentrations can be added without reaching the EC50 or MIC. The volume-dependent partitioning of a potential substrate (S) obeys a hyperbolic function, depending on the volumes of the two phases and the partition coefficient (K) of S in the solvent/water system:
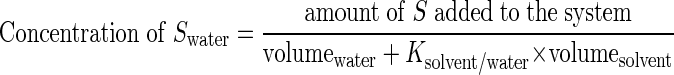 |
Therefore, a phase of 10% (vol/vol) of 1-decanol already contains more than 98% of the added 3-nitrotoluene. Therefore, the application of a solvent such as 1-decanol allows the production of chemical compounds using whole-cell biocatalysis in amounts that allow an economical competition with classical chemical synthesis.
FIG. 4.
Effect of the presence of a second phase of 0% (•), 1% (□), 5% (⧫), and 10% (▵) (vol/vol) 1-decanol on growth inhibition caused by 3-nitrotoluene of shake cultures of P. putida DOT-T1E.
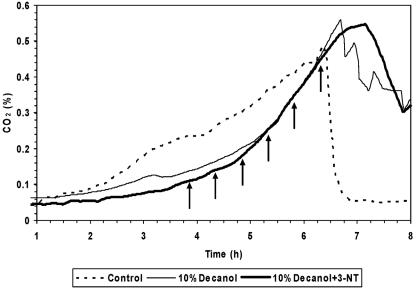
To scale up this system, we continued our studies in 5-liter fermentations with the presence of a 10% (vol/vol) phase of 1-decanol. Also in this scale, the adapted cells of P. putida DOT-T1E showed the same growth behavior in the presence as in the absence of the solvent (Fig. 5). Furthermore, the addition of up to 120 mM of 3-nitrotoluene did not cause any growth inhibition at all (Fig. 5).
FIG. 5.
Growth of P. putida DOT-T1E in a 5-liter bioreactor in the absence and in the presence of a second phase of 10% (vol/vol) 1-decanol and in the presence of 10% (vol/vol) 1-decanol with subsequential additions (as indicated by the arrows) of 20 mM 3-nitrotoluene.
A second solvent phase is beneficial, as it allows a continuous extraction of the product from the aqueous medium phase as well as the presence of very high amounts of the substrate in the system. It keeps the concentration of both the substrates and the products in the aqueous phase below a level that would lead to growth inhibition or even to death of the bacteria. 1-Decanol, in which toxic compounds with a log P between 1 and 4 preferably partition, might be a suitable alternative to hydrophobic solvents such as 1-dodecanol or hexadecane used as the second liquid phase in biotransformations with P. putida DOT-T1E as the biocatalyst.
Additionally, the presented calculations of the maximum membrane concentrations (Table 1) allow a better prediction of whether a potential solvent is tolerated by the biocatalysts. The partitioning of potential substrates and products between 1-decanol and water allows a prediction of maximum concentrations of fine chemicals within the whole fermentation system that will be tolerated by the bacteria. Thus, the application of a solvent-tolerant organism in such a 1-decanol-water system offers new possibilities in coping with toxic products.
P. putida DOT-T1E and related strains such as P. putida S12 set an example for bacteria which can tolerate solvents and are able to produce fine chemicals of interest in economically sound concentrations (14, 38). These bacteria contain at least three different solvent efflux pumps that play a major role in adaptation (32) and are therefore the most effective potential biocatalysts among all P. putida strains. Additionally, these strains are very accessible to genetic manipulation and genes from other microorganisms can easily be introduced, making it possible to obtain a wide range of products. Using this biotransformation system, many important fine chemicals with low molecular weight, including catechols, phenols, and ketones, could be produced in concentrations and with volumetric productivities that will allow competing with classical chemical syntheses.
Acknowledgments
This work was partially supported by contract no. QLRT-2001-00435 of the European Commission within its Fifth Framework Programme.
REFERENCES
- 1.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Bruce, L. J., and A. J. Daugulis. 1991. Solvent selection strategies for extractive biocatalysis. Biotechnol. Prog. 7:116-124. [DOI] [PubMed] [Google Scholar]
- 4.de Bont, J. A. M. 1998. Solvent-tolerant bacteria in biocatalysis. Trends Biotechnol. 16:493-499. [Google Scholar]
- 5.Donlon, B. A., E. Razoflores, J. A. Field, and G. Lettinga. 1995. Toxicity of N-substituted aromatics to acetoclastic methanogenic activity in granular sludge. Appl. Environ. Microbiol. 61:3889-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartig, C., N. Loffhagen, and H. Harms. 2005. Formation of trans fatty acids is not involved in growth-linked membrane adaptation of Pseudomonas putida. Appl. Environ. Microbiol. 71:1915-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmans, S., J. P. Smits, M. J. van der Werf, F. Volkering, and J. A. M. de Bont. 1989. Metabolism of styrene oxide and 2-phenylethanol in the styrene-degrading Xanthobacter strain 124X. Appl. Environ. Microbiol. 55:2850-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heipieper, H. J., P. de Waard, P. van der Meer, J. A. Killian, S. Isken, J. A. M. de Bont, G. Eggink, and F. A. de Wolf. 2001. Regiospecific effect of 1-octanol on cis-trans isomerization of unsaturated fatty acids in the solvent-tolerant strain Pseudomonas putida S12. Appl. Microbiol. Biotechnol. 57:541-547. [DOI] [PubMed] [Google Scholar]
- 9.Heipieper, H. J., R. Diefenbach, and H. Keweloh. 1992. Conversion of cis-unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl. Environ. Microbiol. 58:1847-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heipieper, H. J., B. Loffeld, H. Keweloh, and J. A. M. de Bont. 1995. The cis/trans isomerization of unsaturated fatty acids in Pseudomonas putida S12: an indicator for environmental stress due to organic compounds. Chemosphere 30:1041-1051. [Google Scholar]
- 11.Heipieper, H. J., F. Meinhardt, and A. Segura. 2003. The cis-trans isomerase of unsaturated fatty acids in Pseudomonas and Vibrio: biochemistry, molecular biology and physiological function of a unique stress adaptive mechanism. FEMS Microbiol. Lett. 229:1-7. [DOI] [PubMed] [Google Scholar]
- 12.Heipieper, H. J., G. Meulenbeld, Q. van Oirschot, and J. A. M. de Bont. 1996. Effect of environmental factors on the trans/cis ratio of unsaturated fatty acids in Pseudomonas putida S12. Appl. Environ. Microbiol. 62:2773-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heipieper, H. J., F. J. Weber, J. Sikkema, H. Keweloh, and J. A. M. de Bont. 1994. Mechanisms behind resistance of whole cells to toxic organic solvents. Trends Biotechnol. 12:409-415. [Google Scholar]
- 14.Husken, L. E., M. C. F. Dalm, J. Tramper, J. Wery, J. A. M. de Bont, and R. Beeftink. 2001. Integrated bioproduction and extraction of 3-methylcatechol. J. Biotechnol. 88:11-19. [DOI] [PubMed] [Google Scholar]
- 15.Inoue, A., and K. Horikoshi. 1989. A Pseudomonas thrives in high concentrations of toluene. Nature 338:264-266. [Google Scholar]
- 16.Junker, F., and J. L. Ramos. 1999. Involvement of the cis/trans isomerase Cti in solvent resistance of Pseudomonas putida DOT-T1E. J. Bacteriol. 181:5693-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabelitz, N., P. M. Santos, and H. J. Heipieper. 2003. Effect of aliphatic alcohols on growth and degree of saturation of membrane lipids in Acinetobacter calcoaceticus. FEMS Microbiol. Lett. 220:223-227. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita, T. 1958. Solubility of n-aliphatic alcohols in water at 25°C. Bull. Chem. Soc. Jpn. 31:1081-1082. [Google Scholar]
- 19.Laane, C., S. Boeren, K. Vos, and C. Veeger. 1987. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 30:81-87. [DOI] [PubMed] [Google Scholar]
- 20.Lambert, P. A., and S. M. Hammond. 1973. Potassium fluxes, first indication of membrane damage in micro-organisms. Biochem. Biophys. Res. Commun. 54:796-799. [DOI] [PubMed] [Google Scholar]
- 21.Leon, R., P. Fernandes, H. M. Pinheiro, and J. M. S. Cabral. 1998. Whole-cell biocatalysis in organic media. Enzyme Microb. Technol. 23:483-500. [Google Scholar]
- 22.Morrison, W. R., and L. M. Smith. 1964. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid Res. 5:600-608. [PubMed] [Google Scholar]
- 23.Mosqueda, G., M. I. Ramos-Gonzalez, and J. L. Ramos. 1999. Toluene metabolism by the solvent-tolerant Pseudomonas putida DOT-T1 strain, and its role in solvent impermeabilization. Gene 232:69-76. [DOI] [PubMed] [Google Scholar]
- 24.Ramos, J. L., E. Duque, M. T. Gallegos, P. Godoy, M. I. Ramos-Gonzalez, A. Rojas, W. Teran, and A. Segura. 2002. Mechanisms of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 56:743-768. [DOI] [PubMed] [Google Scholar]
- 25.Ramos, J., E. Duque, M. Huertas, and A. Haidour. 1995. Isolation and expansion of the catabolic potential of a Pseudomonas putida strain able to grow in the presence of high concentrations of aromatic hydrocarbons. J. Bacteriol. 177:3911-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos-Gonzalez, M. I., P. Godoy, M. Alaminos, A. Ben-Bassat, and J. L. Ramos. 2001. Physiological characterization of Pseudomonas putida DOT-T1E tolerance to p-hydroxybenzoate. Appl. Environ. Microbiol. 67:4338-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rekker, R. F., and H. M. de Kort. 1979. The hydrophobic fragmental constant, an extension to a 1000 data point set. J. Eur. Med. Chem. 14:479-488. [Google Scholar]
- 28.Rojas, A., E. Duque, A. Schmid, A. Hurtado, J.-L. Ramos, and A. Segura. 2004. Biotransformation in double-phase systems: physiological responses of Pseudomonas putida DOT-T1E to a double phase made of aliphatic alcohols and biosynthesis of substituted catechols. Appl. Environ. Microbiol. 70:3637-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid, A., J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts, and B. Witholt. 2001. Industrial biocatalysis today and tomorrow. Nature 409:258-268. [DOI] [PubMed] [Google Scholar]
- 30.Schoemaker, H. E., D. Mink, and M. G. Wubbolts. 2003. Dispelling the myths—biocatalysis in industrial synthesis. Science 299:1694-1697. [DOI] [PubMed] [Google Scholar]
- 31.Segura, A., E. Duque, G. Mosqueda, J. L. Ramos, and F. Junker. 1999. Multiple responses of gram-negative bacteria to organic solvents. Environ. Microbiol. 1:191-198. [DOI] [PubMed] [Google Scholar]
- 32.Segura, A., H. J. Heipieper, W. Terán, M. E. Guazzaroni, A. Rojas, E. Duque, M. T. Gallegos, and J. L. Ramos. 2004. Enzymatic activation of the cis-trans isomerase and transcriptional regulation of efflux pumps in solvent tolerance in Pseudomonas putida, p. 479-508. In J. L. Ramos (ed.), The pseudomonads, vol. 2. Kluwer Press, Dordrecht, The Netherlands. [Google Scholar]
- 33.Sikkema, J., J. A. M. de Bont, and B. Poolman. 1994. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 269:8022-8028. [PubMed] [Google Scholar]
- 34.Sikkema, J., J. A. M. de Bont, and B. Poolman. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Sonsbeek, H. M., H. H. Beeftink, and J. Tramper. 1993. Two-liquid-phase bioreactors. Enzyme Microb. Technol. 15:722-729. [DOI] [PubMed] [Google Scholar]
- 36.Vrionis, H. A., A. M. Kropinski, and A. J. Daugulis. 2002. Enhancement of a two-phase partitioning bioreactor system by modification of the microbial catalyst: demonstration of concept. Biotechnol. Bioeng. 79:587-594. [DOI] [PubMed] [Google Scholar]
- 37.Weber, F. J., and J. A. M. de Bont. 1996. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim. Biophys. Acta 1286:225-245. [DOI] [PubMed] [Google Scholar]
- 38.Wery, J., D. I. M. da Silva, and J. A. M. de Bont. 2000. A genetically modified solvent-tolerant bacterium for optimized production of a toxic fine chemical. Appl. Microbiol. Biotechnol. 54:180-185. [DOI] [PubMed] [Google Scholar]