Abstract
Antialgal allelochemicals were isolated from Phragmites communis Tris. The isolated allelopathic fraction showed strong inhibition activity on the growth of Chlorella pyrenoidosa and Microcystis aeruginosa but had no inhibition on Chlorella vulgaris. The 50% effective concentrations (EC50) of the allelopathic fractions on C. pyrenoidosa and M. aeruginosa were 0.49 and 0.79 mg/liter, respectively. The allelopathic activity of the fraction was species-specific. The isolated allelopathic fraction caused metal ion leakage from algal cells. The fraction decreased the activities of antioxidant enzymes, such as superoxide dismutase and peroxidase. The addition of the isolated fraction increased the concentration of unsaturated lipid fatty acids in cell membrane of C. pyrenoidosa and M. aeruginosa. This caused a change in plasma membrane integrity and the leakage of ions in the protoplast. The allelopathic compound was identified by nuclear magnetic resonance and gas chromatography-mass spectrometry as ethyl 2-methylacetoacetate. Synthesized ethyl 2-methylacetoacetate also showed allelopathic activity on C. pyrenoidosa and M. aeruginosa. The EC50 of synthesized ethyl 2-methylacetoacetate on C. pyrenoidosa and M. aeruginosa were 0.49 and 0.65 mg/liter, respectively.
In recent years, harmful algal blooms in eutrophic water bodies have caused serious problems with regard to effective utilization of water resources such as fisheries, water supply, and recruitment. It is estimated that the economic loss from serious water bloom events in the United States is usually over several billion U.S. dollars annually (10, 38). Although some methods have been proposed to control harmful algal growth, only a few of them are applicable due to high cost, secondary pollution, or impracticability (1). The commonly used algicides, such as copper (Cu2+) and herbicide, can inhibit the entire phytoplankton community and cause subsequent water quality deterioration that may stress or kill aquatic animals. Furthermore, copper might accumulate in the bodies of aquatic predators and herbicides might be toxic to all the living things in a body of water.
The discovery of allelopathy—the suppression of neighboring plant growth by the release of toxic compounds (7)—hinted that the antialgal allelochemicals produced by macrophytes might be used as algal growth inhibitors in algal control (5, 14, 38, 41). Macrophytes and algae are known to have an antagonistic relationship in aquatic ecosystems (14, 15, 16, 34, 41, 42). Several experiments have been carried out to evaluate the algal inhibition activity of some macrophytes. The ethyl ether fraction of an extract from a culture solution of the well known troublesome floating macrophyte water hyacinth (Eichhornia crassipes) inhibited Chlorella pyrenoidosa and three other species of algae (43). According to Nakai et al. (25) Cabomba caroliniana and Myriophyllum spicatum inhibited Selenastrum capricornutum and Microcystis aeruginosa coexisting in a culture. Further research studies showed that polyphenols were antialgal allelochemicals and the concentration of polyphenols in M. spicatum was much higher than those in other observed submersed macrophytes of other families (13, 15, 16, 27). Accordingly, a good possibility exists that the growth of noxious algae can be controlled by using antialgal allelopathic compounds extracted from macrophytes. Traditional approaches have focused on the isolation of allelopathic compounds from submerged plants (26, 28) and floating plants (43). There are few evaluations of the antialgal allelopathic potential of emerged macrophytes (18, 34).
Determination of the mode of action of allelochemicals is a challenging endeavor due to the multitude of potential molecular targets. There are no established protocols or standard methods to study the modes of the action of allelochemicals. Each research team develops their own approach that best addresses their needs, and teams often rely on methods with which they are most comfortable. Dehydrozaluzanin C, a natural sesquiterpene lactone, causes rapid plasma membrane leakage in cucumber cotyledon disks (9). The essential oil extracted from palmarosa (Cymbopogon martinii) causes the proportion of membrane fatty acids to change (33). Many kinds of allelochemicals are also known to affect the mitosis of plants (4, 31). The allelochemical fischerellin A, produced by a cyanobacterium of Fischerella muscicola, can inhibit the photosystem II of several plants (17, 39). The activities of enzymes were also affected by allelopathic compounds (8, 23). In photosynthetic organisms, environmental stress can create oxidative stress through increased production of reactive oxygen species (ROS), e.g., singlet oxygen (1O2), hydroxyl radical (HO·), and hydrogen peroxide (H2O2) (2). Reactive oxygen species may cause lipid peroxidation (3), whereas superoxide dismutase (SOD) and peroxidase (POD) inhibit lipid peroxidation (36). There are no studies reporting whether or not allelochemicals increase the production of reactive oxygen species (ROS). In oxidative conditions, plants and microalgae respond by increasing antioxidant defenses, notably enzymes such as SOD and POD. However, excessive ROS may cause a decrease of SOD and POD activities (25).
Our previous research (20, 21) revealed that Phragmites communis demonstrated a very high inhibition activity on the growth of C. pyrenoidosa and M. aeruginosa. Although the controlling of algal growth by adding biomass of P. communis directly to a water body is possible, much research must be carried out to make this a safe and effective method for aquatic ecosystem management. Essential tasks include (i) revealing the allelopathic compounds released from P. communis, (ii) demonstrating the different antialgal activity on different algae species of the compounds, and (iii) exploring the mechanism of the action of the allelochemicals. In the present study, a novel allelochemical was extracted from P. communis. The antialgal activity and the mode of action of the allelochemical were also investigated.
MATERIALS AND METHODS
Materials.
Experiments were performed with the most broadly distributed green alga C. pyrenoidosa, Chlorella vulgaris, and the most undesirable blue-green alga M. aeruginosa. All of the algae used here were provided by the FACHB collection (Freshwater Algae Culture of Hydrobiology Collection [People’s Republic of China]). M. aeruginosa was cultured with sterilized BG11 medium (pH 7.4) (35). The media of C. pyrenoidosa and C. vulgaris contain NaNO3 (25.5 mg), NaHCO3 (15 mg), K2HPO4 (1.04 mg), MgSO4 · 7H2O (14.7 mg), MgCl2 (5.7 mg), CaCl2 · 2H2O (4.41 mg), Na2EDTA · 2H2O (0.3 mg), H3BO4 (0.185 mg), MnCl2 (0.264 mg), ZnCl2 (3.27 × 10−3 mg), CoCl2 · 2H2O (0.78 × 10−3 mg), CuCl2 · 2H2O (0.009 × 10−3 mg), Na2MoO4 · 2H2O (7.26 × 10−3 mg), FeCl3 (0.096 mg), and 1,000 ml of distilled water. Inocula were taken from exponentially growing precultures. The initial cell density in the preculture as well as in the test flasks was adjusted to 108 cells/liter. Specimens of Phragmites communis Tris were collected in summer from a shore area of a pond in Beijing, People’s Republic of China. The specimens were dried at 60°C for 48 h before use. The synthesized compound of ethyl 2-methylacetoacetate (EMA) was purchased from Sigma-Aldrich.
Extraction and separation of allelochemicals from P. communis.
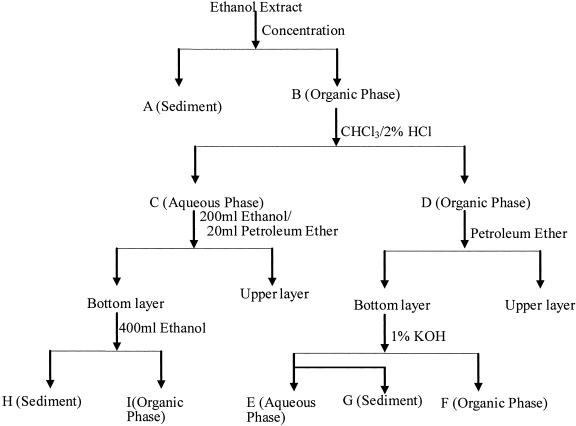
Specimens of P. communis were dried at 60°C for 48 h. The specimens were ground up to fine powder and soaked in 8 liters of ethanol for 24 h at 25°C in the dark. The ethanol extract was separated by using filtration and further fractionated by the following procedure (Fig. 1).
FIG. 1.
Flow chart of the isolation procedure used in the present study.
A small amount of white floccule (fraction A) and brown substance (fraction B) were obtained when the extract was concentrated and dried at 45°C under reduced pressure in an evaporator. Fraction B was redissolved with 300 ml of chloroform and 100 ml of 2% muriatic acid and transferred to a 1,000-ml separation funnel. The mixture was shaken vigorously for 5 min and allowed to stand for 30 min. Aqueous phase (fraction C) and organic phase (fraction D) were obtained. The aqueous phase (fraction C) was evaporated to 10 ml, followed by the addition of 200 ml of ethanol and 20 ml of petroleum ether, and then the resultant mixture was transferred to a 500-ml separation funnel. The mixture was shaken for 5 min and allowed to stand for 5 min. The bottom layer was evaporated to dryness and weighed and redissolved with 400 ml of ethanol (fraction I), and sediment (fraction H) was retained. Then, 10 ml of petroleum ether was added to fraction D. The mixture was separated by using a 500-ml separation funnel. A total of 50 ml of 1% KOH was added in the bottom layer, followed by separation using a separation funnel. The organic phase (fraction F) and aqueous phase (fraction E) and floccule (fraction G) were obtained. All ofthe fractions (fractions E, F, G, H, and I) were redissolved with ethanol (10mg/ml approximately) before bioassays.
Bioassay.
The allelopathic potential of the crude extract and isolated fractions (fractions E, F, G, H, and I) was evaluated by algal bioassays with C. pyrenoidosa, C. vulgaris, and M. aeruginosa. Ethanol solutions (0, 0.2, and 1 ml) of crude extract or isolated fractions were added to Erlenmeyer flasks containing 5 ml of algal inoculant and 195 ml of culture medium. Three replicates were prepared for each treatment. The algae were cultured under 90 μmol of photons m−2 s−1 (light-dark = 14 h-10 h) at 25°C for 96 h.
Dose-response studies.
Effects of different concentrations of the isolated allelochemical and synthesized allelochemical on the growth of C. pyrenoidosa, C.vulgaris, and M. aeruginosa were studied. Different concentration allelochemical (0, 0.2, 0.4, 0.6, 1, 2, 3, and 4 mg/liter) were added to Erlenmeyer flasks containing 5 ml of algal inoculant and 195 ml of culture medium. The algal density was 106 cell/liter level at the beginning of the growth cycle. The algae were cultured under 90 μmol of photons m−2 s−1 (light-dark = 14 h-10 h) at 25°C for96 h.
Mode of action of allelochemical.
In the present study, we investigated the changes of metal ion leakage, membrane fatty acids, and antioxidant enzyme activity caused by allelochemical.
The changes in metal ion leakage of C. pyrenoidosa caused by different concentrations of allelochemical with various contact time were studied. C. pyrenoidosa was cultured to log time (108 cells/liter) followed by the addition of different concentration allelochemical (0, 0.5, and 2 mg/liter). The contact times were 0, 30, 60, and 120 min, respectively. After the addition of the allelochemical, the algal cultures were centrifuged at 3,000 rpm for 10 min, and the concentration of K+ in the supernatant was measured. In a control experiment, the algal cells were heated for 10 min in boiling water to completely destroy the cell membrane absolutely and get the maximum sequestered ions in the algal cells (24). All of the experiments were carried out in triplicate.
In another set of experiments, the effects of different concentrations of allelochemical on the ion leakage of C. pyrenoidosa, C. vulgaris, and M. aeruginosa were studied. C. pyrenoidosa, C. vulgaris, and M. aeruginosa were cultured to log time (108 cells/liter) and then 0, 0.25, 0.5, 1, 2, or 4 mg of allelochemical/liter was added to the algal cultures, followed by a culture for 120 min in the dark. The cultures were centrifuged at 3,000 rpm for 10 min, and the concentrations of K+, Ca2+, and Mg2+ in the supernatant were measured. In a control experiment, the algal cells were heated for 10 min in boiling water to completely destroy the cell membrane and get the maximum sequestered ions in the algal cells.
Effects of different concentrations of allelochemical on the activity of SOD and POD of C. pyrenoidosa, C. vulgaris, and M. aeruginosa were studied. The algae were precultured to log time (the algal density was ca. 108 cells/liter), and then the allelochemical was added. The allelochemical concentrations were 0, 0.25, 0.5, 1, 2, and 4 mg/liter. The contact time was 24 h in the dark. Then the cultures were centrifuged at 3,000 rpm for 10 min to obtain the algal cells for the POD and SOD activity analysis.
The changes in membrane fatty acids caused by the allelochemical were evaluated. The algae were precultured to ca. 108 cells/liter. The allelopathic fraction I was then added to the culture, followed by incubation for 24 h. The alga membrane fatty acids were extracted and measured. A control (without allelochemical) was run in parallel. Algal cells were harvested by centrifugation at 3,000 rpm for 5 min at 4°C and then washed in distilled water thrice. Three replicates were prepared for each treatment. Extraction of membrane fatty acids, preparation of their methyl esters, and final extraction with ether were conducted according to the method of Rose and Veazey (37) but modified by sonication of cells under nitrogen at 14 MHz for 10 min (Soniprep 150; MSE) prior to alkaline saponification. Totals of 2 ml of 2 M KOH in 95% (vol/vol) methanol and 2 ml of benzene were added to the sonicated cells. The headspace was filled with N2 gas, and the mixture was incubated at 80°C for 3 h. On cooling, an equal volume of methanol was added, and the nonsaponified fraction was extracted by shaking with three successive 5-ml aliquots of petroleum ether (60 to 80°C fraction). The lower methanol layer was acidified with 6 M HCl, and the saponified lipids were extracted with petroleum ether (three aliquots of 5 ml). The saponified lipid fraction was evaporated and then derivatized to methyl esters by the addition of 1 ml of 14% BF3 in methanol. The headspace was filled with N2 gas, sealed, and heated at 80°C for 1 h. The contents were added to 5 ml of distilled water and extracted thrice with 5 ml of petroleum ether. The pooled ether fractions were concentrated and analyzed.
Three replicates were given for each treatment in all of the experiments.
Analytical methods.
The algal numbers were counted by using a hemacytometer. Gas chromatography-mass spectrometry (GC-MS) and 1H nuclear magnetic resonance (NMR) were used in the identification of allelochemicals. The constituents of fraction I were analyzed by GC-MS. The GC apparatus used was a Perkin-Elmer 8600 fitted with flame ionization detector. An SE-54 quartz capillary column (30 m) was used. The column temperature was initially held at 60°C for 2 min and then programmed to increase to 300°C at a rate of 5°C/min. The injector temperature was maintained at 280°C, and the injection volume was 1 ml with the splitless mode. Constituents were identified by peak matching against standards in the NIST 95 Computer Library. The relative amounts of constituents were calculated by integrating all peaks with areas of >1%.
NMR analyses of the allelochemicals were carried out on a Pulse Fourier Transform Nuclear Magnetic Resonance Spectroscope (PFT-NMR; JOEL JNM-ECA300 spectrometer, 300 MHz 1H, CDCl3). 1H NMR was recorded on a 2.5-mm inverse detection microprobe head. All chemical shifts are reported as relative δ values.
The concentrations of K+, Ca2+, and Mg2+ in the supernatant were measured by using inductively coupled plasma-MSy (ICP-MS; VG Elemental PQ ExCell ICP-MS). A 500-μl sample of the supernatant was added to 100 μl of internal standard and diluted with 5 ml of 1% ultrapure double-distilled nitric acid. Metal concentrations were calculated by using the ion mass per 109 cells.
The POD activity was obtained by measuring the oxidation of ascorbate in the presence of H2O2 (29). The algal sample was ground in liquid nitrogen and extracted in 2.5 ml of 50 mM potassium phosphate buffer (pH 7.0) containing 10% (wt/vol) polyvinylpyrrolidone-40, 0.25% Triton X-100, and 0.5 mM ascorbate. The extraction was then centrifuged for 5 min at 14,000 rpm at 4°C in order to eliminate debris (Sigma Laborzentrifugen). The oxidation of ascorbate was initiated by adding 50 μl supernatant to 950 μl potassium phosphate buffer (pH 7.0, 50 mM) containing 0.1 mM EDTA, 0.5 mM ascorbate, and 0.1 mM H2O2. After 30 s of the reaction, the decrease in absorbance at 290 nm was measured with a spectrophotometer. The assay was performed at 20°C. One unit of POD was defined as the variance of absorbance per 107 cells (ΔA470/107 cells).
SOD activity was measured according to the method of Paoletti et al. (32) based on the inhibitory action of the enzyme on the rate of NADH oxidation. The algal sample was ground in liquid nitrogen and extracted in 3 to 5 ml of 50 mM potassium phosphate buffer (pH 7.0) containing 10% polyvinylpyrrolidone-40 and 0.25% Triton X-100. The extract was centrifuged for 5 min at 14,000 rpm at 4°C. The supernatant was used for the SOD analysis. For the measurement of SOD activity, the reaction buffer (700 μl) was prepared with 50 mM potassium phosphate buffer (pH 7.8), 0.1 mM EDTA, 18 μM cytochrome c, and 0.1 mM xanthine. Xanthine oxidase was added to the reaction buffer to get an increase of absorbance at 550 nm of ca. 0.025 min−1 at 25°C. Then, 15 to 100 μl of supernatant sample and H2O was added to the reaction buffer, including xanthine oxidase, to give a volume of 800 μl. The rate of cytochrome c was measured at 550 nm for an additional minute. One unit of SOD was defined as the variance of absorbance per min per 107 cells (ΔA550/min 107 cells).
Fatty acids were analyzed by GC-MS. The conditions of GC-MS were the same as those used for the identification of allelochemicals.
Data analysis.
Allelopathic activity on growth inhibition was estimated by inhibition ratio (IR), which is defined as follows: IR = [1 − (N/N0)] × 100, where N0 and N are the numbers of cells in the control and extract-added cultures, respectively.
Origin7.0 software (OriginLab Corp.) was used in calculating the algal growth rate (r) and log time (t). Statistical analysis of data was performed by using the Kruskal-Wallis and/or Student t test.
RESULTS
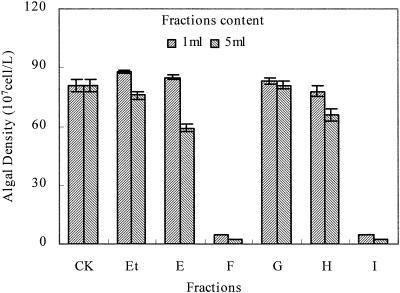
Extraction of allelochemicals from P. communis. Five fractions (E, F, G, H, and I) were isolated from the ethanol extract of P. communis. The antialgal activities of the five fractions were evaluated by using algal bioassays. The inhibitory effect ofthe fractions on algal growth of C. pyrenoidosa is shown in Fig. 2. The algal biomass of the cultures with the additions of fractions F and I were lower than those of the control and fractions E, G, and H (P < 0.001). Fractions F and I exhibited allelopathic activity. The 50% effective concentrations (EC50) of fractions F and I on C. pyrenoidosa, C. vulgaris, and M.aeruginosa are shown in Table 1. Fraction I showed the strongest inhibitory effects on both C. pyrenoidosa and M. aeruginosa, whereas it showed little inhibitory effect on C. vulgaris. Inhibitory effects of fraction I appear to be species specific. So fraction I was chosen for further research.
FIG. 2.
Inhibitory effect on growth of C. pyrenoidosa in fractions. CK, control; Et, ethanol without extracted fractions; E to I, extracted fractions. All error bars correspond to the standard deviation.
TABLE 1.
Summary of inhibitory effects of fractions F and I on C. pyrenoidosa, C. vulgaris, and M. aeruginosa
| Fraction | Mean EC50 (mg/liter) ± SD
|
||
|---|---|---|---|
| C. pyrenoidosa | C. vulgaris | M. aeruginosa | |
| F | 2.13 ± 0.13 | >10 | 4.63 ± 0.24 |
| I | 0.49 ± 0.09 | >10 | 0.79 ± 0.21 |
Effects of fraction I on membrane integrity.
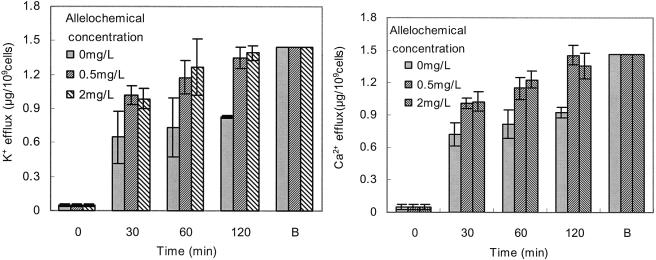
When cells of C.pyrenoidosa were incubated with fraction I, leakage of potassium, magnesium, and calcium ions from the cells was detected. After 120 min of incubation, K+ released from C. pyrenoidosa with additions of 0.5 and 2 mg of fraction I/liter were 1.35 and 1.39 μg/109 cells, respectively. This leakage coincided with the maximum sequestered potassium ions that were obtained from the cells boiled and destroyed cell membrane of C. pyrenoidosa. There were more potassium ions in the supernatant when the incubation time was longer (Fig. 3). The release of Ca2+ and Mg2+ (data not shown) paralleled that of K+. These results indicated that the cell membrane was absolutely destroyed by fraction I after 120 min of incubation.
FIG. 3.
Effect of treatment time on potassium and calcium leakage from cells of C. pyrenoidosa for groups treated with 0.5 and 2 mg of fraction I/liter. B, cells were heated for 10 min in boiling water to completely destroy the cell membrane absolutely. The culture time was 120 min after fraction I added. All error bars correspond to the standard deviation.
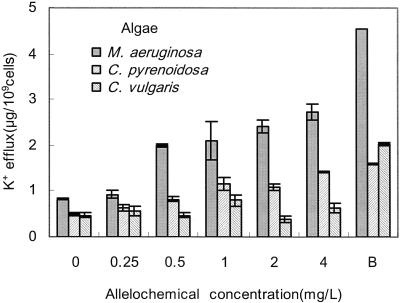
Figure 4 shows the effect of fraction I concentration on potassium leakage from cells of C. pyrenoidosa, C. vulgaris, and M. aeruginosa. The release of K+ from C. vulgaris was less than those from C. pyrenoidosa and M. aeruginosa. Based on the results shown in area B of the graphs, the potassium levels are estimated to be 4.56, 1.59, and 2.03 μg/109 cells inside the cells of M. aeruginosa, C. pyrenoidosa, and C. vulgaris, respectively. When the cells were treated with 4 mg of fraction I/liter for 120 min, >60% potassium ions were released from M. aeruginosa, and ca. 90% potassium ions were released from C. pyrenoidosa, whereas 31.5% were released from C. vulgaris. The results indicate that fraction I caused loss of membrane integrity of C.pyrenoidosa and M. aeruginosa and as a result inhibited their growth.
FIG. 4.
Effect of fraction I concentration on potassium leakage from cells of C. pyrenoidosa, C. vulgaris, and M. aeruginosa. B, cells were heated for 10 min in boiling water to completely destroy the cell membrane absolutely. Culture time was 120 min after fraction I added. All error bars correspond to the standard deviation.
Effects of fraction I on membrane fatty acids.
For the ions to be released from the cells, some changes in the cell membrane may have occurred. The compositions of the cell membrane were determined by analysis of fatty acids. The major components of membrane fatty acids of cells of C. pyrenoidosa and C. vulgaris were C14:0, C16:0, C18:0, C18:2, and C18:3, and those of M. aeruginosa were C16:0, C16:2, C18:0, C18:1, and C18:2 (Table 2). Table 2 shows the effect of fraction I on membrane fatty acid composition, expressed as the mean percentage of each lipid (C14:0, C16:0, C16:2, C18:0, C18:1, C18:2, and C18:3) extracted from cells after incubation with 2 mg of fraction I/liter. Fraction I caused the proportions of C18:2 and C18:3 (unsaturated) in C. pyrenoidosa to increase, whereas the proportions of C14:0, C16:0, and C18:0 (saturated) decreased. The proportions of C18:1 and C18:2 (unsaturated) increased, whereas those of C16:0 and C18:0 (saturated) decreased in M. aeruginosa after the addition of fraction I. Although there was a slightly decrease of the proportion of C16:2, the total proportion of the unsaturated fatty acids (C16:2, C18:1, and C18:2) was increased after the addition of fraction I. The changes of the fatty acid composition indicated a change in membrane fluidity. All of the changes were statistically significant (P < 0.01). However, fraction I did not change the fatty acid composition in C. vulgaris significantly.
TABLE 2.
Effect of fraction I on fatty acids extracted from C. pyrenoidosa, C. vulgaris, and M. aeruginosa
| Fatty acid | Lipid | % of total fatty acids (mean ± SD)a
|
||
|---|---|---|---|---|
| C. pyrenoidosa | C. vulgaris | M. aeruginosa | ||
| With no fraction I | ||||
| C14:0 | Myristic acid | 16.57 ± 0.35 | 8.17 ± 0.12 | 0 ± 0 |
| C16:2 | Hexadecadienoic acid | 0 ± 0 | 0 ± 0 | 11.93 ± 0.44 |
| C16:0 | Cetylic acid | 33.68 ± 0.09 | 26.95 ± 0.21 | 16.8 ± 0.22 |
| C18:3 | Linolenic acid | 23.31 ± 0.64 | 29.56 ± 0.29 | 0 ± 0 |
| C18:2 | Linolic acid | 11.46 ± 0.81 | 30.41 ± 0.06 | 30.26 ± 0.25 |
| C18:1 | Oleinic acid | 0 ± 0 | 0 ± 0 | 18.85 ± 0.14 |
| C18:0 | Stearic acid | 14.98 ± 0.32 | 4.9 ± 0.18 | 22.15 ± 0.31 |
| With fraction I (2 mg/liter) | ||||
| C14:0 | Myristic acid | 4.66 ± 0.06 | 7.77 ± 0.05 | 0 ± 0 |
| C16:2 | Hexadecadienoic acid | 0 ± 0 | 0 ± 0 | 7.21 ± 0.04 |
| C16:0 | Cetylic acid | 22.04 ± 0.13 | 25.04 ± 0.37 | 11.4 ± 0.27 |
| C18:3 | Linolenic acid | 37.68 ± 0.24 | 29.17 ± 0.22 | 0 ± 0 |
| C18:2 | Linolic acid | 25.91 ± 0.51 | 32.1 ± 1.02 | 42.88 ± 1.54 |
| C18:1 | Oleinic acid | 0 ± 0 | 0 ± 0 | 28.46 ± 0.33 |
| C18:0 | Stearic acid | 9.71 ± 0.05 | 5.91 ± 0.65 | 10.05 ± 0.03 |
Data are presented as mean percentages of total fatty acids from three experiments.
Effects of fraction I on the activity of antioxidant enzymes.
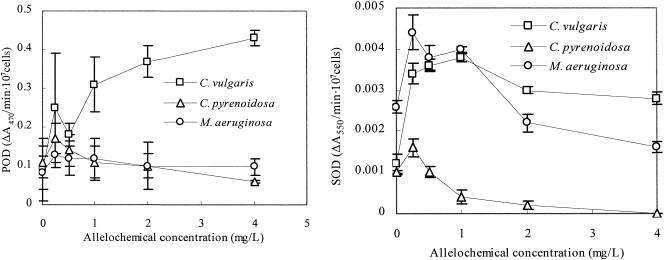
The activity of antioxidant enzymes (SOD and POD) of the algae under fraction I was investigated. The antioxidant enzymes activities behaved differently in C. pyrenoidosa, M.aeruginosa, and C. vulgaris. Low concentration of fraction I (0.25 mg/liter) caused SOD and POD activities reach a peak point in C. pyrenoidosa and M. aeruginosa (Fig. 5). Higher concentrations of fraction I (>0.5 mg/liter) decreased the SOD and POD activities of C. pyrenoidosa and M. aeruginosa. The activities of SOD and POD treated by high concentration fraction I were significantly lower than those treated with 0.25 mg of fraction I/liter in C. pyrenoidosa and M. aeruginosa. The SOD activity in C. pyrenoidosa decreased to 0 with 4 mg of fraction I/liter. POD activity in C. vulgaris was enhanced by the addition of fraction I (up to 430% by 4 mg of fraction I/liter). The SOD activity in C. vulgaris increased more than threefold when fraction I was added.
FIG. 5.
POD and SOD activity changes caused by fraction I. All error bars correspond to the standard deviation.
Identification of allelochemicals.
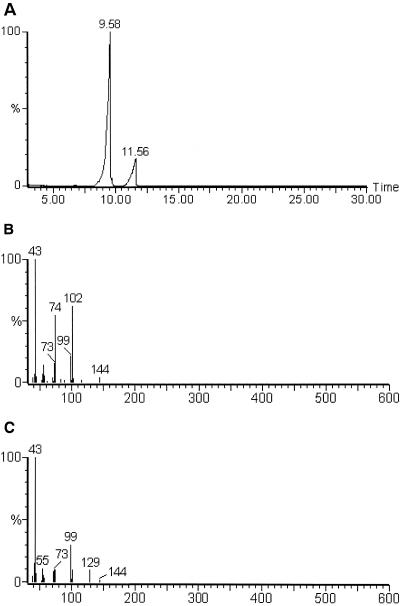
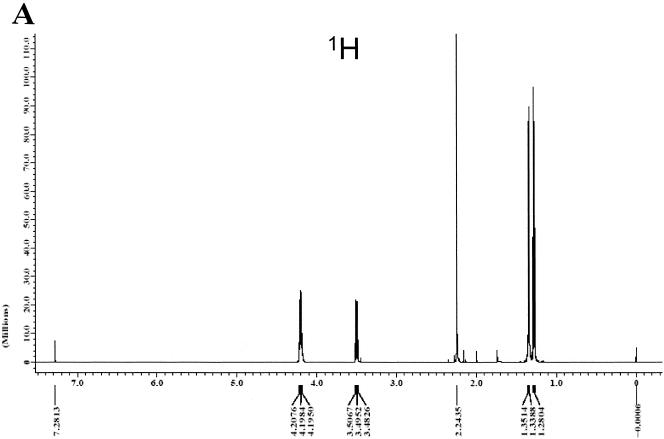
The allelochemicals (fraction I) were identified by using GC-MS and NMR. Figure 6A and B shows the resultant GC image and mass spectrum, respectively, of fraction I. The gas chromatogram showed that there were two compounds in fraction I. The mass spectrum primarily revealed that the compounds were EMA (80%) and ethyl levulinate (18%). They are isomeric compounds. The NMR data were recorded with a JOEL JNM-ECA300 spectrometer, using tetramethyl silane as the internal standard, with 1H NMR (CDCl3) [Fig. 7A: δ4.2(m, 4H), 3.5(m, 2H), 2.24 (s, 1H), 1.35(m, 3H), and 1.28(m, 3H)]. The result of 1H and C NMR (Fig. 7B) revealed that the compound (80%) was EMA.
FIG. 6.
The results of GC-MS analysis of fraction I. (A) Gas chromatogram; (B) mass spectrum of compound with a retention time of 9.58 min; (C) mass spectrum of compound with a retention time of 11.56 min.
FIG. 7.
Result of 1H and 13C NMR analysis of allelopathic EMA in fraction I (PFT-NMR, JOEL JNM-ECA300 spectrometer, 300 MHz, CDCl3).
Antialgal activity of isolated allelochemicals and synthesized allelochemicals.
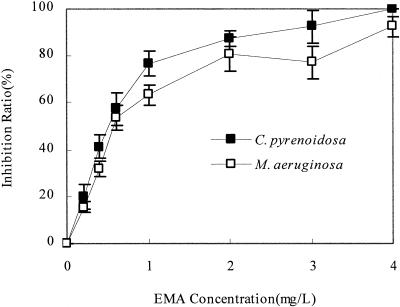
Antialgal activities of the isolated EMA and synthesized EMA and ethyl levulinate were evaluated by using bioassays with C. pyrenoidosa and M. aeruginosa. Synthesized ethyl levulinate showed no antialgal activity on C. pyrenoidosa and M. aeruginosa. The synthesized EMA showed strong inhibitory activity on C. pyrenoidosa and M. aeruginosa (Fig. 8). The EC50 of synthesized EMA on C. pyrenoidosa and M. aeruginosa were 0.49 and 0.65 mg/liter, respectively. The results attested that EMA was the antialgal allelochemical in the allelopathic fraction I extracted from P. communis Tris.
FIG. 8.
Inhibitory effect on the growth of C. pyrenoidosa and M.aeruginosa by synthesized EMA. All error bars correspond to the standard deviation.
DISCUSSION
Isolation and identification of allelochemicals is the main task in the field of allelopathy research (34). The reported antialgal allelochemicals mainly included sesquiterpene lactones, organic acids, phenols, quinines, coumarins, flavonoids, and tannins (19, 28, 34). The antialgal allelochemicals isolated from Myriophyllum spicatum were ellagic, gallic, and pyrogallic acids and (+)-catechin (organic acids) (26). Antialgal phenols and polyphenols were isolated from Zantedeschia aethiopica (12). EMA was isolated as an ester in the present study, making this the first report of EMA as an antialgal allelochemical.
EMA significantly inhibited the growth of C. pyrenoidosa and M. aeruginosa. The EC50 of EMA was 0.49 mg/liter for C.pyrenoidosa and 0.65 mg/liter for M. aeruginosa (Table 3). The EC50 of other reported antialgal allelochemicals shown in Table 3 were higher than 0.5 mg/liter except for catechol. The antialgal activity of EMA was stronger than those of other allelochemicals.
TABLE 3.
Comparison of activities of EMA with other reported antialgal allelochemicals or algicidals
| Allelochemical | Target species | EC50 (mg/liter)a | Source or reference(s) |
|---|---|---|---|
| EMA | C. pyrenoidosa | 0.49 ± 0.07 | This study |
| M. aeruginosa | 0.65 ± 0.13 | This study | |
| C. vulgaris | >10 | This study | |
| Gallic acid | M. aeruginosa | 1.0 | 29 |
| Ellagic acid | M. aeruginosa | 5.1 | 29 |
| Pyrogallic acid | M. aeruginosa | 0.65 | 29 |
| Caffeic acid | M. aeruginosa | 1.5 | 11 |
| Ferulic acid | M. aeruginosa | 4.9 | 11 |
| Catechol | M. aeruginosa | 0.3 | 11 |
| Cu2+ | C. vulgaris | 13.4 | 38 |
| S. quadricauda | 0.27 | 22, 39 | |
| Commercial algicidal | 30-70 | From market | |
| cis,trans-Xanthoxin | Lepidium sativum L. | 1.1 μM | 40 |
| Abscisic acid-β-d-glucopyranosyl ester | M. sativa L., L. sativum L. | 2.1-67 | |
| Sophorolipid | A. tamarense, H. akashiwo | 10-20 | 31 |
Values are in mg/liter except as noted. Mean values ± the standard deviation are given where applicable.
EMA isolated from P. communis in the present study showed strong growth inhibition on C. pyrenoidosa and M.aeruginosa but no inhibition on C. vulgaris. Species specificities of the inhibition effect of other allelopathic compounds were also observed. Sophorolipid showed different inhibition capacities on Alexandrium tamarense, Heterosigma akashiwo, and Cochlodinium polykrikoides (40). Allelochemicals in Myriophyllum spicatum showed strong inhibition on M. aeruginosa but weak inhibition on Anabaena flos-aquae and Phormidium tenue (26). Species specificities were also observed on higher plants; for example, allelochemicals isolated from sunflowers showed species-selective activity on the shoot and root growth of lettuce, cockscomb, tomato, and cat's-eyes (30). The mechanism of the species-specification of allelochemicals should be studied further.
The fatty acid composition of microbial cell membranes affects their ability to survive in various environments (11). The ratio of saturated to unsaturated fatty acids can change in response to environmental conditions. An environment containing EMA increased the proportions of unsaturated fatty acids of the algal cell membrane, leading to increases in membrane fluidity and hence membrane channels.
Allelopathic EMA caused plasma membrane leakage in C.pyrenoidosa and M. aeruginosa. Previous research claimed that many kinds of allelochemicals may cause rapid plasma membrane leakage in cucumber cotyledon disks (4, 9). Palmarosa oil decreased the proportions of unsaturated fatty acids and decreased the membrane integrity of Saccharomyces cerevisiae (33). In the present study, higher concentrations of EMA (>0.5 mg/liter) decreased SOD and POD activity in C. pyrenoidosa and M. aeruginosa. These results indicated that the added allelochemical, which act as an environmental stress, created oxidative stress through increased production of ROS. In oxidative conditions, the algae responded by increasing antioxidant defenses, including SOD and POD. However, higher concentrations of allelochemical and the excessive ROS it creates resulted in decreased SOD and POD activity. The excessive ROS may cause lipid peroxidation (3, 6). The addition of EMA increased the total concentration of unsaturated lipid fatty acids in cell membrane of C. pyrenoidosa and M. aeruginosa. This caused a change in plasma membrane integrity. The loss of plasma membrane integrity caused the leakage of ions in the protoplast.
Acknowledgments
This research was supported partly by the Major State Basic Research Development Program of China (project 2003CN415007).
We thank Cheng-Dui Yang and Hai-Jun Yang (Analysis Center, Tsinghua University) for their kind support.
REFERENCES
- 1.Anderson, D. M. 1997. Turning back the harmful red tides. Nature 38: 513-514. [Google Scholar]
- 2.Asada, K. 1999. The water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Mol. Biol. 50:601-639. [DOI] [PubMed] [Google Scholar]
- 3.Baranenko, V. V. 200l. Pea chloroplasts under clino-rotation: lipid peroxidation and superoxide dismutase activity. Adv. Space Rex. 27:973-976. [DOI] [PubMed] [Google Scholar]
- 4.Dayan, F. E., J. G. Romagni, and S. O. Duke. 2000. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 26:2079-2094. [Google Scholar]
- 5.Duke, S. O., F. E. Dayan, J. G. Romagni, and A. M. Rimando. 2000. Natural products as sources of herbicides: current status and future trends. Weed Res. 40:99-111. [Google Scholar]
- 6.Fargasova, A., A. Bumbalova, and E. Havranek. 1999. Ecotoxicological effects and uptake of metals (Cu+, Cu2+, Mn2+, Mo6+, Ni2+, V5+) in freshwater algal Scenedesmus quadricauda. Chemosphere 38:1165-1173. [Google Scholar]
- 7.Fitter, A. 2003. Making allelopathy respectable. Nature 301:1337-1338. [DOI] [PubMed] [Google Scholar]
- 8.Friebe, A., U. Roth, and P. Kuck. 1997. Effects of 2, 4-dihydroxy-1, 4-benzoxazin-3-ones on the activity of plasma membrane H+-ATPase. Phytochemistry 44:979-983. [Google Scholar]
- 9.Galindo, J. C. G., A. Hernandez, and F. E. Dayan. 1999. Dehydrozaluzanin C, a natural sesquiterpenolide, causes rapid plasma membrane leakage. Phytochemistry 52:805-813. [Google Scholar]
- 10.GEOHAB. 2001. Global ecology and oceanography of harmful algal blooms, p. 87-88 In P. Glibert and G. Pitcher (ed.), Science plan. SCOR/IOC, Baltimore, Md.
- 11.Ghfir, B. L., Y. Koulali, R. Ecalle, and R. Dargent. 1994. Effect of essential oil of Hyssopus officinalis on the lipid composition of Aspergillus fumigatus. Mycopathologia 126:163-167. [DOI] [PubMed] [Google Scholar]
- 12.Greca, M. D., M. Ferrara, A. Fiorentino, P. Monaca, and L. Previtera. Antialgal compounds from Zantedeschia aethiopica. Phytochemistry 49: 1299-1304, 1998. [Google Scholar]
- 13.Gross, E. M., H. Meyer, and G. Schilling. 1996. Release and ecological impact of algicidal hydrolysable polyphenols in Myriophyllum spicatum. Phytochemistry 41:133-138. [Google Scholar]
- 14.Gross, E. M. 2003. Allelopathy of aquatic autotrophs. Crit. Rev. Plant Sci. 22:313-339. [Google Scholar]
- 15.Gross, E. M., C. Feldbaum, and A. Graf. 2003. Epiphyte biomass and elemental composition on submersed macrophytes in shallow eutrophic lakes. Hydrobiologia 506:559-565. [Google Scholar]
- 16.Gross, E. M., D. Erhard, and E. Ivanyi. 2003. Allelopathic activity of Ceratophyllum demersum L. and Najas marina subsp. intermedia (Wolfgang) Casper. Hydrobiologia 506:583-589. [Google Scholar]
- 17.Hagmann, L., and F. Juttner. 1996. Fischerellin A, a novel photosystem-II-inhibiting allelochemical of the cyanobacterium Fischerella muscicola with antifungal and herbicidal activity. Tetrahedron Lett. 37:6539-6542. [Google Scholar]
- 18.He, C. Q., and C. K. Wang. 2001. Allelopathic effect of Acorus tatarinowii upon algae. J. Environ. Sci. 13:481-484. [PubMed] [Google Scholar]
- 19.Hisashi, K. N. 2003. Allelopathic substances in Pueraria thunbergiana. Phytochemistry 63:577-580. [DOI] [PubMed] [Google Scholar]
- 20.Li, F. M., and H. Y. Hu. 2004. Growth inhibition of water bloom algae by allelopathic effects of macrophytes. China Water Wastewater 20:18-21. [Google Scholar]
- 21.Li, F. M., and H. Y. Hu. 2005. Allelopathic effects of different macrophytes on the growth of Microcystis aeruginosa. Allelopathy J. 15:145-152. [Google Scholar]
- 22.Li, K., L. Li, and H. S. Hou. 2002. Study on toxicity of heavy metal ions totwo species of marine unicellular algae. Chin. J. Appl. Environ. Biol. 8: 395-398. [Google Scholar]
- 23.Maffei, M., C. M. Bertea, and F. Garneri. 1999. Effect of benzoic acid hydroxy- and methoxy-ring substituents during cucumber (Cucumis satius L.) germination. I. Isocitrate lyase and catalase activity. Plant Sci. 141:139-147. [Google Scholar]
- 24.McCann, J. H., and K. R. Solomon. 2002. The effect of creosote on membrane ion leakage in Myriophyllum spicatum L. Aquat. Toxicol. 50:275-284. [DOI] [PubMed] [Google Scholar]
- 25.Mishra, N. P., R. K. Mishra, and G. S. Singhal. 1993. Changes in the activities of anti-oxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol. 102:903-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakai, S., Y. Inoue, and M. Hosomi. 1999. Growth inhibition of blue- green algae by allelopathic effects of macrophytes. Water Sci. Technol. 39: 47-53. [Google Scholar]
- 27.Nakai, S., Y. Inoue, and M. Hosomi. 2000. Myrophyllum spicatum-released allelopathic polyphenoles inhibiting growth of blue-green algae Microcystis aeruginosa. Water Res. 34:3026-3032. [Google Scholar]
- 28.Nakai, S., Y. Inoue, and S. Hosomi. 2001. Growth inhibition effects and inducement modes by plant-producing phenols. Water Res. 35:1855-1859. [DOI] [PubMed] [Google Scholar]
- 29.Nakano, Y., and K. Asada. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22: 867-880. [Google Scholar]
- 30.Ohno, S., K. Tomita-Yokotani, S. Kosemura, M. Node, T. Suzuki, M.Amano, K. Yasui, T. Goto, S. Yamamura, and K. Hasegawa. 2001. A species-selective allelopathic substance from germinating sunflower (Helianthus annuus L.) seeds. Phytochemistry 56:577-581. [DOI] [PubMed] [Google Scholar]
- 31.Oliva, A., R. M. Moraes, and S. B. Watson. 2002. Aryltetralin lignans inhibit plant growth by affecting the formation of mitotic microtubular organizing centers. Pesticide Biochem. Physiol. 72:45-54. [Google Scholar]
- 32.Paoletti, F., D. Aldinucci, A. Mocali, and A. Caparrini. 1986. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal. Biochem. 154:536-541. [DOI] [PubMed] [Google Scholar]
- 33.Prashara, A., P. Hilib, R. G. Venessa, and C. S. Evansa. 2003. Antimicrobial action of palmarosa oil (Cymbopogon martinii) on Saccharomyces cerevisiae. Phytochemistry 63:569-575. [DOI] [PubMed] [Google Scholar]
- 34.Rice, E. L. 1984. Allelopathy, 2nd ed. Academic Press, Ltd., London, United Kingdom.
- 35.Rippka, R., J. Deruelles, and J. B. Waterbury. 1979. Generic assignment, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1-61. [Google Scholar]
- 36.Roginsky, V., and T. Barsukova. 2001. Superoxide dismutase inhibits lipid peroxidation in micelles. Chem. Physics Lipids 111:87-91. [DOI] [PubMed] [Google Scholar]
- 37.Rose, A. H., and F. J. Veazey. 1988. Membranes and lipid of yeast, p. 255-275. In I. Campbell (ed.), Yeast: a practical approach. IRL Press, Oxford, England.
- 38.Schrader, K. K., N. P. D. Nanayakkara, C. S. Tucker, A. M. Rimando, M. Ganzera, and B. T. Schaneberg. 2003. Novel derivatives of 9,10-anthraquinone are selective algicides against the musty-odor cyanobacterium Oscillatoria perornata. Appl. Environ. Microbiol. 69:5319-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srivastava, A., F. Juttner, and R. J. Strasser. 1998. Action of the allelochemical, fischerellin A, on photosystem II. Biochim. Biophys. Acta 1364:326-336. [DOI] [PubMed] [Google Scholar]
- 40.Sun, X. X., J. K. Choi, and E. K. Kim. 2004. A preliminary study on the mechanism of harmful algal bloom mitigation by use of sophorolipid treatment. J. Exp. Mar. Biol. Ecol. 304:35-49. [Google Scholar]
- 41.Vyvyan, J. R. 2002. Allelochemicals as leads for new herbicides and agrochemicals. Tetradron 58:1631-1646. [Google Scholar]
- 42.Yeung, P. K. K., F. T. W. Wong, and J. T. Y. Wong. 2002. Mimosine, the allelochemical from the leguminous tree Leucaena leucocephala, selectively enhances cell proliferation in dinoflagellates. Appl. Environ. Microbiol. 68:5160-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu, Z. W., W. H. Sun, and K. Q. Guo. 1992. Allelopathic effects of several aquatic plants on algae. Acta Hydrobiologica Sinica 16:1-7. [Google Scholar]