Abstract
Lichens are slow-growing associations of fungi and green algae or cyanobacteria. This symbiotic association forms a common thallus that does not possess roots or a waxy cuticle and depends mainly on atmospheric input of mineral nutrients. The lifestyle of most lichens is composed of alternating periods of desiccation with low metabolic activity and hydration that induces increase in their metabolism. We have previously shown that rehydration of the naturally desiccated lichen Ramalina lacera resulted in a rapid increase in photosynthesis and was accompanied by a burst of intracellular production of reactive oxygen species and nitric oxide, as well as a transient decrease in water-soluble antioxidant capacity. We report here on enzymatic antioxidants of R. lacera and their response to rehydration. Native gel electrophoresis of crude extracts of R. lacera stained for superoxide dismutase (SOD) activity revealed four Fe-SOD and four Mn-SOD electromorphs that are synthesized by the alga, a Cu/Zn-SOD and a Mn-SOD that are the product of the fungus, and two catalases synthesized one by the fungus and the other by the algae. In addition, we detected glutathione reductase and glucose-6-phosphate dehydrogenase activities in crude extracts of R. lacera. Rehydration of the thalli resulted in a decrease in SOD activity of all forms, and a transient decrease in total catalase activity, as well as a decrease in the antioxidant auxiliary enzymes glutathione reductase and glucose-6-phosphate dehydrogenase.
Lichens are slow-growing associations of fungi (mycobionts) and photosynthetic partners (photobionts) that may be green algae or cyanobacteria. They do not possess roots or waxy cuticles and depend mainly on an atmospheric input of water and mineral nutrients. Consequently, the entire thallus area of lichens is susceptible to penetration and accumulation of airborne elements, some essential for proper functioning of the lichen but others are toxic. These features combined with their ability to grow at a wide geographical range rank lichens among the best bioindicators of air pollution. Absorption and accumulation of airborne elements by lichens, including heavy metals, and their related physiological responses have been studied extensively (17) and utilized to assist in interpretation of epidemiological studies on human respiratory diseases (11).
Lichens are poikilohydric organisms whose water content strongly depends on environmental conditions. In their natural habitats lichens can survive in extreme environments, some of which are rather dry habitats such as hot deserts, arctic tundras, heaths, and tree canopies. They are able to exist a great part of the time in a dry state with a very low level of metabolic activity (19, 54) but can rapidly resume normal physiological activities upon rehydration. It was shown that water loss from lichen thalli is accompanied by almost total inactivation of photosynthetic gas exchange and loss of variable chlorophyll fluorescence (32, 48), whereas rewetting of the thalli with liquid water normally restores photosynthetic activity within minutes (31, 55).
Metabolic activities, mainly respiration and photosynthesis, frequently result in production of reactive oxygen species (ROS) (16, 23). These are enhanced during stresses such nutrition limitation, exposure to xenobiotics, or desiccation and/or rehydration. To evade the potential damaging effects of ROS, cells have evolved protection mechanisms, including antioxidant enzymes, such as superoxide dismutase (SOD), catalase, and peroxidases, and low-molecular-weight antioxidants. This system also includes auxiliary enzymes, such as glutathione reductase (GRX) and glucose 6-phosphate dehydrogenase (G6PD), that replenish the reducing power. Increased production of ROS was shown to upregulate the cellular levels of antioxidants, but their overproduction may overwhelm the antioxidant system and impose oxidative stress that may result in cellular damage (16, 23). The involvement of ROS and antioxidants in desiccation/rehydration damage and protection in plants has attracted a lot of attention (20). However, there are only scant publications on their role in the complex physiological and biochemical processes that occur during the transition between the desiccated and rehydrated states (see, for example, references 2, 27, 31, 32, and 36).
Ramalina lacera is an epiphytic fruticose (shrub-like) lichen that grows in the Mediterranean parts of Israel, on different shrubs and trees. Its thallus contains an ascomycetous fungus and a trebouxioid unicellular green alga. In a previous study (55) we showed that treatment of naturally desiccated R. lacera thalli with water and incubation at 97% humidity caused a rapid increase in photosynthesis. This was accompanied by a burst of intracellular production of ROS by both the photobiont and the mycobiont that was similar in the light and in the dark, as well as production of NO that we detected only in the fungus. These activities did not cause measurable membrane damage but resulted in a transient decrease of water-soluble low-molecular-weight antioxidant capacity. Beckett and coworkers demonstrated that imbibition of some lichens and bryophytes after desiccation stimulated extracellular superoxide production (2, 36). The scant publications on the antioxidant responses of lichens to rehydration revealed that they increased, decreased, or were not affected depending on the species, methods of desiccation and rehydration, and pretreatment of the thalli prior to the assays, as well as the time course of the experiments (2, 7, 13, 25-29, 34, 36, 51).
The objective of the present study was to characterize the antioxidant systems in R. lacera and investigate the effect of rehydration of naturally desiccated thalli on these enzymatic activities. In the present study, we detected in R. lacera different types of SOD and two enzymes with catalase activity and determined their organismal origin. We assessed the cellular activities of SOD and catalase, as well as the auxiliary enzymes GRX and G6PD in naturally desiccated thalli and report on the kinetics of alterations in their activities after rehydration.
MATERIALS AND METHODS
Materials.
Phenylmethylsulfonyl fluoride, Triton X-100, Trizma base, leupeptine, dithiothreitol (DTT), NADP+, NADPH, oxidized glutathione, glucose-6-phosphate, nitroblue tetrazolium (NBT), flavin-mononucleotide (FMN), diaminobenzidine, horseradish peroxidase, polyvinyl-polypyrrolidone (PVPP), bovine serum albumin, and TEMED (N,N,N′,N′-tetramethylethylenediamine) were purchased from Sigma (St. Louis, MO). KCN, sodium azide, H2O2, MgCl2, and EDTA were purchased from Merck (Darmstadt, Germany).
Source and rehydration treatment of lichen material.
R. lacera was collected from the HaZorea forest (Ramot Menashe, northeast Israel, where it grows on twigs of carob trees [Ceratonia siliqua]), frozen promptly in liquid nitrogen, and stored at −80°C. Prior to the experiments, 0.5-g lichen samples were weighed and defrosted for 30 min at room temperature. For the rehydration experiments, the thalli were soaked in deionized water for 5 min, wiped to remove excess water, and placed inside a glass covered, illuminated growth chamber (80 cm by 41 cm by 7.5 cm high, 25°C, 97% relative humidity (RH), 73 μmol of photons m−2 s−1). To maintain high relative humidity conditions inside the growth chamber, trays containing distilled water were placed at the bottom of the growth chamber and ambient air (55% RH, 25°C) was passed through the chamber at a constant rate of 1,063 ml/min. The air stream served to maintain a constant temperature and a fresh atmosphere inside the growth chamber, as well as to prevent water condensation on its inner walls and glass cover. At the end of each treatment, the lichen samples were frozen promptly in liquid nitrogen and stored at −80°C for enzyme determinations. In control experiments we compared all of the parameters tested in this work, by running the experiments with freshly collected as well as frozen thalli collected at the same time, and found no effect of freezing (not shown)
Enrichment for algal cells.
For identification of the organismal origin (alga or fungus) of catalase or superoxide dismutase, algal cells were isolated from the lichen thalli by the method of Bubrick and Galun (5). Thalli were ground in phosphate-buffered saline (PBS) by using a mortar and pestle and then centrifuged at 3,000 × g for 3 min. The pellet was washed with PBS and centrifuged again. The resulting pellet was resuspended in PBS and filtered through a series of Teflon screens of 30-, 20-, and 10-μm pore diameters. The slurry was then centrifuged at 3,000 × g for 3 min, and the pellet was resuspended in a small volume of PBS. The final suspension was found by microscopic observation to contain mostly intact algal cells, some broken algal cells, and a small amount of broken fungal hyphae but no lichen fragments. Crude extract was prepared from this alga-rich suspension in the same way as from the intact thalli (see below).
Preparation of crude extract.
For assessing enzymatic activities, thalli were ground with liquid nitrogen by using mortar and pestle and suspended in 100 mM sodium phosphate buffer (pH 7.4) containing 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptine/ml, 1 mM EDTA, 3 mM DTT, and 0.1% Triton X-100. The slurry was passed through a French press cell (1,000 lb/in2), followed by the addition of PVPP (15 mg/ml), and the samples were sonicated four times for 30 s each time. The samples were centrifuged at 10,000 × g, 4°C for 15 min, and the supernatant was collected and centrifuged at 226,000 × g and 4°C for 1 h. The resulting supernatant was dialyzed overnight by using a 12- to 14-kDa dialysis membrane (Spectra/Por; Spectrum) against 5 mM potassium phosphate buffer (pH 7.0) containing 5 μM EDTA at 4°C and then lyophilized. Prior to the enzymatic assays, the lyophilized material was resuspended in a minimal volume of 5 mM potassium phosphate buffer (pH 7.0) containing protease inhibitors and 1 mM EDTA. Compared to other methods described in the literature, our method was significantly more efficient in extracting soluble proteins from the cells on the basis of the amount of protein obtained per gram (dry weight) (not shown). The protein concentration was determined using the Bradford method (4) with bovine serum albumin as a standard.
Enzymatic assays. (i) SOD.
Assessment of SOD activity and identification of the various forms of the enzyme and their organismal origin were conducted by gel electrophoresis and staining for SOD activity according to the method of Butow et al. (6) and E. Tel-Or (personal communication). Native polyacrylamide gel electrophoresis (PAGE) was carried out on 14% nondenaturing gels (30), with 20 μg of protein for each lane. Staining for SOD activity was performed by soaking the gels in 100 mM potassium phosphate buffer (pH 7.8) for 10 min, followed by a 30-min immersion in the same buffer containing 2.45 mM nitroblue tetrazolium (NBT), 0.028 mM flavin-mononucleotide (FMN), and 16 mM TEMED in the dark at room temperature. The gels were then illuminated for 5 to 15 min until achromatic bands of SOD were revealed. The metal ions in the active centers of the enzymes were identified by adding inhibitors to the staining solutions: 2 mM KCN to inhibit Cu/Zn-SOD and 2 mM H2O2 to inhibit Fe-SOD (6). Stained gels were scanned with an ImageScanner densitometer and LabScan v3.00 software (Amersham Biosciences), and their image was analyzed quantitatively by using an ImageMaster 1D Prime v3.01 image analysis software (Amersham Biosciences). The background reading was subtracted from the intensity of each enzyme band. The absolute activities of the bands were determined by comparing their intensity with that of purified, recombinant Cu/Zn-SOD (provided by Bio-Technology General, Ltd., Rehovot, Israel) that was applied on each gel. To ensure that the activities assessed from gels correlated with the actual enzymatic rates, activity of the recombinant SOD was assayed by the cytochrome c method (35), and each gel included three lanes with three different concentrations of this standard. The intensities of the bands in these lanes were linear with the number of units in each of them. In addition, for each extract we loaded 2 to 3 lanes whose intensities were linear with the amount of applied protein.
(ii) Catalase.
Catalase activity was assayed polarographically using a Clark-type oxygen electrode (Yellowsprings, OH) by monitoring the initial, linear rate of oxygen production from H2O2 according to the method of Goldberg and Hochman (18). The assay was performed at 30°C in a reaction mixture containing 100 mM potassium phosphate buffer (pH 6.3) and 20 mM H2O2. One unit of enzyme activity was defined as the amount of enzyme that catalyzes the decomposition of 1 μmol of H2O2/min at an initial concentration of 20 mM H2O2. Gel electrophoresis to separate the catalase enzymes and staining for activity was performed according to the method of Goldberg and Hochman (18).
(iii) GRX.
GRX activity was assayed spectrophotometrically (using a microplate reader Spectramax 190 [Molecular Devices Co.]) in 96-well microtiter plates (Costar; Corning, Inc.) at 30°C by monitoring the oxidation of NADPH at 340 nm according to the method of Carlberg and Mannervik (8) with slight modifications. The reaction mixture contained 0.2 mM NADPH, 100 mM potassium phosphate buffer (pH 7.4), 1 mM EDTA, 2 mM oxidized glutathione, and 5 to 20 μg of sample protein in a total volume of 200 μl. One unit of enzyme activity was defined as the amount of enzyme that catalyzes the oxidation of 1 μmol of NADPH per min.
(iv) G6PD.
Glucose 6-phosphate dehydrogenase (G6PD) activity was assayed spectrophotometrically (using the microplate reader Spectramax 190) in 96-well microtiter plates (Costar) at 30°C by monitoring the reduction of NADP+ at 340 nm according to the method of Ben-Bassat and Goldberg (3) with slight modifications. The reaction mixture contained: 4 mM glucose 6-phosphate, 40 mM Tris-HCl (pH 8.2), 5 mM MgCl2, 0.6 mM NADP+, and 2 to 8 μg of sample protein in a total volume of 200 μl. One unit of enzyme activity was defined as the amount of enzyme that catalyzes the reduction of 1 μmol of NADP+ per min.
Statistical analysis.
The results were evaluated by using SPSS software (version 10). One-way analysis of variance (ANOVA) and Tukey honestly significant difference tests were applied to determine the significance (P < 0.05) of the differences between the treatments.
RESULTS
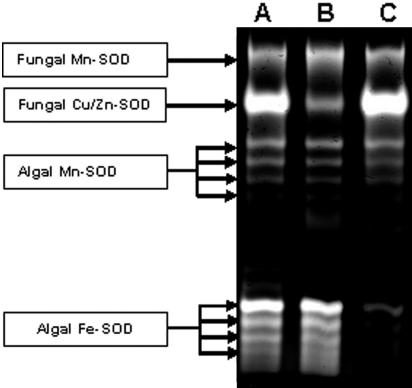
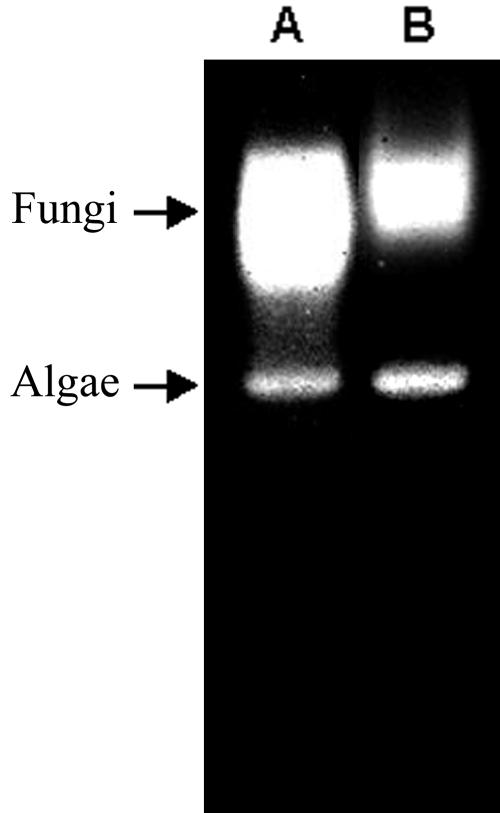
R. lacera synthesizes at least 10 electromorphs displaying SOD activity, as detected by native gel electrophoresis of crude extracts prepared from whole thalli (Fig. 1, lane A). After enrichment for algal cells, there was decrease in the intensities of the two upper bands (Fig. 1, lane B), indicating that these two electromorphs are synthesized by the fungus while the other eight electromorphs are product of the alga. Using specific inhibitors, H2O2 for Fe-SOD and KCN for Cu/Zn-SOD (6), it was found that the two fungal electromorphs are Cu/Zn-SOD and Mn-SOD (Fig. 2, lane B), whereas four of the algal electromorphs are Fe-SODs and four are Mn-SODs (Fig. 2, lane C). Electropherograms of crude extracts from R. lacera stained for catalase activity revealed two distinct catalase bands (Fig. 3, lane A). In a preparation enriched for algal cells there was a relative decrease in the upper band (Fig. 3, lane B), indicating that this electromorph is the product of the fungus, whereas the lower one is synthesized by the alga.
FIG. 1.
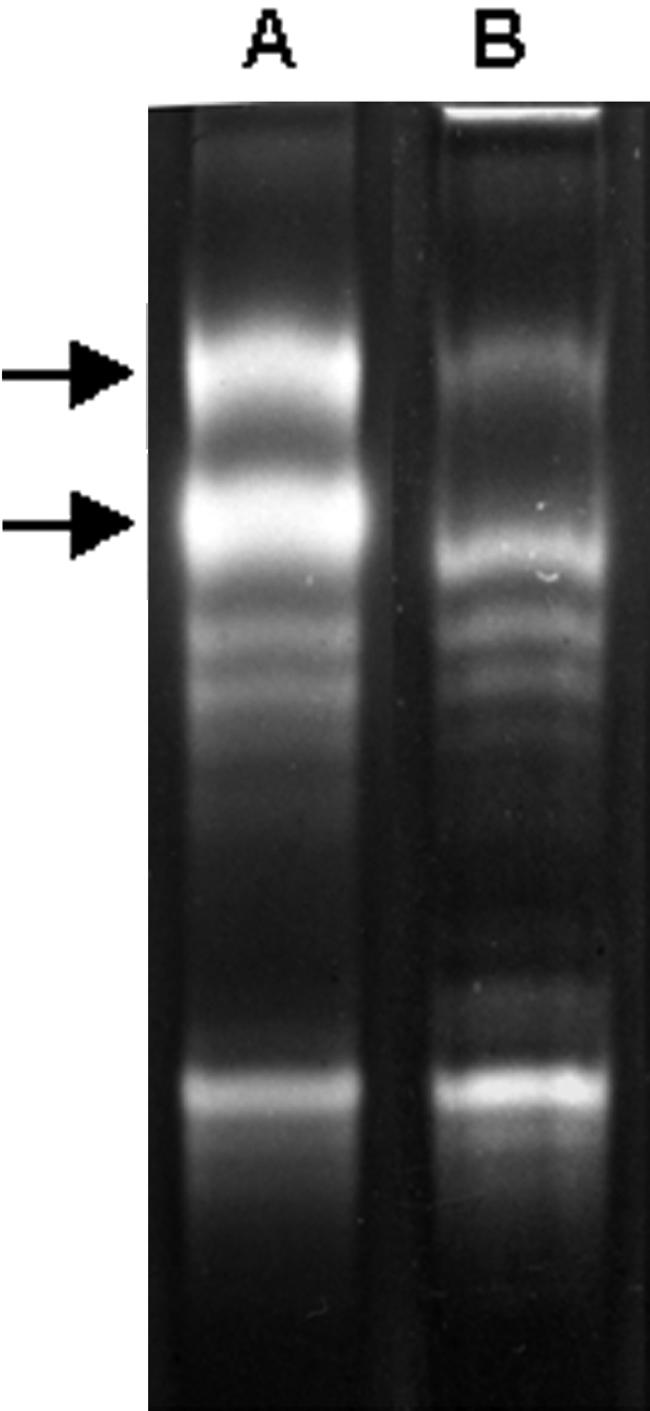
Identification of fungal and algal electromorphs of superoxide dismutase in R. lacera. Native-PAGE stained for SOD activity of crude extract from R. lacera (lane A) and algal enriched extracts (lane B). A total of 20 μg of proteins were loaded in each lane. Arrows indicate fungal electromorphs.
FIG. 2.
Identification of the various forms of superoxide dismutase in R. lacera. Native-PAGE stained for SOD activity of crude extract (shown for comparison) (lane A), crude extract treated with 2 mM KCN to inhibit Cu/Zn-SOD (lane B), and crude extract treated with 2 mM H2O2 to inhibit Fe-SOD (lane C). A total of 20 μg of proteins was loaded into each lane.
FIG. 3.
Identification of fungal and algal catalases in R. lacera. Native-PAGE stained for catalase activity of crude extract from R. lacera (lane A) and algal enriched extracts (lane B). A total of 20 μg of proteins were loaded into each lane. The arrow indicates fungal catalase.
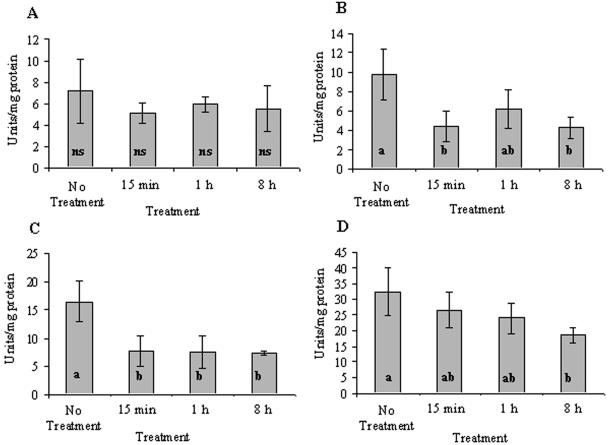
Rehydration of the thalli resulted in a decrease in SOD activity of all forms (Fig. 4). Algal Mn-SODs had a total activity of 7 U/mg of protein in untreated thalli (Fig. 4A). This activity decreased nonsignificantly (probably due to high standard deviation) to 72% of the untreated thalli after 15 min and remained lower than the control for the rest of the experiment. Algal Fe-SODs in untreated thalli that had a total activity of 9.8 U/mg of protein decreased to 45% (P = 0.018) of the untreated thalli and remained low for the rest of the experiment (Fig. 4B). Fungal Mn-SOD in untreated thalli had an activity of 16.5 U/mg of protein, which decreased to 46% (P = 0.013) of the untreated thalli value 15 min after rehydration and remained at this level until the end of the experiment (Fig. 4C). Fungal Cu/Zn-SOD in untreated thalli had a relatively high activity of 32 U/mg of protein, which decreased with time reaching 58% (P = 0.049) of the initial value 8 h after rehydration (Fig. 4D).
FIG. 4.
Activity of the various forms of algal and fungal SOD in untreated and rehydrated R. lacera placed in the growth chamber: algal Mn-SOD (A), algal Fe-SOD (B), fungal Mn-SOD (C), and fungal Cu/Zn-SOD (D). Lichen samples were soaked in deionized water for 5 min, wiped to remove excess water, and placed inside a growth chamber (25°C, 97% RH, 73 μmol of photons m−2 s−1). The results are expressed as means ± the standard deviation (SD) (n = 4). The values of treatments denoted by different letters differ significantly by one-way ANOVA and Tukey HSD test: a, nonsignificant (ns); b, P = 0.018, F ratio = 5.753; c, P = 0.013, F ratio = 7.627; d, P = 0.049, F ratio = 3.741.
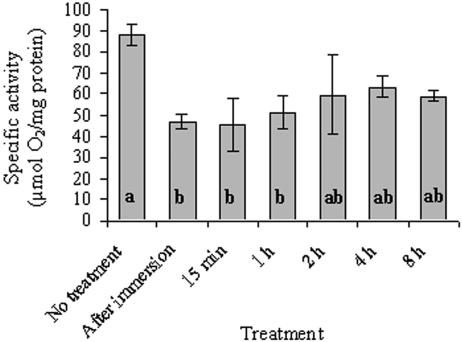
Untreated thalli of R. lacera had a relatively high total catalase activity, 88 U/mg of protein (Fig. 5). Rehydration of the thalli resulted in a significant (P = 0.004) decrease in catalase activity to ca. 50% of the control after immersion, and it lasted up to 1 h. After 1 h this activity increased somewhat to values that were not significantly lower relative to the control. In crude extracts prepared from hydrated thalli we could not detect the algal catalase activity band on PAGE. This suggests that total catalase activity that we measured polarographically in rehydrated thalli is contributed mostly by the fungus.
FIG. 5.
Catalase activity in untreated and rehydrated R. lacera placed in the growth chamber. Lichen samples were soaked in deionized water for 5 min, wiped to remove excess water, and placed inside a growth chamber (25°C, 97% RH, 73 μmol of photons m−2 s−1). The results are expressed as means ± the SD (n = 4). The values of treatments denoted by different letters differ significantly by one-way ANOVA and Tukey HSD test (P = 0.004, F ratio = 6.129).
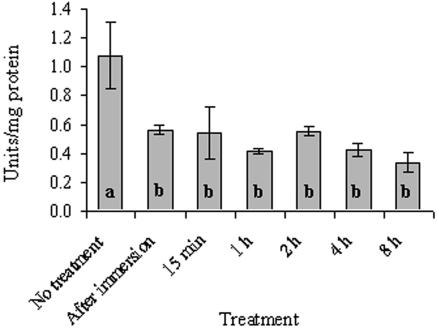
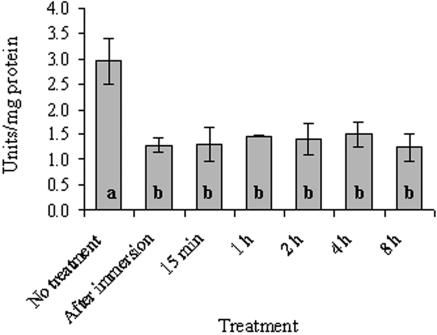
Activity levels of GRX and G6PD also responded to rehydration by a decrease in activities. After a 5-min soaking in water, GRX decreased to 52% (P < 0.001) (Fig. 6) and G6PD decreased to 44% (P < 0.001) (Fig. 7) of the control values (1.08 and 2.95 U/mg of protein, respectively). The activity of both enzymes remained at these lower levels up to 8 h of incubation in the growth chamber.
FIG. 6.
GRX activity in untreated and rehydrated R. lacera placed in the growth chamber. Lichen samples were soaked in deionized water for 5 min, wiped to remove excess water, and placed inside a growth chamber (25°C, 97% RH, 73 μmol of photons m−2 s−1). The results are expressed as means ± the SD (n = 4). The values of treatments denoted by different letters differ significantly by one-way ANOVA and Tukey HSD test (P < 0.001, F ratio = 13.346).
FIG. 7.
G6PD activity in untreated and rehydrated R. lacera placed in the growth chamber. Lichen samples were soaked in deionized water for 5 min, wiped to remove excess water, and placed inside a growth chamber (25°C, 97% RH, 73 μmol of photons m−2 s−1). The results are expressed as means ± the SD (n = 4). The values of treatments denoted by different letters differ significantly by one-way ANOVA and Tukey HSD test (P < 0.001, F ratio = 12.648).
DISCUSSION
In the present study we present novel findings on the various forms of SOD and catalase in R. lacera, and their organismal origin. We characterized in this lichen cellular activities and the effect of rehydration on these antioxidant enzymes, as well as the auxiliary enzymes GRX and G6PD.
We have found that total SOD activity in untreated R. lacera was 65 U/mg of protein, which is within the wide range of such activities, 32 to 697 U/mg of protein (24, 34), reported for other lichens. This total activity was composed of one fungal Mn-SOD, one fungal Cu/Zn-SOD, four algal Mn-SOD electromorphs, and four algal Fe-SOD electromorphs. At present it is not clear whether the four algal electrophoretic bands displaying Mn-SOD or Fe-SOD were protein products of four respective different genes or gene duplicates that underwent some modifications. Alternatively, this multiplicity may be the result of posttranslational modifications. Posttranslational modifications of SODs were shown to be induced, among others, by ROS or aging, in rats, plants, drosophila, yeast, and fungi (1, 12, 21, 22, 33, 44). Lichens grow slowly, and a thallus may contain young cells as well as older ones that are indistinguishable by their appearance from the rest of the thallus. Furthermore, since thalli undergo cycles of desiccation and rehydration, they are continuously exposed to ROS (55). Multiple forms of SOD were previously reported in other lichens (46, 51), but there is only one publication on an attempt to identify and characterize its various forms in each of the symbionts. Silberstein et al. (51) detected in R. lacera collected from the HaZorea forest (the same lichen species and collection site as in the present study) only three such enzymes: a fungal Mn-SOD, an algal Fe-SOD, and a third one that was not identified. These authors did not detect a Cu/Zn-type SOD, and there was no report on the cellular activities of these enzymes. We suggest that these authors were not able to detect the full complement of enzymes due to one or more differences in their experimental procedure. The thalli were rehydrated before the experiment, and the methods for preparation of crude extract and assaying the activity were not optimal. In the present study we used naturally desiccated thalli and a complex technique for cell disruption to ensure maximum efficiency in breaking the cells and the organelles: grinding in liquid nitrogen, passage through a French press cell, and sonication. Furthermore, the extraction buffer included reagents to minimize inactivation and degradation of the enzymes such as DTT, protease inhibitors, and PVPP. Silberstein et al. (51), on the other hand, ground the thalli with glass beads in phosphate buffer containing only EDTA and sodium ascorbate.
We detected in R. lacera two enzymes displaying catalase activity, with a total activity of 88 U/mg of protein in untreated thalli. One major enzyme was synthesized by the fungus, which contributed 90% to the total activity, and a minor enzyme activity was contributed by the algal cells. Catalase activity in lichens was shown to vary greatly between species and depends on the experimental conditions (see, for example, references 34 and 51). To the best of our knowledge, the present study is the first to report on the differential contribution of each of the symbionts to catalase activity in a lichen.
Our findings show that rehydration of R. lacera caused a rapid decrease of 50 to 70% in activities of SOD, catalase, GRX, and G6PD. This decrease in enzymatic antioxidants may be simply the result of inactivation of the enzymes by interaction with ROS that are produced during rehydration (55). ROS such as hydroxyl radical and hydrogen peroxide were shown to cause inactivation and fragmentation of SOD enzymes (9). Catalase may be converted to its inactive derivatives “compound II” and “compound III” by interaction with H2O2 (47). The fact that we could not detect the algal catalase enzyme in the hydrated state may suggest that it is more sensitive to inactivation. It does not imply that the algae are less protected against ROS since it is well documented that peroxidases can function efficiently as a hydrogen peroxide scavenging enzyme in these organisms (50). GRX and G6PD were also shown previously to be inactivated and/or degraded by exposure to ROS (15, 37, 40, 45).
An apparent decrease in cellular activities of antioxidant enzymes in the hydrated thalli or their increase after desiccation may reflect general changes in cellular protein concentrations. Selective decrease in total cell proteins during desiccation takes place as part of the preparation for survival in the dried state. Such effects were reported for plants and seeds undergoing dehydration and were dependent on an increase in proteolytic activities (10, 14, 49). Various proteases were identified and characterized whose transcript and activities increased in response to desiccation or rehydration and, in some cases, their activity was mediated by ubiquitin (38, 39). It was proposed that the role of these changes in protein concentration was to eliminate proteins that were denatured because of the stresses or to mobilize amino acids required for synthesis of new proteins (53). Accordingly, the decrease in enzyme activities upon rehydration may reflect selective enhanced protein synthesis that resulted in increase in total cellular proteins, which in turn was manifested as an apparent decrease in specific cellular activities of the enzymes. Alternatively, the decrease in activities may be the expression of readjustment of cellular concentrations of these enzymes to a metabolically active state. We propose that during desiccation in the field, and sometimes under laboratory conditions, there is an increase in cellular activities of antioxidant enzymes, which upon rehydration resume a lower activity typical of hydrated thalli. The increase could be a response to ROS produced during the process of drying as it is well documented that cellular antioxidants are upregulated under conditions of enhanced production of ROS (see, for example, references 42, 43, and 56). ROS production upon desiccation is not yet documented in lichens but was found in other organisms such as yeasts and plants (see, for example, references 41 and 52) and, in accordance with this, it was shown that desiccation caused an increase in antioxidant enzymes, including in lichens (see, for example, references 41 and 29).
There are several reports in the literature on the effect of rehydration on antioxidant enzymes of lichens, but the extent and the direction of the response vary with lichen species and the experimental conditions (25-28, 34). For example, Mayaba and Beckett (34) reported that SOD and catalase activities in the desert lichen Teloschistes capensis increased significantly after rehydration with air at 100% RH for 24 h, followed by contact with wet filter paper for a further 24 h. However, in the same study, these activities in the lichen Ramalina celastri from a moderately xeric habitat and Peltigera polydactyla from a moist habitat did not change significantly after rehydration. The effects of the experimental conditions on the apparent activities of antioxidant enzymes subjected to rehydration may be exemplified by a set of experiments with the lichen Pseudevernia furfuracea, a canopy species, which is often exposed to extreme desiccation. Thalli were collected in the field, dried in a cool room for 2 days and then desiccated over silica gel. After desiccation for at least 3 days, rehydration in liquid water for 30 s resulted in a decrease in G6PD and GRX activities, whereas after rehydration for 2 min the GRX activity increased back to the level of dry thalli and G6PD activity increase (27). In a different study, desiccation of this lichen in a desiccator for 2 days, followed by rehydration for 5 min, resulted in an increase in G6PD activity but not GRX (26). However, in another study (25), thalli of the same lichen were exposed to air of 100% RH for 12 h, allowed to dry at ambient air for 12 h, and then placed in a desiccator for 2 days. Rehydration of this lichen for 5 min at the end of this procedure resulted in an increase of GRX and G6PD activities, but after 15 min rehydration it decreased and increased again after 30 min of rehydration.
In summary, we characterized here SOD and catalase activities in R. lacera, and identified their organismal origin. In experiments conducted with an attempt to simulate natural environmental conditions by using in situ-dried thalli, we showed that rehydration of this lichen resulted in a decrease in the activities of the antioxidant enzymes SOD and catalase, as well as the auxiliary enzymes GRX and G6PD. These changes may result either from inactivation of the enzymes by ROS, from an increase in activities during dehydration, or from degradation of total cellular proteins during desiccation and their resynthesis upon rehydration, as part of adaptation of the thalli to a new survival regime. In addition, our findings should serve as a cautionary suggestion for scientists engaged in lichen research regarding pretreatments as well as treatment of thalli for conducting experiments. R. lacera used in our study is a moderately xeric species. In its natural habitat it may encounter long days of desiccation and get rehydrated by dew during the night or by rain during the rainy season. In order to simulate natural conditions, we collected dry thalli from the field and kept them in this state until further treatments. Furthermore, in the rehydration protocol we made sure that saturation of water content has been attained. In other studies (25, 27, 57), the pretreatments may have altered the physiology and biochemistry of the thalli, and the results of increase or decrease of enzymatic activities reflected the effects of these pretreatments. In addition, the differences among previous studies and between them and our data may reflect the variation of extraction methods. However, we believe that in the present study we attempted to simulate in laboratory experiments as close as possible the changes in antioxidant enzymes that take place in R. lacera upon rehydration under natural conditions.
Acknowledgments
This research was supported by grant 2001238 from the United States-Israel Binational Science Foundation, Jerusalem, Israel.
We thank Haya Lehr for technical assistance. We also thank Rami Nimrod from Bio-technology General, Ltd., Rehovot, Israel, for kindly providing recombinant SOD.
REFERENCES
- 1.Arking, R., V. Burde, K. Graves, R. Hari, E. Feldman, A. Zeevi, S. Soliman, A. Saraiya, S. Buck, J. Vettraino, and K. Sathrasala. 2000. Identical longevity phenotypes are characterized by different patterns of gene expression and oxidative damage. Exp. Gerontol. 35:353-373. [DOI] [PubMed] [Google Scholar]
- 2.Beckett, R. P., F. V. Minibayeva, N. N. Vylegzhanina, and T. Tolpysheva. 2003. High rates of extracellular superoxide production by lichens in the suborder Peltigerineae correlate with indices of high metabolic activity. Plant Cell Environ. 26:1827-1837. [Google Scholar]
- 3.Ben-Bassat, A., and I. Goldberg. 1980. Purification and properties of glucose-6-phospate dehydrogenase (NADP+/NAD+) and 6-phosphogluconate dehydrogenase (NADP+/NAD+) from methanol-grown Pseudomonas. Biochim. Biophys. Acta 611:1-10. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Bubrick, P., and M. Galun. 1980. Symbiosis in lichens: differences in cell wall properties of freshly isolated and cultured phycobionts. FEMS Microbiol. Lett. 7:311-313. [Google Scholar]
- 6.Butow, B. J., D. Wynne, and E. Tel-Or. 1997. Superoxide dismutase activity in Peridinium gatunense in Lake Kinneret: effect of light regime and carbon dioxide concentration. J. Phycol. 33:787-793. [Google Scholar]
- 7.Calatayud, A., V. I. Deltoro, A. Abadia, J. Abadia, and E. Barreno. 1999. Effects of ascorbate feeding on chlorophyll fluorescence and xanthophyll cycle components in the lichen Parmelia quercina (Willd.) Vainio exposed to atmospheric pollutants. Physiol. Plant 105:679-684. [Google Scholar]
- 8.Carlberg, I., and B. Mannervik. 1985. Glutathione reductase. Methods Enzymol. 113:484-490. [DOI] [PubMed] [Google Scholar]
- 9.Casano, L. M., L. D. Gomez, H. R. Lascano, C. A. Gonzalez, and V. S. Trippi. 1997. Inactivation and degradation of CuZn-SOD by active oxygen species in wheat chloroplasts exposed to photooxidative stress. Plant Cell Physiol. 38:433-440. [DOI] [PubMed] [Google Scholar]
- 10.Chaitanya, K. S. K., S. Keshavkant, and S. C. Naithani. 2000. Changes in total protein and protease activity in dehydrating recalcitrant sal (Shorea robusta) seeds. Silva Fenn. 34:71-77. [Google Scholar]
- 11.Cislaghi, C., and P. L. Nimis. 1997. Lichens, air pollution, and lung cancer. Nature 387:463-464. [DOI] [PubMed] [Google Scholar]
- 12.Cyrne, L., L. Martins, L. Fernandes, and H. S. Marinho. 2003. Regulation of antioxidant enzymes gene expression in the yeast Saccharomyces cerevisiae during stationary phase. Free Rad. Biol. Med. 34:385-393. [DOI] [PubMed] [Google Scholar]
- 13.Deltoro, V. I., C. Gimeno, A. Calatayud, and E. Barreno. 1999. Effects of SO2 fumigations on photosynthetic CO2 gas exchange, chlorophyll a fluorescence emission, and antioxidant enzymes in the lichens Evernia prunastri and Ramalina farinacea. Physiol. Plant 105:648-654. [Google Scholar]
- 14.Dhindsa, R. S. 1991. Drought stress, enzymes of glutathione metabolism, oxidation injury, and protein-synthesis in Tortula ruralis. Plant Physiol. 95:648-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dincer, Y., T. Akcay, Z. Alademir, and H. Ilkova. 2002. Effect of oxidative stress on glutathione pathway in red blood cells from patients with insulin-dependent diabetes mellitus. Metabolism 51:1360-1362. [DOI] [PubMed] [Google Scholar]
- 16.Fridovich, I. 1999. Fundamental aspects of reactive oxygen species, or what's the matter with oxygen? Ann. N. Y. Acad. Sci. 893:13-18. [DOI] [PubMed] [Google Scholar]
- 17.Garty, J. 2001. Biomonitoring atmospheric heavy metals with lichens: theory and application. Crit. Rev. Plant Sci. 20:309-371. [Google Scholar]
- 18.Goldberg, I., and A. Hochman. 1989. Purification and characterization of a novel type of catalase from the bacterium Klebsiella pneumoniae. Biochim. Biophys. Acta 991:330-336. [DOI] [PubMed] [Google Scholar]
- 19.Green, T. G. A., and O. L. Lange. 1995. Photosynthesis in poikilohydric plants: a comparison of lichens and bryophytes, p. 319-341. In E. D. Schulze and M. M. Caldwell (ed.), Ecophysiology of photosynthesis. Springer, Berlin, Germany.
- 20.Hoekstra, F. A., E. A. Golovina, and J. Buitink. 2001. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 6:431-438. [DOI] [PubMed] [Google Scholar]
- 21.Hollander, J., J. Bejma, T. Ookawara, H. Ohno, and L. L. Ji. 2000. Superoxide dismutase gene expression in skeletal muscle: fiber-specific effect of age. Mech. Ageing Dev. 116:33-45. [DOI] [PubMed] [Google Scholar]
- 22.Jacob, C., M. Courbot, A. Brun, H. M. Steinman, J. P. Jacquot, B. Botton, and M. Chalot. 2001. Molecular cloning, characterization and regulation by cadmium of a superoxide dismutase from the ectomycorrhizal fungus Paxillus involutus. Eur. J. Biochem. 268:3223-3232. [DOI] [PubMed] [Google Scholar]
- 23.Kohen, R., and A. Nyska. 2002. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 30:620-650. [DOI] [PubMed] [Google Scholar]
- 24.Kong, F. X., W. Hu, S. Y. Chao, W. L. Sang, and L. S. Wang. 1999. Physiological responses of the lichen Xanthoparmelia mexicana to oxidative stress of SO2. Environ. Exp. Bot. 42:201-209. [Google Scholar]
- 25.Kranner, I. 2002. Glutathione status correlates with different degrees of desiccation tolerance in three lichens. New Phytol. 154:451-460. [DOI] [PubMed] [Google Scholar]
- 26.Kranner, I., and D. Grill. 1997. Desiccation of lichens: changes in the glutathione status, p. 253-255. In W. J. Cram, L. J. D. Kok, I. Stulen, C. Brunold, and H. Rennenberg (ed.), Sulphur metabolism in higher plants. Backhuys Publishers, Leiden, The Netherlands.
- 27.Kranner, I., and D. Grill. 1994. Rapid changes of the glutathione status and the enzymes involved in the reduction of glutathione-disulfide during the initial stage of wetting of lichens. Crypt. Bot. 4:203-206. [Google Scholar]
- 28.Kranner, I., and D. Grill. 1997. The glutathione status during recovery of desiccated lichens: is desiccation tolerance correlated with a high reducing capacity for glutathione disulphide?, p. 249-252. In W. J. Cram, L. J. D. Kok, I. Stulen, C. Brunold, and H. Rennenberg (ed.), Sulphur metabolism in higher plants. Backhuys Publishers, Leiden, The Netherlands.
- 29.Kranner, I., M. Zorn, B. Turk, S. Wornik, R. P. Beckett, and F. Batic. 2003. Biochemical traits of lichens differing in relative desiccation tolerance. New Phytol. 160:167-176. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 31.Lange, O. L., W. Bilger, S. Rimke, and U. Schreiber. 1989. Chlorophyll fluorescence of lichens containing green and blue-green algae during hydration by water vapor uptake and by addition of liquid water. Bot. Acta 102:306-313. [Google Scholar]
- 32.Lange, O. L., and J. D. Tenhunen. 1982. Water relations and photosynthesis of desert lichens. J. Hattori Bot. Lab. 53:309-313. [Google Scholar]
- 33.Macmillan-Crow, L. A., and D. L. Cruthirds. 2001. Invited review: manganese superoxide dismutase in disease. Free Rad. Res. 34:325-336. [DOI] [PubMed] [Google Scholar]
- 34.Mayaba, N., and R. P. Beckett. 2001. The effect of desiccation on the activities of antioxidant enzymes in lichens from habitats of contrasting water status. Symbiosis 31:113-121. [Google Scholar]
- 35.McCord, J. M., and I. Fridovich. 1969. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244:6049-6055. [PubMed] [Google Scholar]
- 36.Minibayeva, F., and R. P. Beckett. 2001. High rates of extracellular superoxide production in bryophytes and lichens, and an oxidative burst in response to rehydration following desiccation. New Phytol. 152:333-341. [Google Scholar]
- 37.Ninfali, P., M. Ditroilo, S. Capellacci, and E. Biagiotti. 2001. Rabbit brain glucose-6-phosphate dehydrogenase: biochemical properties and inactivation by free radicals and 4-hydroxy-2-nonenal. Neurochemistry 12:4149-4153. [DOI] [PubMed] [Google Scholar]
- 38.Oliver, M. J., T. Zultan, and B. T. Mishler. 2000. The evolution of vegetative desiccation tolerance in land plants. Plant Ecol. 151:85-100. [Google Scholar]
- 39.O'Mahony, P. J., and M. J. Oliver. 1999. Characterization of a desiccation-responsive small GTP-binding protein (Rab2) from the desiccation-tolerant grass Sporobolus stapfianus. Plant Mol. Biol. 39:809-821. [DOI] [PubMed] [Google Scholar]
- 40.Park, J. E., J. H. Yang, S. J. Yoon, J. H. Lee, E. S. Yang, and J. W. Park. 2003. Lipid peroxidation-mediated cytotoxicity and DNA damage in U937 cells. Biochimie 84:1198-1204. [DOI] [PubMed] [Google Scholar]
- 41.Pereira, E. D., A. D. Panek, and E. C. A. Eleutherio. 2003. Protection against oxidation during dehydration of yeast. Cell Stress Chaperon. 8:120-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinheiro, R., I. Belo, and M. Mota. 2002. Oxidative stress response of Kluyveromyces marxianus to hydrogen peroxide, paraquat and pressure. Appl. Microbiol. Biotechnol. 58:842-847. [DOI] [PubMed] [Google Scholar]
- 43.Rabinowitch, H. D., D. A. Clare, J. D. Crapo, and I. Fridovich. 1983. Positive correlation between superoxide dismutase and resistance to paraquat toxicity in the green alga Chlorella sorokiniana. Arch. Biochem. Biophys. 225:640-648. [DOI] [PubMed] [Google Scholar]
- 44.Rubio, M. C., J. Ramos, K. J. Webb, F. R. Minchin, E. Gonzalez, C. Arrese-Igor, and M. Becana. 2001. Expression studies of superoxide dismutase in nodules and leaves of transgenic alfalfa reveal abundance of iron-containing isozymes, posttranslational regulation, and compensation of isozyme activities. Mol. Plant Microbe 14:1178-1188. [DOI] [PubMed] [Google Scholar]
- 45.Savvides, S. N., M. Scheiwein, C. C. Bohme, G. E. Arteel, P. A. Karplus, K. Becker, and R. H. Schirmer. 2002. Crystal structure of the antioxidant enzyme glutathione reductase inactivated by peroxynitrite. J. Biol. Chem. 277:2779-2784. [DOI] [PubMed] [Google Scholar]
- 46.Schlee, D., R. Kandzia, H. Tintemann, and R. Turk. 1995. Activity of superoxide dismutase and malondialdehyde content in lichens along an altitude profile. Phyton (Horn) 35:233-242. [Google Scholar]
- 47.Schonbaum, G. R., and B. Chance. 1976. Catalase, p. 363-408. In P. D. Boyer (ed.), The enzymes, 2nd ed., vol. 13. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 48.Schroeter, B., P. Jacobsen, and L. Kappen. 1991. Thallus moisture and microclimatic control of CO2 exchange of Peltigera aphthosa (L) Willd on Disko Island (West Greenland). Symbiosis 11:131-146. [Google Scholar]
- 49.Sgherri, C. L. M., B. Loggini, S. Puliga, and F. Navari-izzo. 1994. Antioxidant system in Sporobolus stapfianus: changes in response to desiccation and rehydration. Phytochemistry 35:561-565. [Google Scholar]
- 50.Shigeoka, S., T. Ishikawa, M. Tamoi, Y. Miyagawa, T. Takeda, Y. Yabuta, and K. Yoshimura. 2002. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 53:1305-1319. [PubMed] [Google Scholar]
- 51.Silberstein, L., B. Z. Siegel, S. M. Siegel, A. Mukhtar, and M. Galun. 1996. Comparative studies on Xanthoria parietina, a pollution-resistant lichen, and Ramalina duriaei, a sensitive species: II. Evaluation of possible air pollution-protection mechanisms. Lichenologist 28:367-383. [Google Scholar]
- 52.Smirnoff, N. 1993. Tansley review. 52. The role of active oxygen in the response of plants to water-deficit and desiccation. New Phytol. 125:27-58. [DOI] [PubMed] [Google Scholar]
- 53.Stroeher, V. L., J. L. Maclagan, and A. G. Good. 1997. Molecular cloning of a Brassica napus cysteine protease gene inducible by drought and low temperature stress. Physiol. Plant. 101:389-397. [Google Scholar]
- 54.Sundberg, B., K. Palmqvist, P. A. Esseen, and K. E. Renhorn. 1997. Growth and vitality of epiphytic lichens. II. Modelling of carbon gain using field and laboratory data. Oecologia 109:10-18. [DOI] [PubMed] [Google Scholar]
- 55.Weissman, L., J. Garty, and A. Hochman. 2005. Rehydration of the lichen Ramalina lacera results in production of reactive oxygen species and nitric oxide and a decrease in antioxidants. Appl. Environ. Microbiol. 71:2121-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu, X. Q., L. P. Li, and S. Q. Pan. 2001. Feedback regulation of an Agrobacterium catalase gene katA involved in Agrobacterium-plant interaction. Mol. Microbiol. 42:645-657. [DOI] [PubMed] [Google Scholar]
- 57.Zorn, M., H. W. Pfeifhofer, D. Grill, and I. Kranner. 2001. Responses of plastid pigments to desiccation and rehydration in the desert lichen Ramalina maciformis. Symbiosis 31:201-211. [Google Scholar]